Synthesis and Electrochemical Energy Storage Applications of Micro/Nanostructured Spherical Materials
Abstract
:1. Introduction
2. Synthesis of Micro/nanostructured Spherical Materials
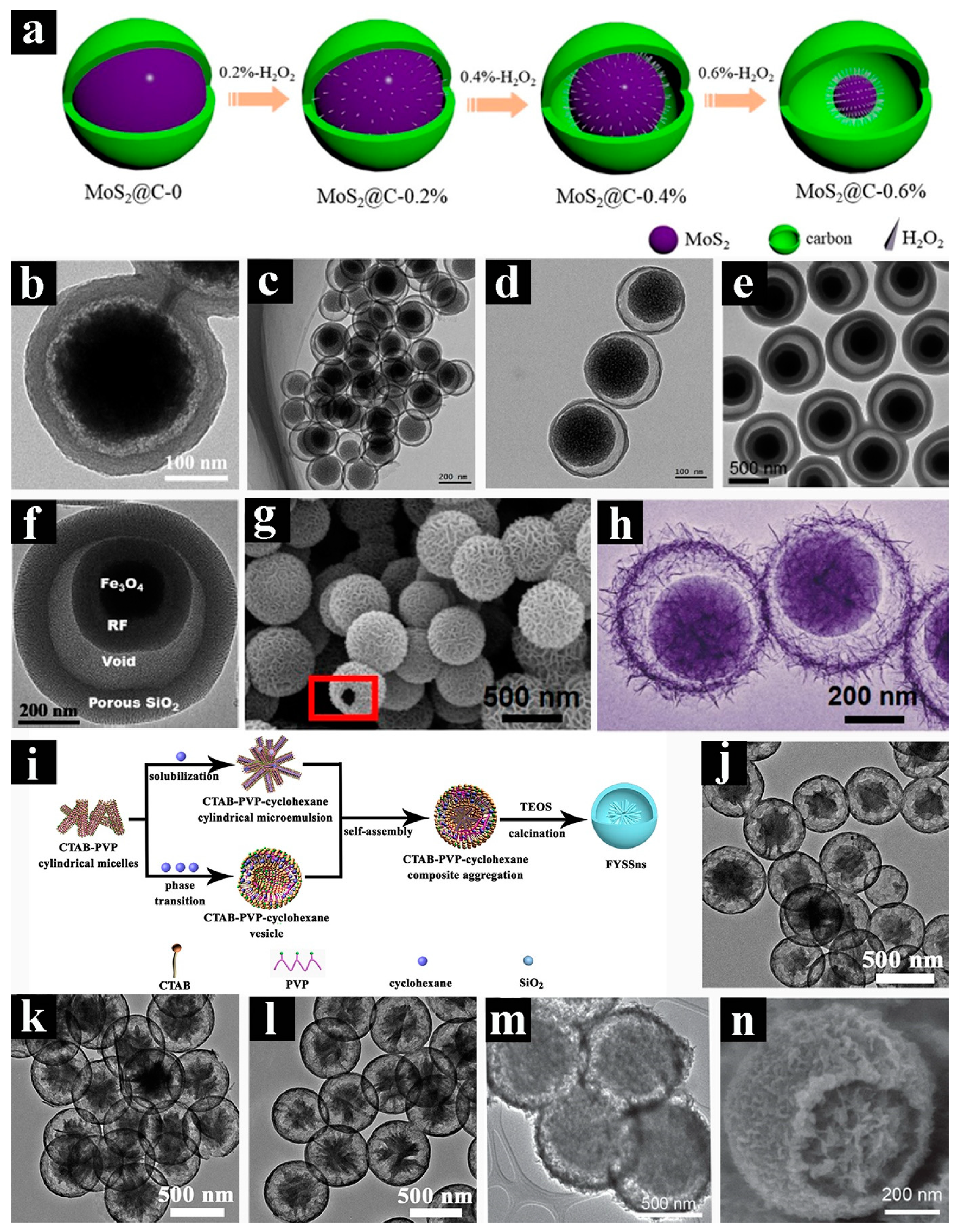
2.1. Hollow Spheres with Complex Architectures
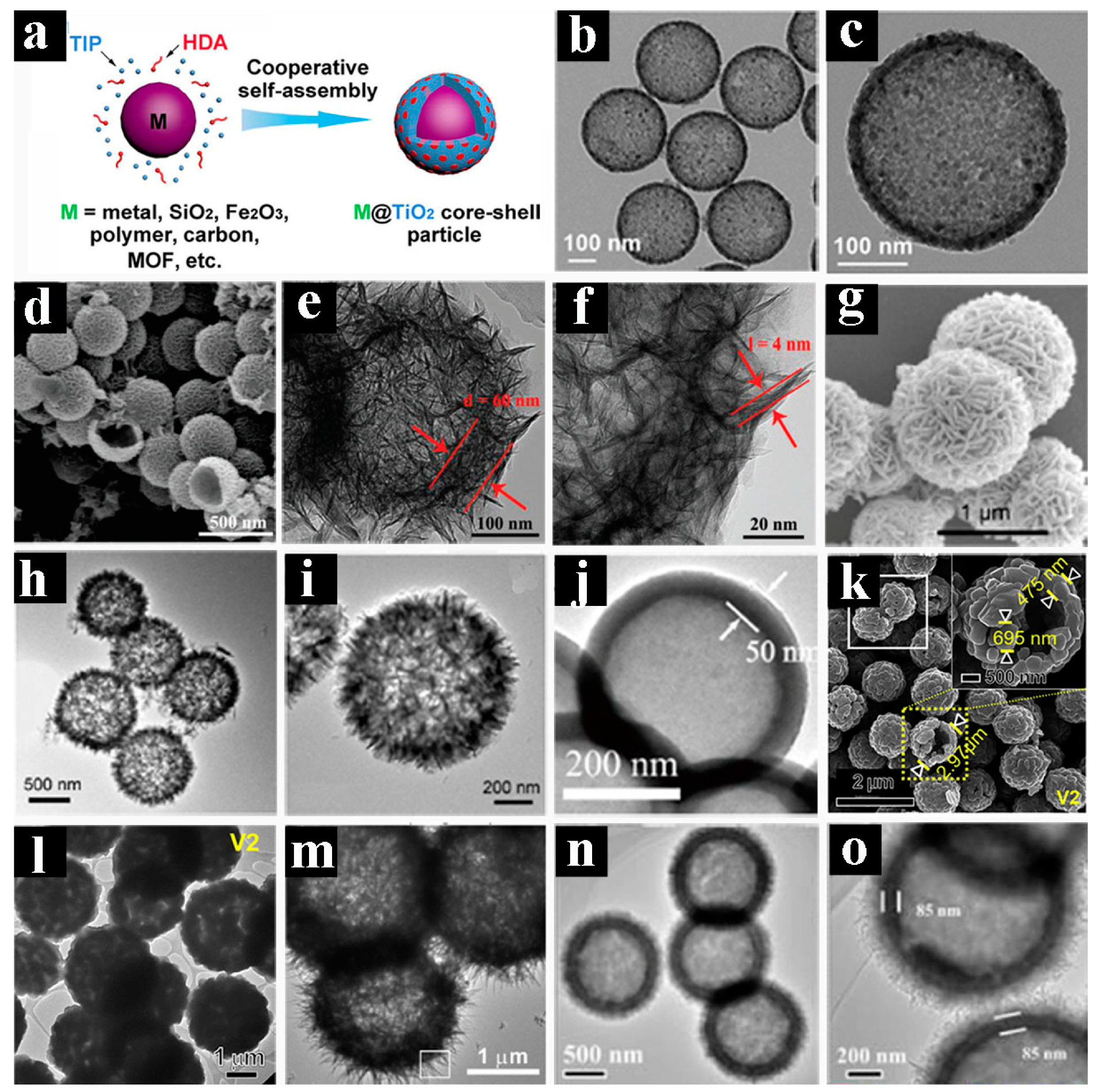
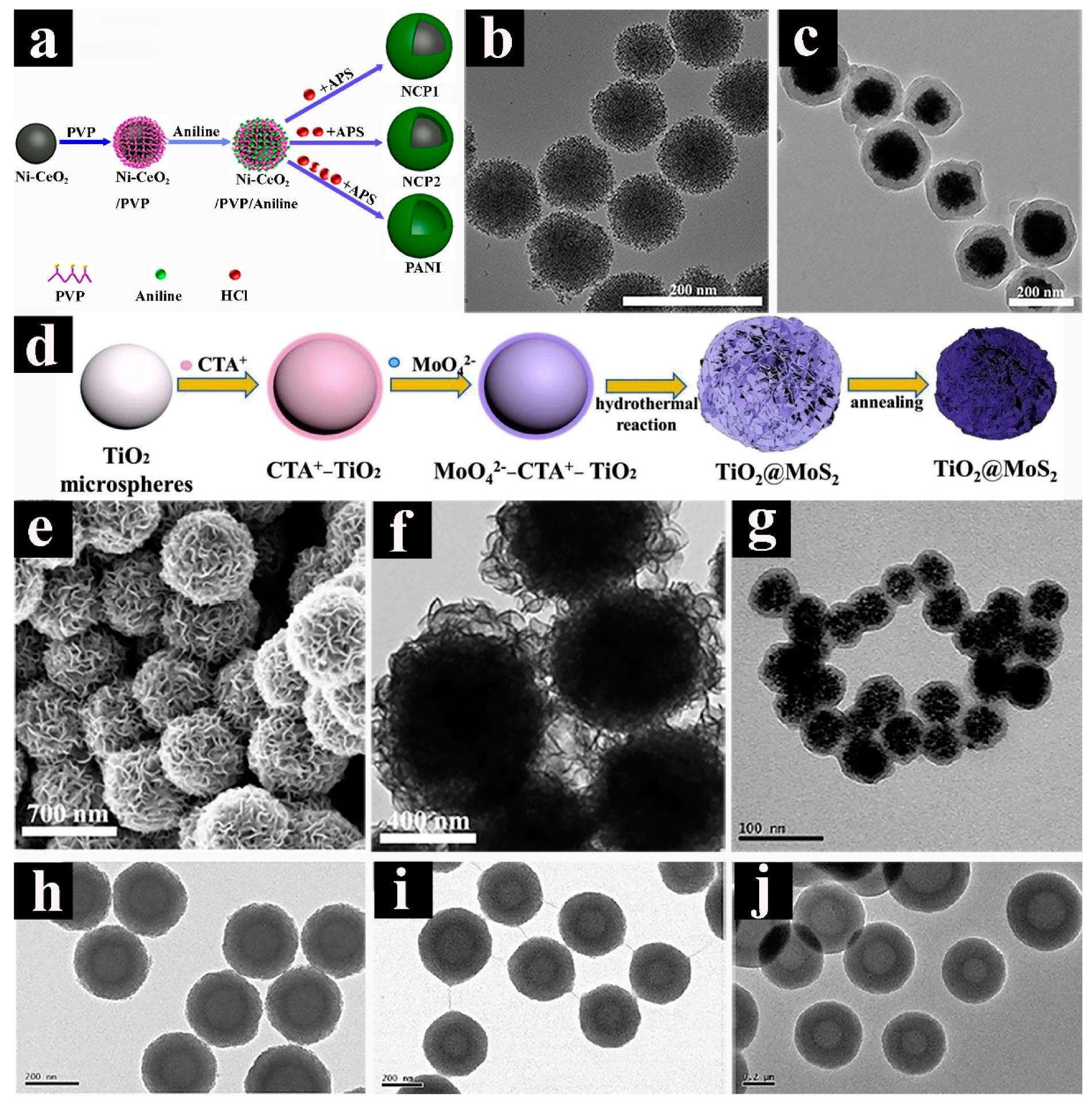
2.2. Core-shelled Spheres with Complex Architectures
2.3. Yolk-shelled Spheres with Complex Architectures
2.4. Double-shelled Spheres with Complex Architectures
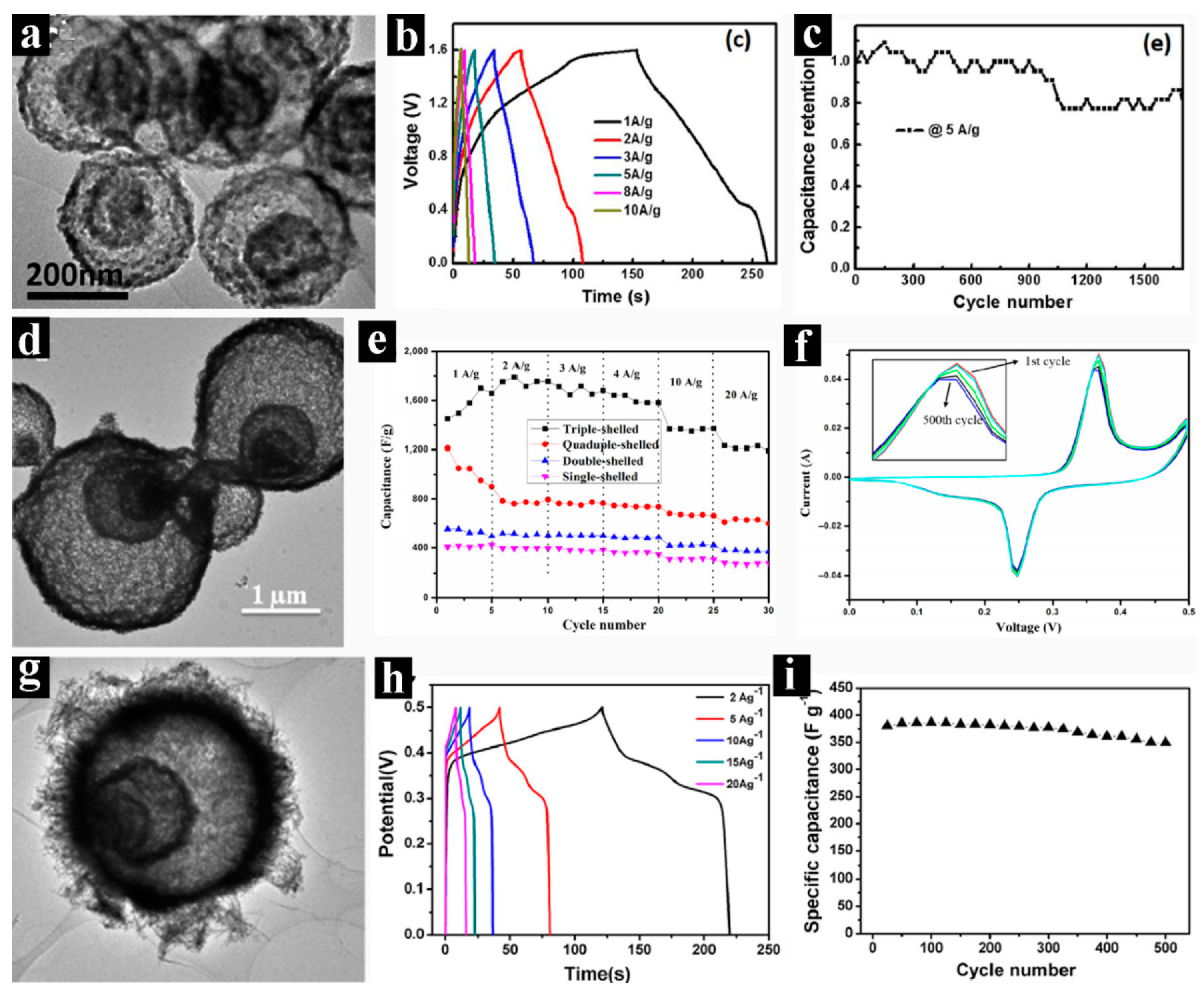
2.5. Multi-shelled Spheres with Complex Architectures
2.5.1. Hard-Templating Method
2.5.2. Soft-Templating Method
2.5.3. Self-Templating Method
3. Applications of Micro/nanostructured Spherical Materials in Energy Storage
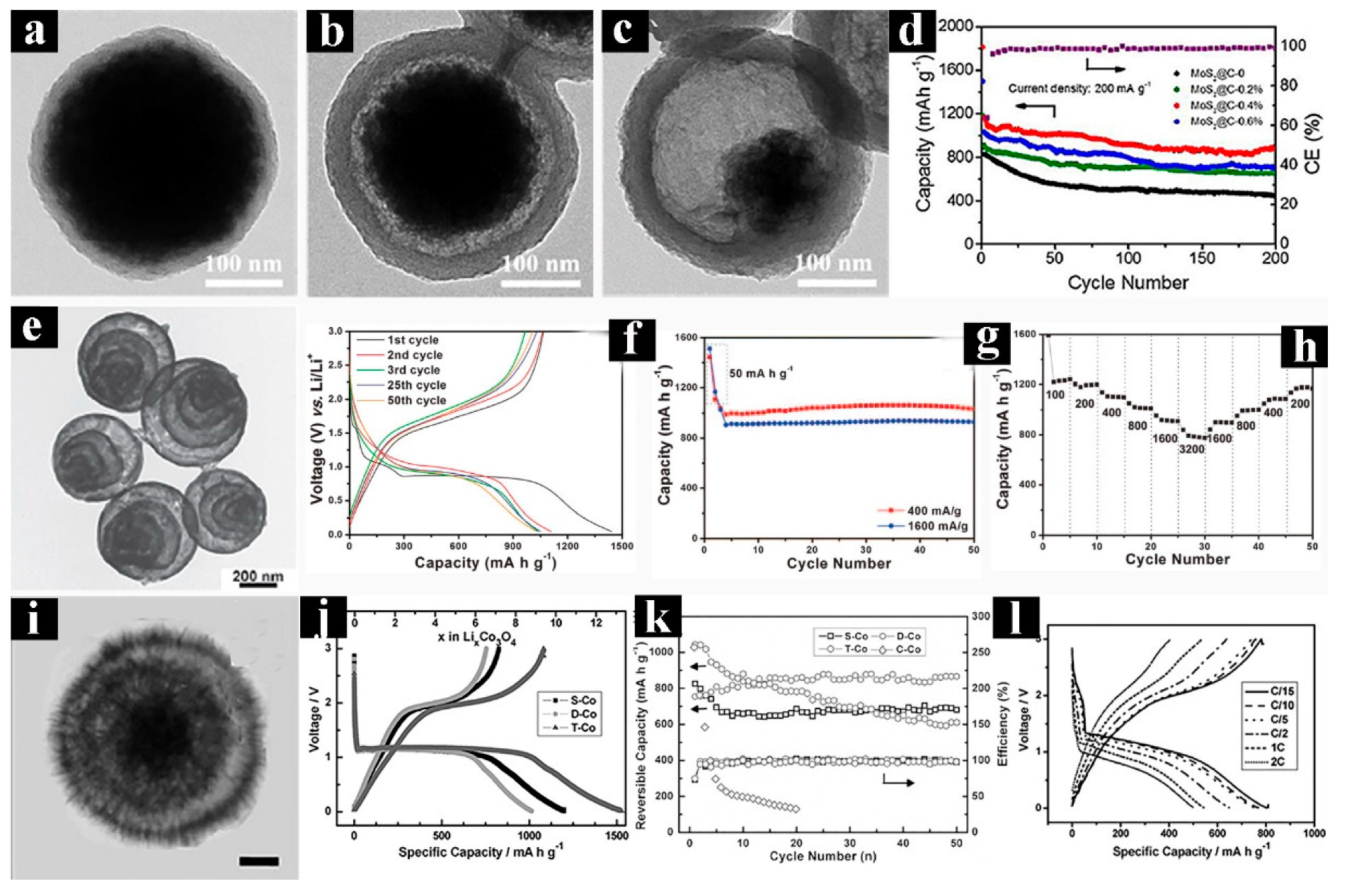
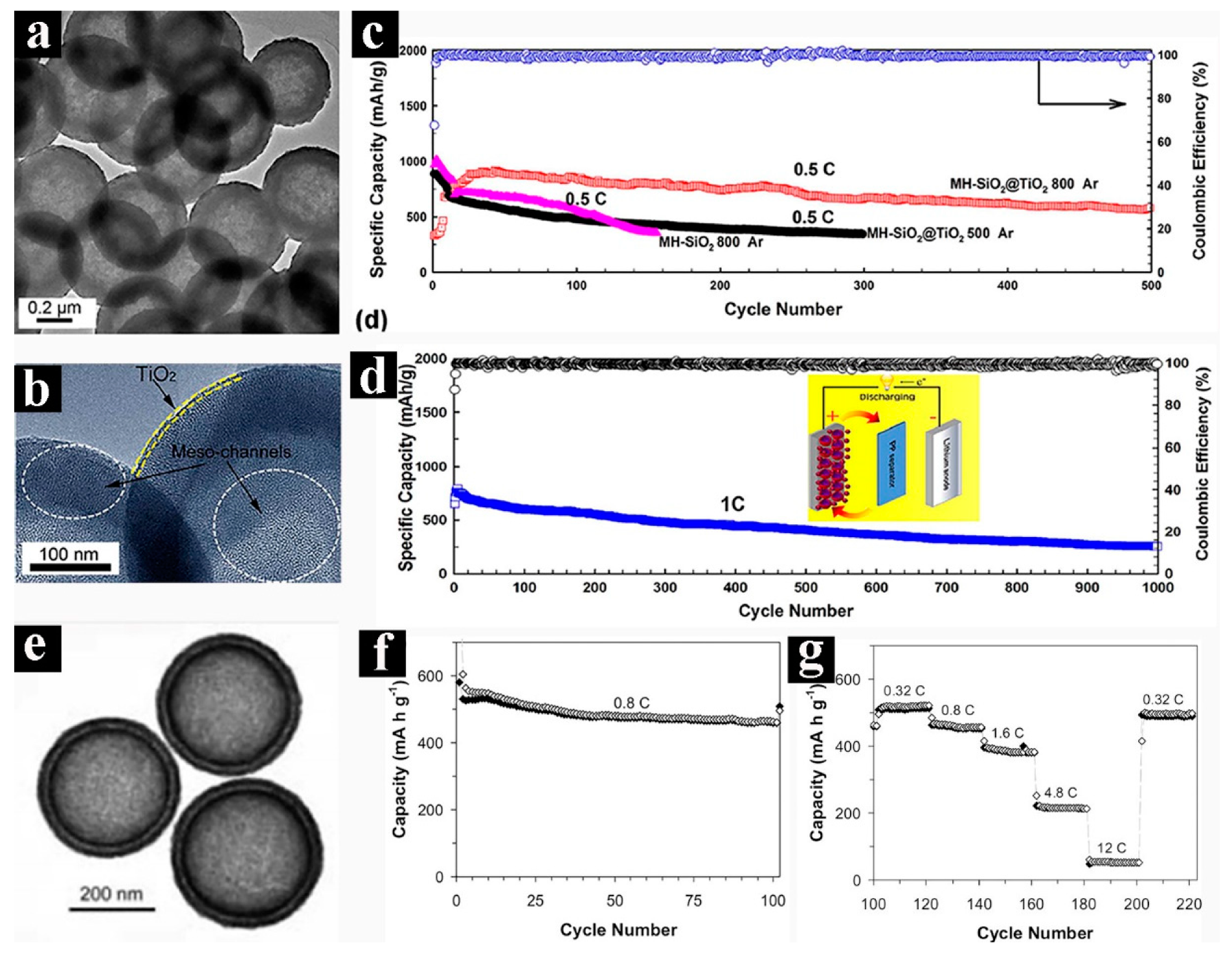
3.1. Lithium-Ion Batteries
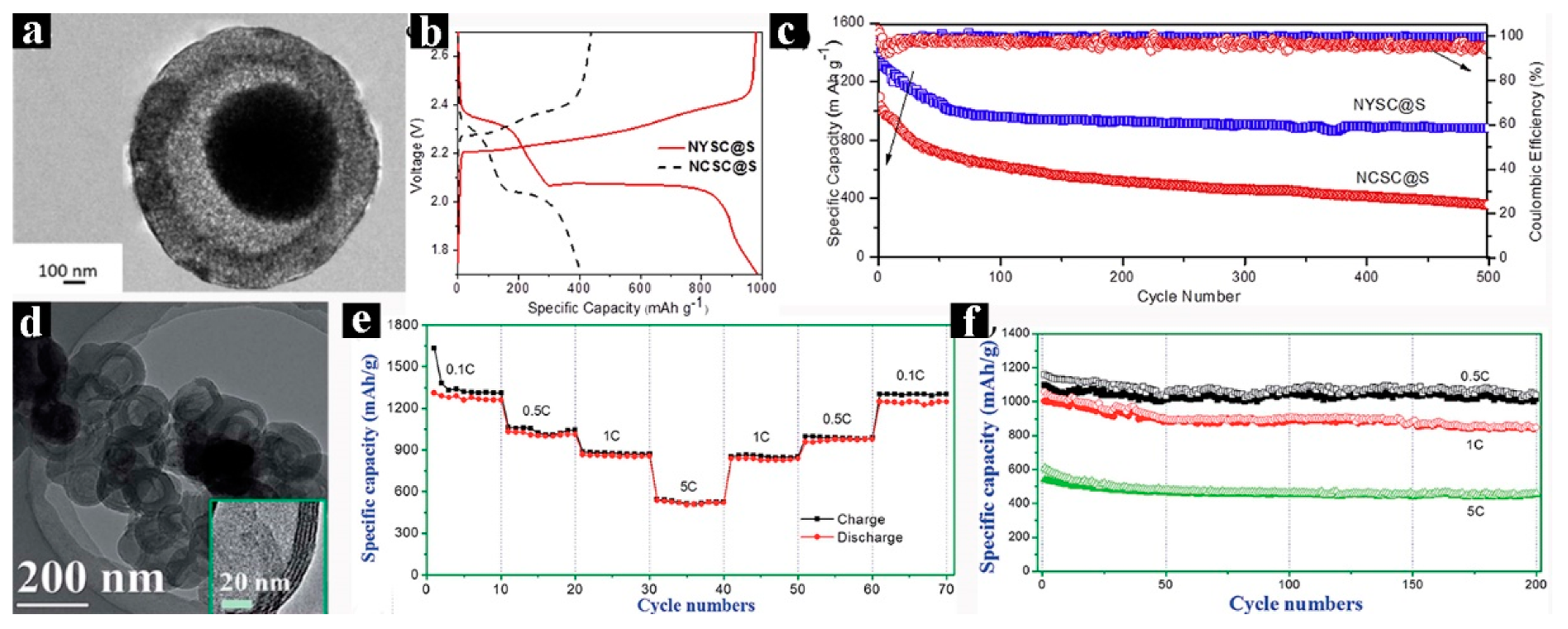
3.2. Lithium–Sulfur Batteries
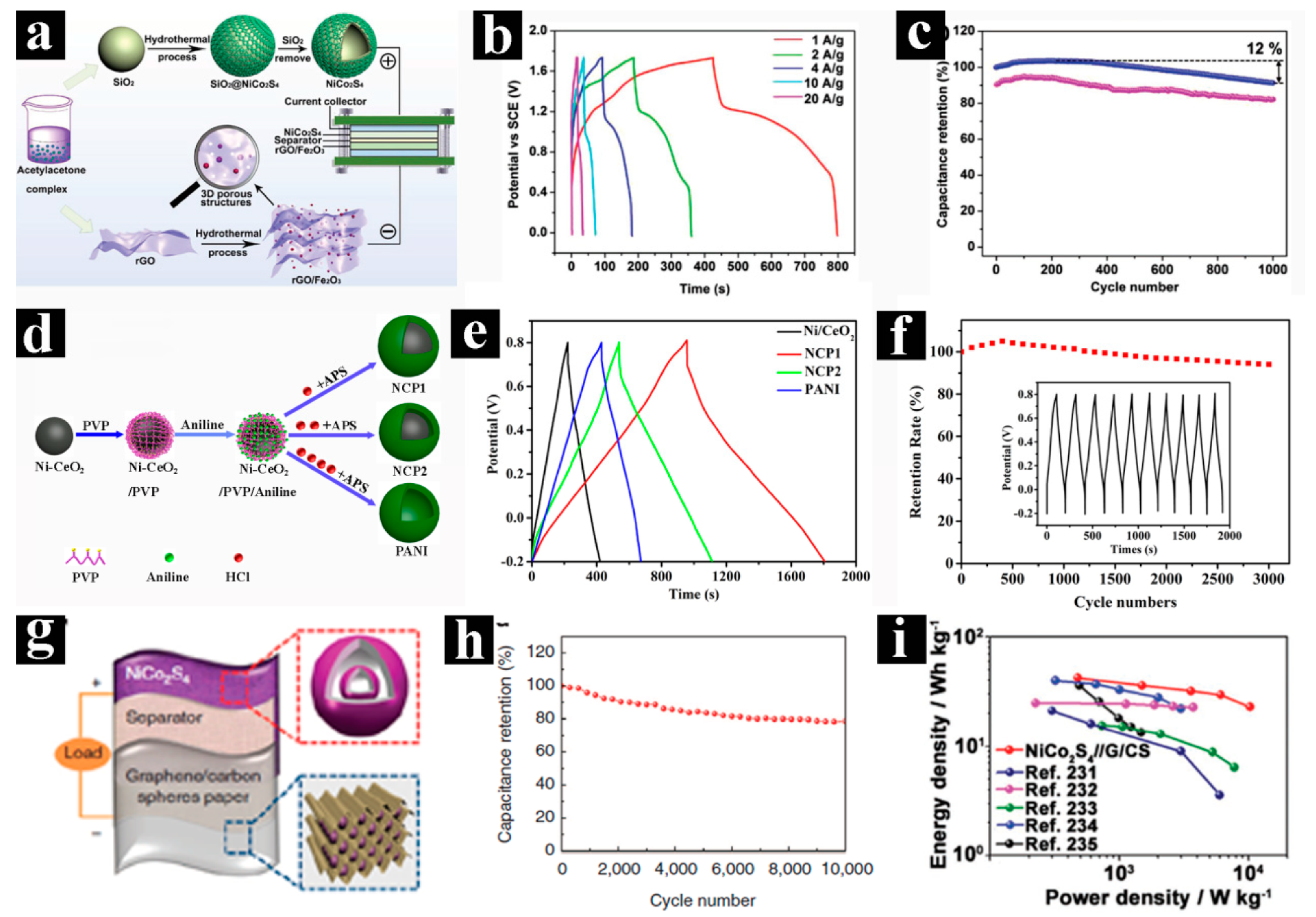
3.3. Supercapacitors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, G.M.; Zhao, Y.B.; Manthiram, A. Dual-confined flexible sulfur cathodes Encapsulated in nitrogen-doped double-shelled hollow carbon spheres and wrapped with graphene for Li-S batteries. Adv. Energy Mater. 2015, 5, 1402263. [Google Scholar] [CrossRef]
- Chen, S.G.; Wei, Z.D.; Qi, X.Q.; Dong, L.C.; Guo, Y.G.; Wan, L.J.; Shao, Z.G.; Li, L. Nanostructured polyaniline-decorated Pt/C@PANI core–shell catalyst with enhanced durability and activity. J. Am. Chem. Soc. 2012, 134, 13252–13255. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D.Y. Core-shell structured titanium dioxide nanomaterials for solar energy utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tong, X.; Ai, Y.F.; Liu, D.S.; Yu, P.; Wu, J.; Wang, Z.M. A Review enhanced anodes of Li/Na-Ion batteries based on yolk-shell structured nanomaterials. Nano-Micro Lett. 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.J.; Gao, T.T.; Sun, X.F.; Cao, P.; Zhou, G.W. Flower-shaped TiO2/C microspheres embedded with fish-scale-like MoS2 as anodes for lithium-ion batteries. Ceram. Int. 2018, 44, 8550–8555. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Zhang, B.X.; Anbalgam, K.; Thomas, T.; Yang, M.H. Synthesis and application of nano-structured metal nitrides and carbides: A review. Prog. Solid. State. Chem. 2018, 50, 1–15. [Google Scholar] [CrossRef]
- Pei, F.; An, T.H.; Zang, J.; Zhao, X.J.; Fang, X.L.; Zheng, M.S.; Dong, Q.F.; Zheng, N.F. From hollow carbon spheres to N-doped hollow porous carbon bowls rational design of hollow carbon host for Li-S batteries. Adv. Energy Mater. 2016, 6, 1502539. [Google Scholar] [CrossRef]
- Li, Y.S.; Shi, J.L. Hollow-structured mesoporous materials chemical synthesis, functionalization and applications. Adv. Mater. 2014, 26, 3176–3205. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Lai, X.Y.; Wang, J.Y.; Tang, H.J.; Ren, H.; Yang, Y.; Jin, Q.; Zhang, L.J.; Yu, R.B.; Ma, G.H.; et al. Multi-shelled hollow micro-nanostructures. Chem. Soc. Rev. 2015, 44, 6749–6773. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.M.; Liu, H.; Liao, M.L. Novel Porous Nitrogen Doped Graphene/Carbon Black Composites as Efficient Oxygen Reduction Reaction Electrocatalyst for Power Generation in Microbial Fuel Cell. Nanomaterials 2019, 9, 836. [Google Scholar] [CrossRef] [PubMed]
- Tareen, A.K.; Priyanga, G.S.; Behara, S.; Thomas, T.; Yang, M.H. Mixed ternary transition metal nitrides: a comprehensive review of synthesis, electronic structure, and properties of engineering relevance. Prog. Solid. State Chem. 2019, 53, 1–26. [Google Scholar] [CrossRef]
- Qi, J.; Chen, J.; Li, G.D.; Li, S.X.; Gao, Y.; Tang, Z.Y. Facile synthesis of core-shell Au@CeO2 nanocomposites with remarkably enhanced catalytic activity for CO oxidation. Energy Environ. Sci. 2012, 5, 8937–8941. [Google Scholar] [CrossRef]
- Rai, P.; Majhi, S.M.; Yu, Y.T.; Lee, J.H. Noble Metal@metal oxide semiconductor core@shell nano-architectures as a new platform for gas sensor applications. RSC Adv. 2015, 5, 76229–76248. [Google Scholar] [CrossRef]
- Geng, P.B.; Zheng, S.S.; Tang, H.; Zhu, R.M.; Zhang, L.; Cao, S.; Xue, H.G.; Pang, H. Transition metal sulfides based on graphene for electrochemical energy storage. Adv. Energy Mater. 2018, 8, 1703259. [Google Scholar] [CrossRef]
- Cui, Z.M.; Chen, Z.; Cao, C.Y.; Jiang, L.; Song, W.G. A yolk-shell structured Fe2O3@mesoporous SiO2 nanoreactor for enhanced activity as a fenton catalyst in total oxidation of dyes. Chem. Commun. 2013, 49, 2332–2334. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.N.; Choi, S.H.; Park, S.B.; Kang, Y.C. Hierarchical MoSe2 yolk-shell microspheres with superior Na-ion storage properties. Nanoscale 2014, 6, 10511–10515. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Qi, B.; Moore, T.; Wang, F.L.; Colvin, D.C.; Sanjeewa, L.D.; Gore, J.C.; Hwu, S.J.; Mefford, O.T.; Alexis, F.; et al. Multifunctional yolk-in-shell nanoparticles for pH-triggered drug release and imaging. Small 2014, 10, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Zu, L.H.; Su, Q.M.; Zhu, F.; Chen, B.J.; Lu, H.H.; Peng, C.X.; He, T.; Du, G.H.; He, P.F.; Chen, K.; et al. Antipulverization electrode based on low-carbon triple-shelled superstructures for lithium-ion batteries. Adv. Mater. 2017, 29, 1701494. [Google Scholar] [CrossRef] [PubMed]
- Rehman, W.; Xu, Y.L.; Sun, X.F.; Ullah, I.; Zhang, Y.; Li, L. Bouquet-like Mn2SnO4 nanocomposite engineered with graphene sheets as an advanced lithium-ion battery anode. ACS Appl. Mater. Interfaces 2018, 10, 17963–17972. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Hu, A.P.; Tang, Q.L.; Zhang, S.Y.; Deng, W.N.; Li, Y.H.; Liu, Z.; Fan, B.B.; Xiao, K.K.; Liu, J.L.; et al. Compact-nanobox engineering of transition metal oxides with enhanced initial coulombic efficiency for lithium-ion battery anodes. ACS Appl. Mater. Interfaces 2018, 10, 8955–8964. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Xu, N.; Qian, T.; Liu, J.; Shen, X.W.; Yan, C.L. High coulumbic efficiency and high-rate capability lithium sulfur batteries with low-solubility lithium polysulfides by using alkylene radicals to covalently connect sulfur. Nano Energy 2017, 41, 758–764. [Google Scholar] [CrossRef]
- Ni, X.Y.; Qian, T.; Liu, X.J.; Xu, N.; Liu, J.; Yan, C.L. High lithium ion conductivity LiF/GO solid electrolyte interphase inhibiting the shuttle of lithium polysulfides for long-life Li-S battery. Adv. Funct. Mater. 2018, 28, 1706513. [Google Scholar] [CrossRef]
- Liu, J.; Qian, T.; Wang, M.F.; Liu, X.J.; Xu, N.; You, Y.Z.; Yan, C.L. Molecularly imprinted polymer enables high-efficiency recognitionand trapping Lithium Polysulfides for Stable Lithium Sulfur Battery. Nano Lett. 2017, 17, 5064–5070. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Xu, N.; Qian, T.; Liu, J.; Shen, X.W.; Yan, C.L. Preventing polysulfide dissolution for lithium-sulfur batteries by stabilized lithium-sulfur batteries by covalently binding sulfur onto the thiol-terminated polymeric matrices. Small 2017, 13, 1702104. [Google Scholar] [CrossRef] [PubMed]
- Du, P.C.; Wei, W.L.; Liu, D.; Kang, H.X.; Liu, P. Fabrication of hierarchical carbon layer encapsulated polyaniline core-shell structure nanotubes and application in supercapacitors. Chem. Eng. J. 2018, 335, 373–383. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, C.; Tjiu, W.W.; Pramoda, K.P.; He, C.; Liu, T.X. Graphene-wrapped polyaniline hollow spheres as novel hybrid electrode materials for supercapacitor applications. ACS Appl. Mater. Interfaces 2013, 5, 3382–3391. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Kim, K.-W.; Park, Y.-K.; An, K.-H.; Park, S.-J.; Kim, B.-J. Activated Carbons from Thermoplastic Precursors and Their Energy Storage Applications. Nanomaterials 2019, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Lia, H.L.; Hea, Y.; Pavlinekb, V.; Cheng, Q.L.; Saha, P.; Lia, C.Z. MnO2 nanoflakes/polyaniline nanorods hybrid nanostructures on graphene paper for high-performance flexible supercapacitor electrodes. J. Mater. Chem. A 2015, 3, 17165–17171. [Google Scholar] [CrossRef]
- Wang, R.H.; Xu, C.H.; Sun, J.; Liu, Y.Q.; Gao, L.; Lin, C.C. Free-standing and binder-free lithium-ion electrodes based on robust layered assembly of graphene and Co3O4 nanosheets. Nanoscale 2013, 5, 6960–6967. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Luo, J.; Pan, B.Y.; Qiu, Z.Q.; Huang, S.M.; Tang, Y. Hierarchical shell/core CuO nanowire/carbon fiber composites as binder-free anodes for lithium-ion batteries. Electrochim. Acta 2017, 241, 261–271. [Google Scholar] [CrossRef]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gómez-Romero, P. Hybrid energy storage: the merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, J.; Bai, Y.; Zhang, Q.; Yin, Y. Synthesis, Properties, and applications of hollow micro-nanostructures. Chem. Rev. 2016, 116, 10983–11060. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Dai, S.; Qiao, S.Z. Self-supported electrocatalysts for advanced energy conversion processes. Mater. Today 2016, 19, 265–273. [Google Scholar] [CrossRef]
- Liu, J.; Yang, T.Y.; Wang, D.W.; Lu, G.Q.; Zhao, D.Y.; Qiao, S.Z. A facile soft-template synthesis of mesoporous polymeric and carbonaceous nanospheres. Nat. Commun. 2013, 4, 2798. [Google Scholar] [CrossRef]
- Li, X.X.; Zheng, S.S.; Jin, L.; Li, Y.; Geng, P.B.; Xue, H.G.; Pang, H.; Xu, Q. Metal-organic framework-derived carbons for battery applications. Adv. Energy Mater. 2018, 8, 1800716. [Google Scholar] [CrossRef]
- Cho, W.; Lee, Y.H.; Lee, H.J.; Oh, M. Multi ball-in-ball hybrid metal oxides. Adv. Mater. 2011, 23, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wickramaratne, N.P.; Qiao, S.Z.; Jaroniec, M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 2015, 14, 763–774. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Wang, M.W.; Pareek, V.K.; Liu, J.; Jaroniec, M. Synthesis and applications of porous non-silica metal oxide submicrospheres. Chem. Soc. Rev. 2016, 45, 6013–6047. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Guan, B.Y.; Xia, B.Y.; Lou, X.W. Designed formation of Co3O4/NiCo2O4 double-shelled nanocages with enhanced pseudocapacitive and electrocatalytic properties. J. Am. Chem. Soc. 2015, 137, 5590–5595. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous hollow carbon@sulfur composites for high-power lithium-sulfur batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Yu, R.B.; Wang, J.Y.; Jin, Q.; Yang, M.; Mao, D.; Kisailus, D.; Zhao, H.J.; Wang, D. Multishelled TiO2 hollow microspheres as anodes with superior reversible capacity for lithium ion batteries. Nano Lett. 2014, 14, 6679–6684. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.M.; Hessel, C.M.; Ren, H.; Yu, R.B.; Jin, Q.; Yang, M.; Zhao, H.J.; Wang, D. α-Fe2O3 multi-shelled hollow microspheres for lithium ion battery anodes with superior capacity and charge retention. Energy Environ. Sci. 2014, 7, 632–637. [Google Scholar] [CrossRef]
- Yec, C.C.; Zeng, H.C. Synthetic architecture of multiple core–shell and yolk–shell structures of (Cu2O@)nCu2O (n = 1–4) with centricity and eccentricity. Chem. Mater. 2012, 24, 1917–1929. [Google Scholar] [CrossRef]
- Meng, X.B.; Yang, X.Q.; Sun, X.L. Emerging applications of atomic layer deposition for lithium-ion battery studies. Adv. Mater. 2012, 24, 3589–3615. [Google Scholar] [CrossRef]
- Chen, J.S.; Lou, X.W. SnO2-based nanomaterials synthesis and application in lithium-ion batteries. Small 2013, 9, 1877–1893. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Zhi, L.J. Design and construction of three dimensional graphene-based composites for lithium ion battery applications. Energy Environ. Sci. 2015, 8, 456–477. [Google Scholar] [CrossRef]
- Tang, Y.X.; Zhang, Y.Y.; Li, W.L.; Ma, B.; Chen, X.D. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 44, 5926–5940. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.L.; Li, J.C.; Xiao, X.C.; Lott, A.; Lu, W.Q.; Sheldon, B.W.; Wu, J. Silicon-based nanomaterials for lithium-ion batteries: a review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Sun, Y.M.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Fu, K.; Wang, Z.Y.; Yan, C.Y.; Liu, Z.; Yao, Y.G.; Dai, J.Q.; Hitz, E.; Wang, Y.B.; Luo, W.; Chen, Y.N.; et al. All-component transient lithium-ion batteries. Adv. Energy Mater. 2016, 6, 1502496. [Google Scholar] [CrossRef]
- Ong, S.P.; Chevrier, V.L.; Hautier, G.; Jain, A.; Moore, C.; Kim, S.; Ma, X.H.; Ceder, G. Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy Environ. Sci. 2011, 4, 3680–3688. [Google Scholar] [CrossRef] [Green Version]
- Li, G.M.; Li, Y.; Chen, J.; Zhao, P.P.; Li, D.G.; Dong, Y.H.; Zhang, L.P. Synthesis and research of egg shell-yolk NiO/C porous composites as lithium-ion battery anode material. Electrochim. Acta 2017, 245, 941–948. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.E.; Lee, M.J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Xie, J.J.; Liu, L.; Xia, J.; Zhang, Y.; Li, M.; Ouyang, Y.; Nie, S.; Wang, X.Y. Template-free synthesis of Sb2S3 hollow microspheres as anode materials for lithium-ion and sodium-ion batteries. Nano-Micro Lett. 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.N.; Kang, Y.C.; Park, S.B. A new strategy for synthesizing yolk-shell V2O5 powders with low melting temperature for high performance Li-ion batteries. Nanoscale 2013, 5, 8899–8903. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Chung, S.H.; Zu, C.X. Lithium-sulfur batteries progress and prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Liu, X.F.; Zhang, Q.; Tian, G.L.; Huang, J.Q.; Zhu, W.C.; Wei, F. Graphene/single-walled carbon nanotube hybrids one-step catalytic growth and applications for high-rate Li-S batteries. ACS Nano 2012, 6, 10759–10769. [Google Scholar] [CrossRef]
- Yang, Z.H.; Xu, F.F.; Zhang, W.X.; Mei, Z.S.; Pei, B.; Zhu, X. Controllable preparation of multishelled NiO hollow nanospheres via layer-by-layer self-assembly for supercapacitor application. J. Power Sources 2014, 246, 24–31. [Google Scholar] [CrossRef]
- Wang, G.P.; Zhang, L.; Zhang, J.J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Khoo, E.; Sumboja, A.; Lee, P.S. Facile coating of manganese oxide on tin oxide nanowires with high-performance capacitive behavior. ACS Nano 2010, 4, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Rakhi, R.B.; Chen, W.; Cha, D.; Alshareef, H.N. Substrate dependent self-organization of mesoporous cobalt oxide nanowires with remarkable pseudocapacitance. Nano Lett. 2012, 12, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.H.; Xie, X.; Pan, L.J.; Bao, Z.N.; Cui, Y. Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2013, 2, 213–234. [Google Scholar] [CrossRef]
- Li, R.Z.; Wang, Y.M.; Zhou, C.; Wang, C.; Ba, X.; Li, Y.Y.; Huang, X.T.; Liu, J.P. Carbon-stabilized high-capacity ferroferric oxide nanorod array for flexible solid-state alkaline battery-supercapacitor hybrid device with high environmental suitability. Adv. Funct. Mater. 2015, 25, 5384–5394. [Google Scholar] [CrossRef]
- Wang, Y.P.; Pan, A.Q.; Zhu, Q.Y.; Nie, Z.W.; Zhang, Y.F.; Tang, Y.; Liang, S.Q.; Cao, G.Z. Facile synthesis of nanorod-assembled multi-shelled Co3O4 hollow microspheres for high-performance supercapacitors. J. Power Sources 2014, 272, 107–112. [Google Scholar] [CrossRef]
- Yang, J.Q.; Duan, X.C.; Guo, W.; Li, D.; Zhang, H.L.; Zheng, W.J. Electrochemical performances investigation of NiS/rGO composite as electrode material for supercapacitors. Nano Energy 2014, 5, 74–81. [Google Scholar] [CrossRef]
- Yang, J.Q.; Duan, X.C.; Qin, Q.; Zheng, W.J. Solvothermal synthesis of hierarchical flower-like β-NiS with excellent electrochemical performance for supercapacitors. J. Mater. Chem. A 2013, 1, 7880–7884. [Google Scholar] [CrossRef]
- Yang, J.; Yu, C.; Fan, X.M.; Qiu, J.S. 3D architecture materials made of NiCoAl-LDH nanoplates coupled with NiCo-carbonate hydroxide nanowires grown on flexible graphite paper for asymmetric supercapacitors. Adv. Energy Mater. 2014, 4, 1400761. [Google Scholar] [CrossRef]
- Lim, E.; Kim, H.; Jo, C.; Chun, J.; Ku, K.; Kim, S.; Lee, H.I.; Nam, I.S.; Yoon, S.; Kang, K.; et al. Advanced hybrid supercapacitor based on a mesoporous niobium pentoxide/carbon as high-performance anode. ACS Nano 2014, 8, 8968–8978. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zhao, Y.L.; He, R.H.; Luo, W.; Meng, J.S.; Yu, Q.; Zhao, D.Y.; Zhou, L.; Mai, L.Q. Yolk@Shell SiOx/C microspheres with semi-graphitic carbon coating on the exterior and interior surfaces for durable lithium storage. Energy Storage Mater. 2019, 19, 299–305. [Google Scholar] [CrossRef]
- Fei, J.B.; Cui, Y.; Yan, X.H.; Qi, W.; Yang, Y.; Wang, K.W.; He, Q.; Li, J.B. Controlled preparation of MnO2 hierarchical hollow nanostructures and their application in water treatment. Adv. Mater. 2008, 20, 452–456. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Li, J.; Lou, X.W. A Universal Cooperative assembly-directed method for coating of mesoporous TiO2 nanoshells with enhanced lithium storage properties. Sci. Adv. 2016, 2, e1501554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.X.; Lei, T.; Ai, Y.F.; Peng, Z.K.; Yan, X.Y.; Li, H.; Zhang, J.J.; Wang, Z.M.; Chueh, Y.L. Hollow NiCo2S4 nanospheres hybridized with 3D hierarchical porous rGO/Fe2O3 composites toward high-performance energy storage device. Adv. Energy Mater. 2018, 8, 1703453. [Google Scholar] [CrossRef]
- Cao, A.M.; Hu, J.S.; Liang, H.P.; Wan, L.J. Self-assembled vanadium pentoxide (V2O5) hollow microspheres from nanorods and their application in lithium-ion batteries. Angew. Chem. Int. Ed. 2005, 44, 4391–4395. [Google Scholar] [CrossRef]
- Shao, M.F.; Ning, F.Y.; Zhao, J.W.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical layered double hydroxide microspheres with largely enhanced performance for ethanol electrooxidation. Adv. Funct. Mater. 2013, 23, 3513–3518. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.S.; Wu, H.B.; Wang, Z.Y.; Lou, X.W. Quasiemulsion-templated formation of α-Fe2O3 hollow spheres with enhanced lithium storage properties. J. Am. Chem. Soc. 2011, 133, 17146–17148. [Google Scholar] [CrossRef]
- Sun, Y.G.; Piao, J.Y.; Hu, L.L.; Bin, D.S.; Lin, X.J.; Duan, S.Y.; Cao, A.M.; Wan, L.J. Controlling the reaction of nanoparticles for hollow metal oxides nanostructures. J. Am. Chem. Soc. 2018, 140, 9070–9073. [Google Scholar] [CrossRef]
- Kim, K.; Lee, M.J. Template-assisted solvothermal assembly of size-controlled hierarchical V2O5 hollow microspheres with tunable nanoscale building blocks and their enhanced lithium storage properties. Electrochim. Acta 2017, 258, 942–950. [Google Scholar] [CrossRef]
- Yu, X.Y.; Yao, X.Z.; Luo, T.; Jia, Y.; Liu, J.H.; Huang, X.J. Facile synthesis of urchin-like NiCo2O4 hollow microspheres with enhanced electrochemical properties in energy and environmentally related applications. ACS Appl. Mater. Inter. 2014, 6, 3689–3695. [Google Scholar] [CrossRef]
- Wang, J.B.; Liu, Z.W.; Yang, W.J.; Han, L.J.; Wei, M.D. A one-step synthesis of porous V2O3@C hollow spheres as a high-performance anode for lithium-ion batteries. Chem. Commun. 2018, 54, 7346–7349. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Guo, X.T.; Luo, Y.Q.; Li, Q.; Zhu, R.M.; Pang, H. Core-shell materials for advanced batteries. Chem. Eng. J. 2019, 355, 208–237. [Google Scholar] [CrossRef]
- Wei, S.Y.; Wang, Q.; Zhu, J.H.; Sun, L.Y.; Lin, H.F.; Guo, Z.H. Multifunctional composite core-shell nanoparticles. Nanoscale 2011, 3, 4474–4502. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.G.; Juste, J.P.; Liz-Marzán, L.M. Recent progress on silica coating of nanoparticles and related nanomaterials. Adv. Mater. 2011, 22, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Su, L.W.; Jing, Y.; Zhou, Z. Li ion battery materials with core-shell nanostructures. Nanoscale 2011, 3, 3967–3983. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.K.; Yang, J.P.; Li, X.M.; Zhou, L.; Zhang, R.Y.; Li, W.; Chen, L.; Wang, R.; Zhang, F.; Zhao, D.Y. Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres. Nano Lett. 2014, 14, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.Z.; Biradar, A.V.; Peng, D.L.; Zboril, R.; Varma, R.S. Core-shell nanoparticles synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, Z.L.; Li, Z.; Hu, Y.; Chen, C.; Yang, C.H.; Du, B.S.; Sun, Y.; Besenbacher, F.; Yu, M. Design and mechanism of core-shell TiO2 nanoparticles as a high-performance photothermal agent. Nanoscale 2017, 9, 16183–16192. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Hofman, E.; Li, J.; Davis, A.H.; Tung, C.H.; Wu, L.Z.; Zheng, W.W. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Adv. Funct. Mater. 2018, 28, 1704288. [Google Scholar] [CrossRef]
- Gong, Q.H.; Li, Y.J.; Huang, H.; Zhang, J.; Gao, T.T.; Zhou, G.W. Shape-controlled synthesis of Ni-CeO2@PANI nanocomposites and their synergetic effects on supercapacitors. Chem. Eng. J. 2018, 344, 290–298. [Google Scholar] [CrossRef]
- Xu, W.; Wang, T.; Yu, Y.; Wang, S. Synthesis of core-shell TiO2@MoS2 composites for lithium-ion battery anodes. J. Alloys Compd. 2016, 689, 460–467. [Google Scholar] [CrossRef]
- Zhang, B.H.; Yu, X.Y.; Ge, C.Y.; Dong, X.M.; Fang, Y.P.; Li, Z.S.; Wang, H.Q. Novel 3-D superstructures made up of SnO2@C core-shell nanochains for energy storage applications. Chem. Commun. 2010, 46, 9188–9190. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.Y.; Li, L.Y.; Li, Y.; Wang, C.C. Fabrication of hydroxyl group modified monodispersed hybrid silica particles and the h-SiO2/TiO2 core/shell microspheres as high performance photocatalyst for dye degradation. J. Colloid Interface 2011, 354, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, J.; Wang, M.G.; Guo, R. Fe3O4/PANI/MnO2 core-shell hybrids as advanced adsorbents for heavy metal ions. J. Mater. Chem. A 2017, 5, 4058–4066. [Google Scholar] [CrossRef]
- Li, Z.K.; Yao, Y.; Zheng, Y.J.; Gao, T.T.; Liu, Z.H.; Zhou, G.W. Fabrication of core-shell Fe3O4@C@MnO2 microspheres and their application in supercapacitors. J. Electrochem. Soc. 2018, 165, E58–E63. [Google Scholar] [CrossRef]
- Li, Z.L.; Zhao, H.L.; Lv, P.P.; Zhang, Z.J.; Zhang, Y.; Du, Z.H.; Teng, Y.Q.; Zhao, L.N.; Zhu, Z.M. Watermelon-like structured SiOx-TiO2@C nanocomposite as a high-performance lithium-ion battery anode. Adv. Funct. Mater. 2018, 28, 1605711. [Google Scholar] [CrossRef]
- Li, J.F.; Wang, J.Z.; Wexler, D.; Shi, D.Q.; Liang, J.W.; Liu, H.K.; Xiong, S.L.; Qian, Y.T. Simple synthesis of yolk-shelled ZnCo2O4 microspheres towards enhancing the electrochemical performance of lithiumion batteries in conjunction with a sodium carboxymethyl cellulose binder. J. Mater. Chem. A 2013, 1, 15292–15299. [Google Scholar] [CrossRef]
- Hong, Y.J.; Son, M.Y.; Kang, Y.C. One-pot facile synthesis of double-shelled SnO2 yolk-shell-structured powders by continuous process as anode materials for Li-ion batteries. Adv. Mater. 2013, 25, 2279–2283. [Google Scholar] [CrossRef]
- Li, J.F.; Wang, J.Z.; Liang, X.; Zhang, Z.J.; Liu, H.K.; Qian, Y.T.; Xiong, S.L. Hollow MnCo2O4 submicrospheres with multilevel interiors: from mesoporous spheres to yolk-in-double-shell structures. ACS Appl. Mater. Inter. 2014, 6, 24–30. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.X.; Li, X.H.; Guo, H.J.; Li, H.K.; Shih, K.; Yan, G.C.; Wang, J.X. Accurate construction of a hierarchical nickel-cobalt oxide multishell yolk-shell structure with large and ultrafast lithium storage capability. J. Mater. Chem. A 2017, 5, 14996–15001. [Google Scholar] [CrossRef]
- Son, M.Y.; Hong, Y.J.; Kang, Y.C. Superior electrochemical properties of Co3O4 yolk-shell powders with a filled core and multishells prepared by a one-pot spray pyrolysis. Chem. Commun. 2013, 49, 5678–5680. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kopold, P.; Wu, C.; Aken, P.A.V.; Maierb, J.; Yu, Y. Uniform yolk-shell Sn4P3@C nanospheres as high-capacity and cycle-stable anode materials for sodium-ion batteries. Energy Environ. Sci. 2015, 8, 3531–3538. [Google Scholar] [CrossRef]
- Pan, Y.M.; Zhang, J.J.; Lu, H.B. Uniform Yolk-shell MoS2@carbon microsphere anodes for high performance lithium-ion batteries. Chem. Eur. J. 2017, 23, 9937–9945. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.C.; Hu, X.; Hou, Y.; Wen, Z.H. Tunable synthesis of yolk-shell porous silicon@carbon for optimizing SiC-based anode of lithium-ion batteries. ACS Appl. Mater. Inter. 2017, 9, 42084–42092. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Guan, B.Y.; Yu, L.; Lou, X.W. Rational design of three-layered TiO2@Carbon@MoS2 hierarchical nanotubes for enhanced lithium storage. Adv. Mater. 2017, 29, 1702724. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Li, J.; Zhang, Y.; Cheng, X.; Chen, X.; Pan, P.; Su, J.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y.; et al. Plasmolysis-inspired nanoengineering of functional yolk-shell microspheres with magnetic core and mesoporous silica shell. J. Am. Chem. Soc. 2017, 139, 15486–15493. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, J.; Ho, G.W. Self-supported yolk-shell nanocolloids towards high capacitance and excellent cycling performance. Nano Energy 2015, 18, 273–282. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Wang, D.D.; Li, Z.K.; Gao, T.T.; Zhou, G.W. Laccase biosensor fabricated on flower-shaped yolk-shell SiO2 nanospheres for catechol detection. Colloid. Surface. A 2018, 538, 202–209. [Google Scholar] [CrossRef]
- Peng, S.J.; Li, L.L.; Tan, H.T.; Cai, R.; Shi, W.H.; Li, C.C.; Mhaisalkar, S.G.; Srinivasan, M.; Ramakrishna, S.; Yan, Q.Y. MS2 (M = Co and Ni) hollow spheres with tunable interiors for high-performance supercapacitors and photovoltaics. Adv. Funct. Mater. 2014, 24, 2155–2162. [Google Scholar] [CrossRef]
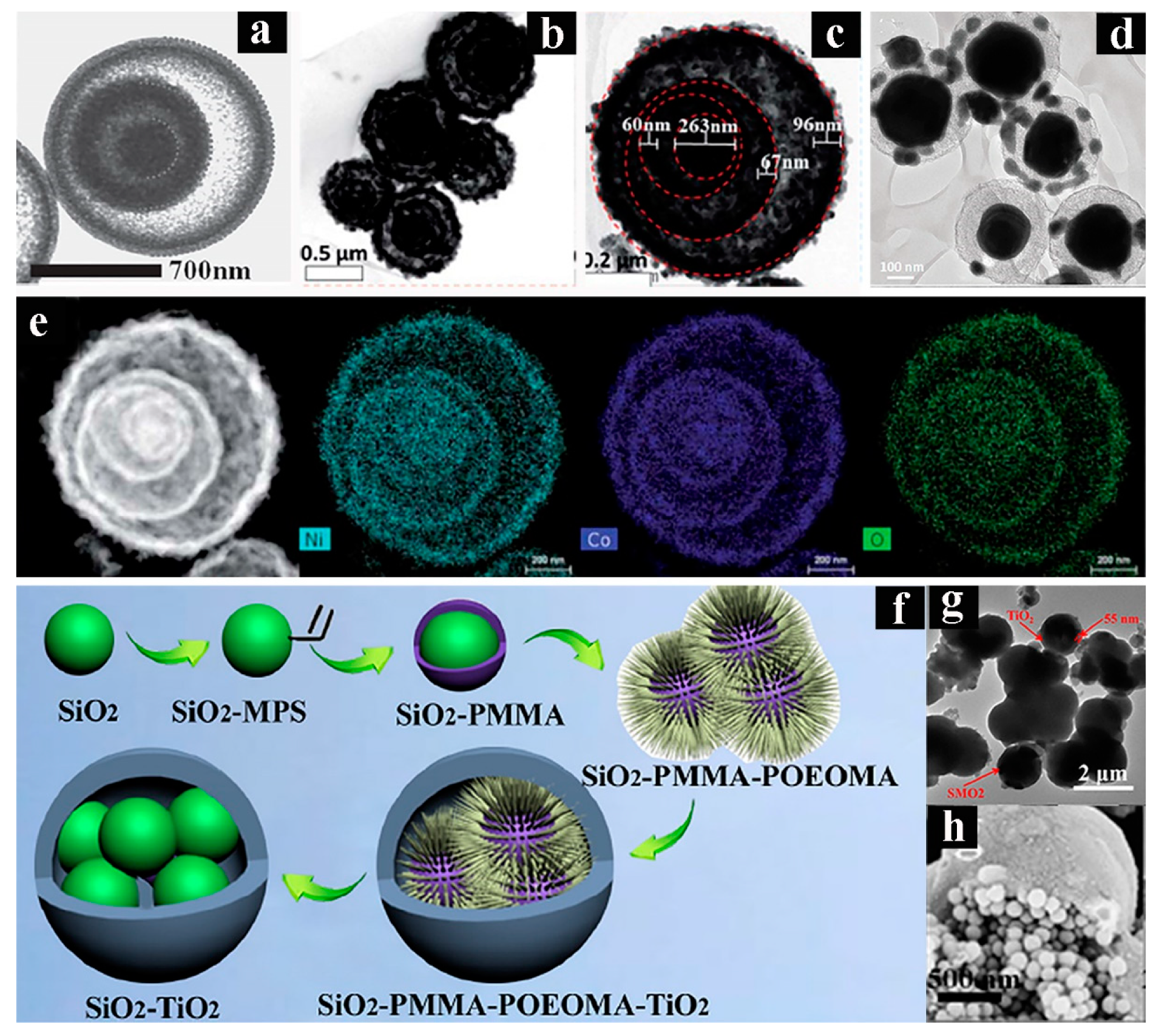
- Chen, X.F.; Pang, J.L.; Zhou, G.W.; Sun, B. Synthesis and characterization of SiO2-PMMA-POEOMA structuresand SiO2-TiO2 pomegranate-like hybrid microspheres for the photodecomposition of methyl orange. Colloid. Surface. A 2015, 481, 176–185. [Google Scholar] [CrossRef]
- Wang, H.; Shi, L.Y.; Yan, T.T.; Zhang, J.P.; Zhong, Q.D.; Zhang, D.S. Design of graphene-coated hollow mesoporous carbon spheres as high performance electrodes for capacitive deionization. J. Mater. Chem. A 2014, 2, 4739–4750. [Google Scholar] [CrossRef]
- Li, S.X.; Chen, J.; Zheng, F.Y.; Li, Y.C.; Huang, F.Y. Synthesis of the double-shell anatase-rutile TiO2 hollow spheres with enhanced photocatalytic activity. Nanoscale 2013, 5, 12150–12155. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.W.; Li, C.M.; Archer, L.A. Designed synthesis of coaxial SnO2@carbon hollow nanospheres for highly reversible lithium storage. Adv. Mater. 2009, 21, 2536–2539. [Google Scholar] [CrossRef]
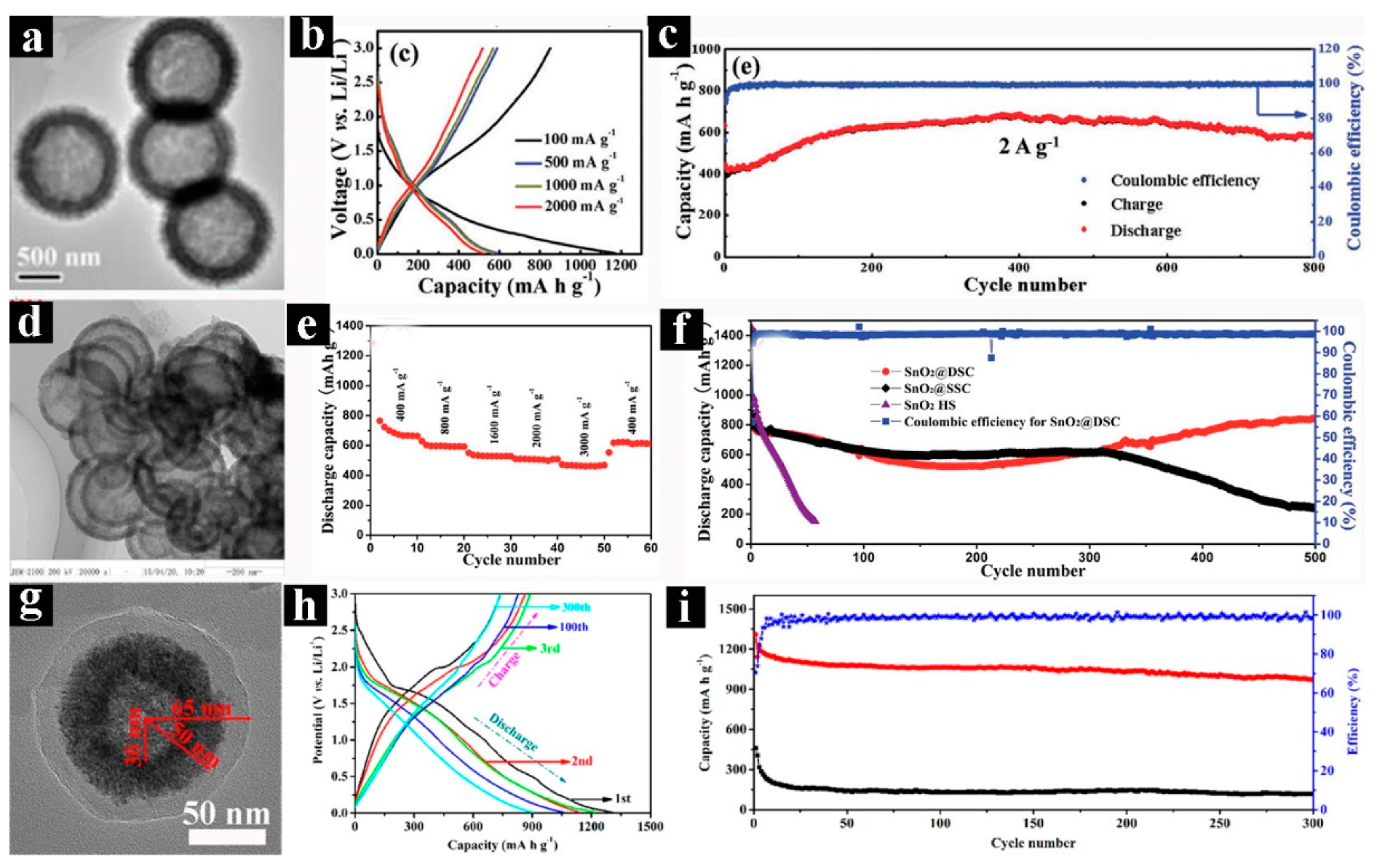
- Tian, Q.H.; Tian, Y.; Zhang, Z.X.; Yang, L.; Hirano, S. Double-shelled support and confined void strategy for improving lithium storage properties of SnO2/C anode materials for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 18036–18044. [Google Scholar] [CrossRef]
- Zhang, C.F.; Wu, H.B.; Yuan, C.Z.; Guo, Z.P.; Lou, X.W. Confining sulfur in double-shelled hollow carbon spheres for lithium-sulfur batteries. Angew. Chem. 2012, 124, 9730–9733. [Google Scholar] [CrossRef]
- Wang, H.Q.; Gong, Q.H.; Huang, H.; Gao, T.T.; Yuan, Z.W.; Zhou, G.W. P-n heterostructured TiO2/NiO double-shelled hollow spheres for the photocatalytic degradation of papermaking wastewater. Mater. Res. Bull. 2018, 107, 397–406. [Google Scholar] [CrossRef]
- Zhang, K.L.; Li, X.N.; Liang, J.W.; Zhu, Y.C.; Hu, L.; Cheng, Q.S.; Guo, C.; Lin, N.; Qian, Y.T. Nitrogen-doped porous interconnected double-shelled hollow carbon spheres with high capacity for lithium ion batteries and sodium ion batteries. Electrochim. Acta 2015, 155, 174–182. [Google Scholar] [CrossRef]
- Gong, Q.H.; Gao, T.T.; Huang, H.; Wang, R.X.; Cao, P.; Zhou, G.W. Double-shelled CeO2@C hollow nanospheres as enhanced anode materials for lithium-ion batteries. Inorg. Chem. Front. 2018, 5, 3197–3204. [Google Scholar] [CrossRef]
- Xu, H.L.; Wang, W.Z. Template synthesis of multishelled Cu2O hollow spheres with a single-crystalline shell wall. Angew. Chem., Int. Ed. 2007, 46, 1489–1492. [Google Scholar] [CrossRef]
- Liu, J.; Xia, H.; Xue, D.F.; Lu, L. Double-shelled nanocapsules of V2O5-based composites as high-performance anode and cathode materials for Li ion batteries. J. Am. Chem. Soc. 2009, 131, 12086–12087. [Google Scholar] [CrossRef]
- Shen, L.F.; Yu, L.; Wu, H.B.; Yu, X.Y.; Zhang, X.G.; Lou, X.W. Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties. Nat. Commun. 2015, 6, 6694–6701. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.W.; Yuan, C.L.; Archer, L.A. Double-walled SnO2 nano-cocoons with movable magnetic cores. Adv. Mater. 2007, 19, 3328–3332. [Google Scholar] [CrossRef]
- Hwang, S.H.; Yun, J.; Jang, J. Multi-shell porous TiO2 hollow nanoparticles for enhanced light harvesting in dye-sensitized solar cells. Adv. Funct. Mater. 2015, 24, 7619–7626. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, S.H.; Yun, J.; Jang, J. Fabrication of SiO2/TiO2 double-shelled hollow nanospheres with controllable size via sol-gel reaction and sonication-mediated etching. ACS Appl. Mater. Inter. 2014, 6, 15420–15426. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.Y.; Li, J.; Korgel, B.A.; Dong, Z.H.; Li, Z.M.; Su, F.B.; Du, J.; Wang, D. General synthesis and gas-sensing properties of multiple-shell metal oxide hollow microspheres. Angew. Chem. Int. Ed. 2011, 50, 2738–2741. [Google Scholar] [CrossRef] [PubMed]
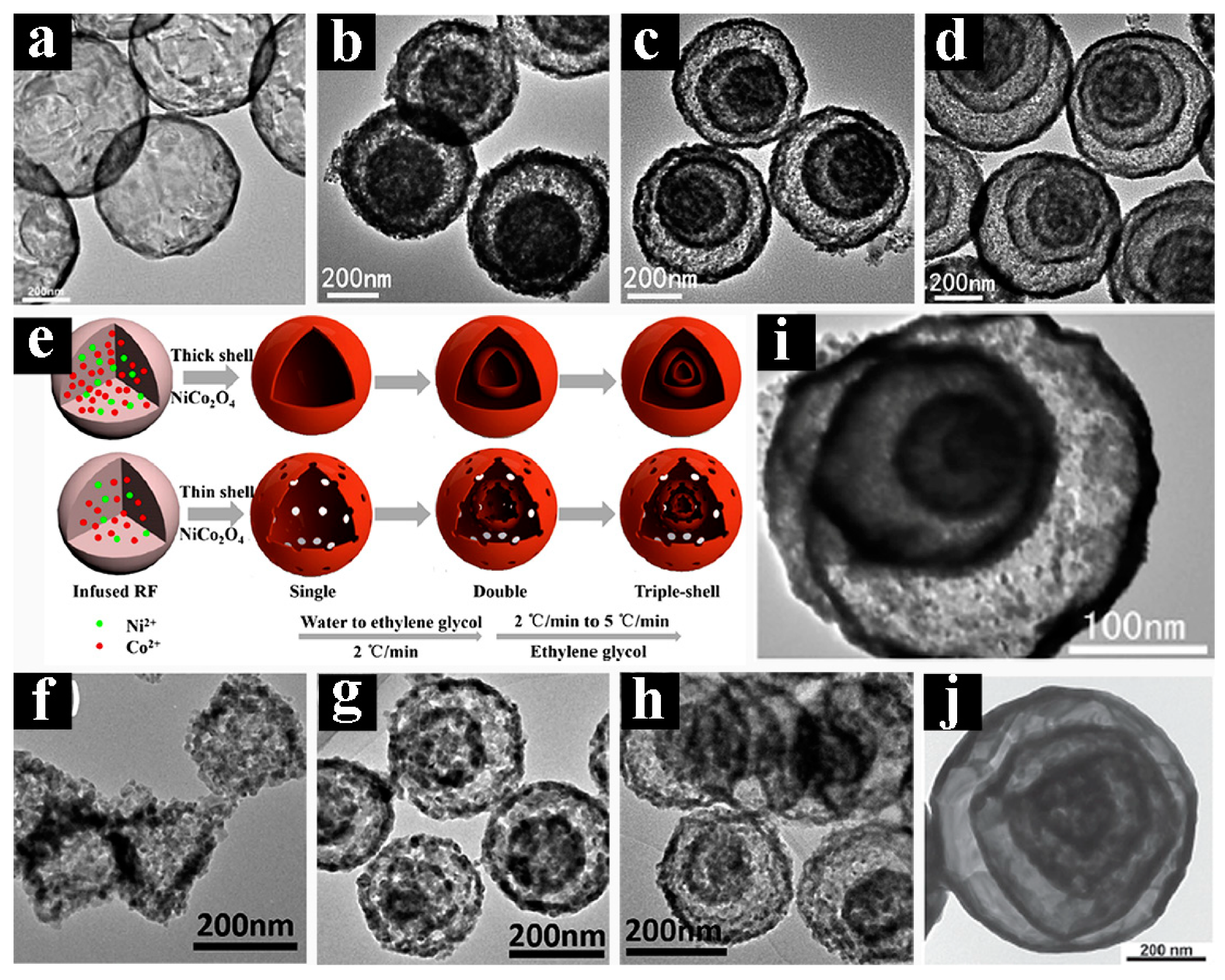
- Zhao, X.X.; Yu, R.B.; Tang, H.J.; Mao, D.; Qi, J.; Wang, B.; Zhang, Y.; Zhao, H.J.; Hu, W.P.; Wang, D. Formation of septuple-shelled (Co23Mn13)(Co56Mn16)2O4 hollow spheres as electrode material for alkaline rechargeable battery. Adv. Mater. 2017, 29, 1700550. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Tang, H.J.; Ren, H.; Yu, R.B.; Qi, J.; Mao, D.; Zhao, H.J.; Wang, D. pH-regulated synthesis of multi-shelled manganese oxide hollow microspheres as supercapacitor electrodes using carbonaceous microspheres as templates. Adv. Sci. 2014, 1, 1400011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Lou, X.W. General synthesis of multi-shelled mixed metal oxide hollow spheres with superior lithium storage properties. Angew. Chem. Int. Ed. 2014, 53, 9041–9044. [Google Scholar] [CrossRef]
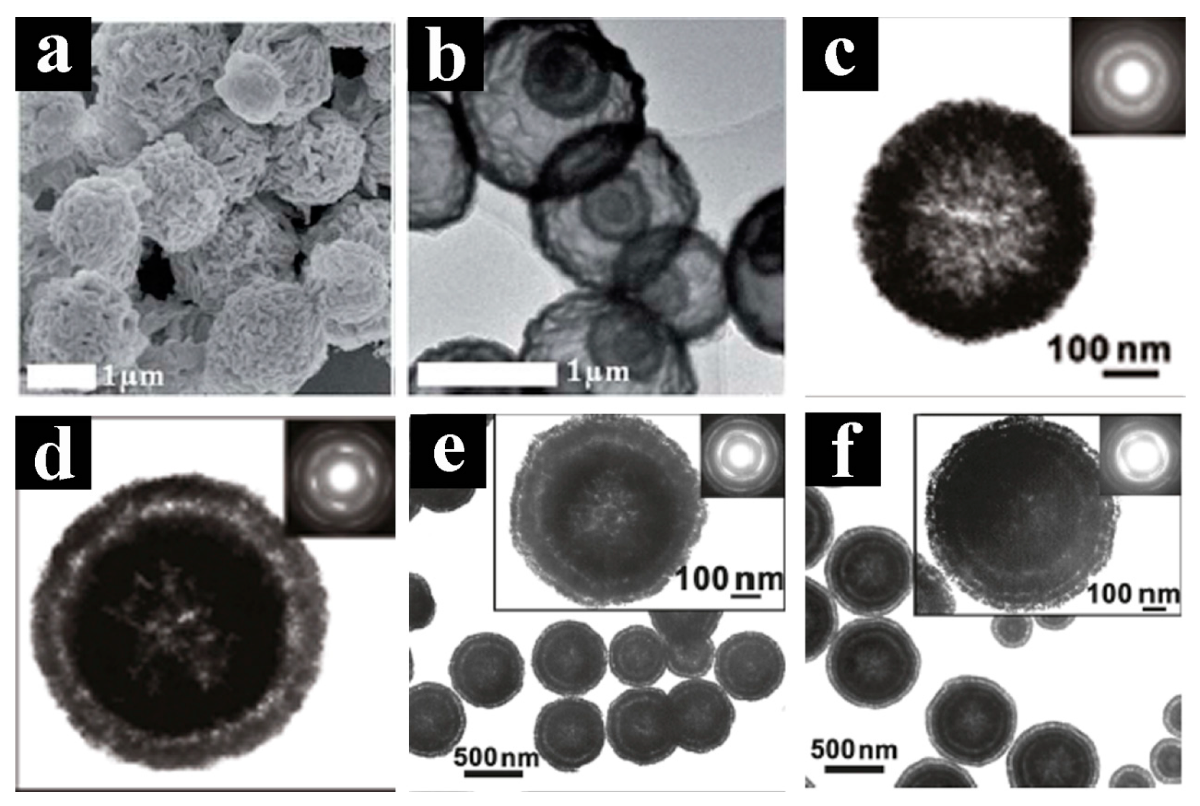
- Wang, J.Y.; Yang, N.L.; Tang, H.J.; Dong, Z.H.; Jin, Q.; Yang, M.; Kisailus, D.; Zhao, H.J.; Tang, Z.Y.; Wang, D. Accurate control of multishelled Co3O4 hollow microspheres as high-performance anode materials in lithium-ion batteries. Angew. Chem. Int. Ed. 2013, 52, 6417–6420. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.H.; Zheng, W.J.; He, G.H.; Tian, T.F.; Du, N.X.; Wang, L. NiCo2O4 Hollow microspheres with tunable numbers and thickness of shell for supercapacitors. Chem. Eng. J. 2017, 309, 426–434. [Google Scholar] [CrossRef]
- Xi, G.C.; Yan, Y.; Ma, Q.; Li, J.F.; Yang, H.F.; Lu, X.J.; Wang, C. Synthesis of multiple-shell WO3 hollow spheres by a binary carbonaceous template route and their applications in visible light photocatalysis. Chem. Eur. J. 2012, 18, 13949–13953. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, H.Y.; Zhang, H.W.; Yang, J.; Hartono, S.B.; Qian, K.; Zou, J.; Yu, C.Z. Cheap and scalable synthesis of α-Fe2O3 multi-shelled hollow spheres as high-performance anode materials for lithium ion batteries. Chem. Commun. 2013, 49, 8695–8697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.W.; Chen, Y.J.; Yang, S.H. Comparative studies on catalytic properties of immobilized candida rugosa lipase in ordered mesoporous rod-like silica and vesicle-like silica. Micropor. Mesopor. Mat. 2009, 119, 223–229. [Google Scholar] [CrossRef]
- Zhou, G.W.; Fung, K.K.; Wong, L.W.; Chen, Y.J.; Renneberg, R.; Yang, S.H. Immobilization of glucose oxidase on rod-like and vesicle-like mesoporous silica for enhancing current responses of glucose biosensors. Talanta 2011, 84, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.W.; Chen, Y.J.; Yang, J.H.; Yang, S.H. From cylindrical-channel mesoporous silica to vesicle-like silica with welldefined multilamella shells and large inter-shell mesopores. J. Mater. Chem. 2007, 17, 2839–2844. [Google Scholar] [CrossRef]
- Wu, C.C.; Zhou, G.W.; Jiang, X.J.; Ma, J.Y.; Zhang, H.Y.; Song, H.B. Active biocatalysts based on candida rugosa lipase immobilized in vesicular silica. Process Biochem. 2012, 47, 953–959. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.W.; Sun, B.; Zhao, M.N.; Zhang, J.Y.; Chen, F.J. A cationic-cationic co-surfactant templating route for synthesizing welldefined multilamellar vesicular silica with adjustable number of layers. ChemComm 2014, 50, 2907–2909. [Google Scholar]
- Wang, S.M.; Gao, T.T.; Li, Y.; Li, S.C.; Zhou, G.W. Fabrication of vesicular polyaniline using hard templates and composites with graphene for supercapacitor. J. Solid. State. Electrochem. 2017, 21, 705–714. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, W.; Jiang, M.L.; Chen, C.; Li, Y.D. One-step accurate synthesis of shell controllable CoFe2O4 hollow microspheres as high-performance electrode materials in supercapacitor. Nano Res. 2016, 9, 2026–2033. [Google Scholar] [CrossRef]
- Liu, J.; Hartono, S.B.; Jin, Y.G.; Li, Z.; Lu, G.Q.; Qiao, S.Z. A facile vesicle template route to multi-shelled mesoporous silica hollow nanospheres. J. Mater. Chem. 2010, 20, 4595–4601. [Google Scholar] [CrossRef]
- Gu, D.; Bongard, H.; Deng, Y.H.; Feng, D.; Wu, Z.X.; Fang, Y.; Mao, J.J.; Tu, B.; Schüth, F.; Zhao, D.Y. An aqueous emulsion route to synthesize mesoporous carbon vesicles and their nanocomposites. Adv. Mater. 2010, 22, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Hwang, S.H.; Yun, J.; Jang, J. Enhanced electroresponsive performance of double-shell SiO2/TiO2 hollow nanoparticles. ACS Nano 2015, 9, 4939–4949. [Google Scholar] [CrossRef]
- Wu, X.J.; Xu, D.S. Soft template synthesis of yolk silica shell particles. Adv. Mater. 2010, 22, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.D.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of hollow nanocrystals through the nanoscale kirkendall effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.B.; Zhang, J.T.; Zhang, L.L.; Kumar, N.A.; Zhao, X.S. Functionalization of chemically derived graphene for improving its electrocapacitive energy storage properties. Energy Environ. Sci. 2016, 9, 1891–1930. [Google Scholar] [CrossRef]
- Wang, H.Q.; Lin, H.F.; Long, Y.; Ni, B.; He, T.; Zhang, S.M.; Zhu, H.H.; Wang, X. Titanocene dichloride (Cp2TiCl2) as a precursor for template-free fabricating hollow TiO2 nanostructures with enhanced photocatalytic hydrogen production. Nanoscale 2017, 9, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Yec, C.C.; Zeng, H.C. Synthesis of complex nanomaterials via Ostwald Ripening. J. Mater. Chem. A 2014, 2, 4843–4851. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, X.Q.; Zhao, W.Y.; Deng, J.S.; Fan, B.B.; Shao, G.; Bai, Z.Y.; Zhang, R. Facile synthesis of yolk-shell Ni@void@SnO2(Ni3Sn2) ternary composites via galvanic Replacement/Kirkendall effect and their enhanced microwave absorption properties. Nano Res. 2017, 10, 331–343. [Google Scholar] [CrossRef]
- Gao, J.H.; Liang, G.L.; Zhang, B.; Kuang, Y.; Zhang, X.X.; Xu, B. FePt@CoS2 yolk-shell nanocrystals as a potent agent to kill hela cells. J. Am. Chem. Soc. 2007, 129, 1428–1433. [Google Scholar] [CrossRef]
- Zhang, H.G.; Zhu, Q.S.; Zhang, Y.; Wang, Y.; Zhao, L.; Yu, B. One-pot synthesis and hierarchical assembly of hollow Cu2O microspheres with nanocrystals-composed porous multishell and their gas-sensing properties. Adv. Funct. Mater. 2007, 17, 2766–2771. [Google Scholar] [CrossRef]
- Wu, C.Z.; Zhang, X.D.; Ning, B.; Yang, J.L.; Xie, Y. Shape evolution of new-phased lepidocrocite VOOH from single-shelled to double-shelled hollow nanospheres on the basis of programmed reaction-temperature strategy. Inorg. Chem. 2009, 48, 6044–6054. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhao, K.; Li, G.D.; Gao, Y.; Zhao, H.J.; Yu, R.B.; Tang, Z.Y. Multi-shelled CeO2 hollow microspheres as superior photocatalysts for water oxidation. Nanoscale. 2014, 6, 4072–4077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H. Interior structural tailoring of Cu2O shell-in-shell nanostructures through multistep Ostwald Ripening. J. Phys. Chem. C. 2011, 115, 18479–18485. [Google Scholar] [CrossRef]
- Xiong, S.L.; Zeng, H.C. Serial ionic exchange for the synthesis of multishelled copper sulfide hollow spheres. Angew. Chem. Int. Ed. 2012, 51, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.G.; Su, X.D.; Zheng, Y.Y.; Zhang, J.J.; Liu, Y.; Wang, S.J.; Wu, J.; Chen, G.T.; Wang, J.D.; Zhao, D.Y.; et al. A facile multi-interface transformation approach to monodisperse multiple-shelled periodic mesoporous organosilica hollow spheres. J. Am. Chem. Soc. 2015, 137, 7935–7944. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Arbiol, J.; Puntes, V.F. Carving at the nanoscale sequential galvanic exchange and Kirkendall growth at room temperature. Science 2011, 334, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.Y.; Chen, T.; Wang, L.; Ma, L.B.; . Hu, Y.; Chen, R.P.; Wang, Y.R.; Wang, C.X.; Yan, W.; Tie, Z.X.; et al. High energy density hybrid lithium-ion capacitor enabled by Co3ZnC@N-doped carbon nanopolyhedra anode and microporous carbon cathode. Energy Storage Mater. 2018, 14, 246–252. [Google Scholar] [CrossRef]
- Xia, H.C.; Li, K.X.; Guo, Y.Y.; Guo, J.H.; Xu, Q.; Zhang, J.N. CoS2 nanodots trapped within the graphitic structured N-doped carbon spheres with efficient performances for lithium storage. J. Mate. Chem. A 2018, 6, 7148–7154. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.S.; Ma, Y.T.; Qiu, Y.L.; Xie, Q.S.; Chen, Y.Z.; Peng, D.L. Facile synthesis and microwave absorption properties of yolk-shell ZnO-Ni-C/RGO composite materials. Chem. Eng. J. 2018, 333, 92–100. [Google Scholar] [CrossRef]
- Ding, X.; Huang, X.B.; Jin, J.L.; Ming, H.; Wang, L.M.; Ming, J. Advanced and safer lithium-ion battery based on sustainable electrodes. J. Power Sources. 2018, 379, 53–59. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, C.K.; Gao, X.; Chen, S.; Hu, Y.R.; Guan, H.Q.; Ma, Y.R.; Zhang, J.; Zhou, H.H.; Qi, L.M. SnO2@PANI core-shell nanorod arrays on 3D graphite foam: A high-performance integrated electrode for lithium-ion batteries. ACS Appl. Mater. Inter. 2017, 7, 9620–9629. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.Y.; Wang, L.; Lin, H.N.; Ma, L.B.; Zhao, P.Y.; Hu, Y.; Chen, T.; Chen, R.P.; Wang, Y.R.; Tie, Z.X.; et al. Walnut-like multicore-shell MnO encapsulated nitrogen rich carbon nanocapsules as anode material for long-cycling and soft-packed lithium-ion batteries. Adv. Funct. Mater. 2018, 28, 1800003. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Barnett, B.; Xu, K. Before Li ion batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.C.; Zhu, K.J.; Xu, Y.; Bian, K.; Wang, J.; Tai, G.A.; Gao, Y.F.; Luo, H.J.; Lu, L.; Liu, J.S. Hierarchical porous and intercalation-type V2O3 for high-performance anode materials of Li-ion batteries. Chem. Eur. J. 2017, 23, 7538–7544. [Google Scholar] [CrossRef]
- Choi, S.H.; Kang, Y.C. Ultrafast synthesis of yolk-shell and cubic NiO nanopowders and application in lithium ion batteries. ACS Appl. Mater. Inter. 2014, 6, 2312–2316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, X.L.; Guo, Y.G.; Zhong, Y.T.; Cao, X.Q.; Ma, Y.; Yao, J.N. Synthesis and lithium storage properties of Co3O4 nanosheet-assembled multishelled hollow spheres. Adv. Funct. Mater. 2010, 20, 1680–1686. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, D.Y.; Lou, X.W. Double-shelled CoMn2O4 hollow microcubes as high-capacity anodes for lithium-ion batteries. Adv. Mater. 2012, 24, 745–748. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.B.; Lou, X.W. Metal-Organic-Frameworks-Derived general formation of hollow structures with high complexity. J. Am. Chem. Soc. 2013, 135, 10664–10672. [Google Scholar] [CrossRef]
- Chen, W.; Lei, T.Y.; Qian, T.; Lv, W.Q.; He, W.D.; Wu, C.Y.; Liu, X.J.; Liu, J.; Chen, B.; Yan, C.L.; et al. A new hydrophilic binder enabling strongly anchoring polysulfides for high-performance sulfur electrodes in lithium sulfur battery. Adv. Energy Mater. 2018, 8, 1702889. [Google Scholar] [CrossRef]
- Chen, W.; Qian, T.; Xiong, J.; Xu, N.; Liu, X.J.; Liu, J.; Zhou, J.Q.; Shen, X.W.; Yang, T.Z.; Chen, Y.; et al. A new type of multifunctional polar binder: toward practical application of high energy lithium sulfur batteries. Adv. Mater. 2017, 29, 1605160. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lei, T.Y.; Wu, C.Y.; Deng, M.; Gong, C.H.; Hu, K.; Ma, Y.C.; Dai, L.P.; Lv, W.Q.; He, W.D.; et al. Designing safe electrolyte system for high-stability lithium-sulfur battery. Adv. Energy Mater. 2018, 8, 1702348. [Google Scholar] [CrossRef]
- Tao, Y.Q.; Wei, Y.J.; Liu, Y.; Wang, J.T.; Qiao, W.M.; Ling, L.C.; Long, D.H. Kinetically-enhanced polysulfide redox reactions by Nb2O5 nanocrystals for high-rate lithium–sulfur battery. Energy Environ. Sci. 2016, 10, 3230–3239. [Google Scholar] [CrossRef]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium–sulfur batteries: electrochemistry, materials, and prospects. Angew. Chem. 2013, 52, 13186–13200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, W.; Zhang, W.G.; Zheng, X.Y.; Wu, M.B.; Wei, W.; Tao, Y.; Li, Z.J.; Yang, Q.H. Reduction of graphene oxide by hydrogen sulfide: A promising strategy for pollutant control and as an electrode for Li-S batteries. Adv. Energy Mater. 2014, 4, 1301565. [Google Scholar] [CrossRef]
- Seh, Z.W.; Yu, J.H.; Li, W.; Hsu, P.C.; Wang, H.; Sun, Y.; Yao, H.; Zhang, Q.; Cui, Y. Two-dimensional layered transition metal disulphides for effective encapsulation of high-capacity lithium sulphide cathodes. Nat. Commun. 2014, 5, 5017. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yu, B.C.; Park, J.S.; Choi, J.W.; Kim, C.; Sung, Y.E.; Goodenough, J.B. Tungsten disulfide catalysts supported on a carbon cloth interlayer for high performance Li-S battery. Adv. Energy Mater. 2017, 7, 1602567. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L.Q. Nanostructured metal oxides and sulfides for lithium-sulfur batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.B.; Chen, X.; Zhao, X.X.; Cheng, M.R.; Xiong, P.X.; He, Y.W.; Zhang, Q.; Xu, Y.H. A polysulfide-immobilizing polymer retards the shuttling of polysulfide intermediates in lithium-sulfur batteries. Adv. Mater. 2018, 30, 1804581. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.D.; Shterenberg, I.; Gofer, Y.; Gershinsky, G.; Pour, N.; Aurbach, D. Mg rechargeable batteries: an on-going challenge. Energy Environ. Sci. 2013, 6, 2265–2279. [Google Scholar] [CrossRef]
- Liang, Y.; Yoo, H.D.; Li, Y.; Shuai, J.; Calderon, H.A.; Hernandez, F.C.R.; Grabow, L.C.; Yao, Y. Interlayer-expanded molybdenum disulfide nanocomposites for electrochemical magnesium storage. Nano Lett. 2015, 15, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Zong, X.L.; Liang, Z.; Yu, X.Y.; Gao, J.; Xun, C.C.; Li, P.Y.; Wang, Y.L. Double-shelled hollow carbon sphere with microporous outer shell towards high performance lithium-sulfur battery. Electrochim. Acta. 2018, 284, 89–97. [Google Scholar] [CrossRef]
- Xue, W.J.; Yan, Q.B.; Xu, G.Y.; Suo, L.M.; Chen, Y.M.; Wang, C.; Wang, C.A.; Li, J. Double-oxide sulfur host for advanced lithium-sulfur batteries. Nano Energy. 2017, 38, 12–18. [Google Scholar] [CrossRef]
- Yu, X.Y.; Yu, L.; Lou, X.W. Metal sulfide hollow nanostructures for electrochemical energy storage. Adv. Energy Mater. 2016, 6, 1501333. [Google Scholar] [CrossRef]
- Xu, J.; Fan, H.B.; Su, D.W.; Wang, G.X. Nitrogen doped yolk-shell carbon spheres as cathode host for lithium-sulfur battery. J. Alloy. Compd. 2018, 747, 283–292. [Google Scholar] [CrossRef]
- Chen, S.Q.; Huang, X.D.; Sun, B.; Zhang, J.Q.; Liu, H.; Wang, G.X. Multi-shelled hollow carbon nanospheres for lithium-sulfur batteries with superior performances. J. Mater. Chem. A. 2014, 2, 16199–16207. [Google Scholar] [CrossRef]
- Choi, S.H.; Kang, Y.C. Synergetic effect of yolk-shell structure and uniform mixing of SnS-MoS2 nanocrystals for improved Na-ion storage capabilities. ACS Appl. Mater. Inter. 2015, 7, 24694–24702. [Google Scholar] [CrossRef]
- Ren, L.; Chen, J.; Wang, X.Q.; Zhi, M.J.; Wu, J.W.; Zhang, X.H. Facile synthesis of flower-like CoMn2O4 microspheres for electrochemical supercapacitors. RSC Adv. 2015, 5, 30963–30969. [Google Scholar] [CrossRef]
- Zhu, B.G.; Tang, S.C.; Vongehr, S.; Xie, H.; Zhu, J.; Meng, X.K. FeCo2O4 submicron-tube arrays grown on Ni foam as high rate-capability and cycling-stability electrodes allowing superior energy and power densities with symmetric supercapacitors. Chem. Commun. 2016, 52, 2624–2627. [Google Scholar] [CrossRef]
- Jabeen, N.; Xia, Q.Y.; Yang, M.; Xia, H. Unique core-shell nanorod arrays with polyaniline deposited into mesoporous NiCo2O4 support for high-performance supercapacitor electrodes. ACS Appl. Mater. Inter. 2016, 8, 6093–6100. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, C.; Liang, S.X.; Li, S.F.; Huang, H.W.; Han, X.T.; Zhao, C.T.; Song, X.D.; Hao, C.; Ajayan, P.M.; et al. Bridging of ultrathin NiCo2O4 nanosheets and graphene with polyaniline: a theoretical and experimental study. Chem. Mater. 2016, 28, 5855–5863. [Google Scholar] [CrossRef]
- Kwon, H.; Hong, D.J.; Ryu, I.; Yim, S.Y. Supercapacitive properties of 3D-arrayed polyaniline hollow nanospheres encaging RuO2 nanoparticles. ACS Appl. Mater. Inter. 2017, 9, 7412–7423. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.F.; Xin, S.; Yang, Z.C.; Li, Y.T.; Zhang, D.W.; Yao, P. A facile synthesis of MoS2/Reduced graphene oxide@polyaniline for high-performance supercapacitors. ACS Appl. Mater. Inter. 2016, 8, 21373–21380. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, G.W. Preparation of zinc–nickel–cobalt ternary oxide nanosheets for supercapacitors. Chem. Res. Chinese U. 2017, 33, 939–945. [Google Scholar] [CrossRef]
- Shen, L.F.; Yu, L.; Yu, X.Y.; Zhang, X.G.; Lou, X.W. Self-templated formation of uniform NiCo2O4 hollow spheres with complex interior structures for lithium-ion batteries and supercapacitors. Angew. Chem. Int. Ed 2015, 54, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-dimensional layered MoS2: rational design, properties and electrochemical applications. Energy Environ. Sci. 2016, 9, 1190–1209. [Google Scholar] [CrossRef]
- Wang, X.; Ding, J.; Yao, S.; Wu, X.; Feng, Q.; Wang, Z.; Geng, B. High supercapacitor and adsorption behaviors of flower-like MoS2 nanostructures. J. Mater. Chem. A 2014, 2, 15958–15963. [Google Scholar] [CrossRef]
- Wang, S.Z.; Zhu, J.Y.; Shao, Y.L.; Li, W.R.; Wu, Y.Z.; Zhang, L.; Hao, X.P. Three-dimensional MoS2@CNT/RGO network composites for high-performance flexible supercapacitors. Chem. Eur. J. 2017, 23, 3438–3446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Sun, W.P.; Yang, D.; Zhang, Y.; Hoon, H.H.; Zhang, H.; Yan, Q.Y. Multifunctional architectures constructing of PANI nanoneedle arrays on MoS2 thin nanosheets for high-energy supercapacitors. Small. 2015, 11, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Sha, C.H.; Lu, B.; Mao, H.Y.; Cheng, J.P.; Pan, X.H.; Lu, J.G.; Ye, Z.Z. 3D ternary nanocomposites of molybdenum disulfide/polyaniline/reduced graphene oxide aerogel for high performance supercapacitors. Carbon. 2016, 99, 26–34. [Google Scholar] [CrossRef]
- Choudhary, N.; Patel, M.; Ho, Y.H.; Dahotre, N.B.; Lee, W.; Hwang, J.Y.; Choi, W. Directly deposited MoS2 thin film electrodes for high performance supercapacitors. J. Mater. Chem. A. 2015, 3, 24049–24054. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Chung, H.S.; Moore, J.; Thomas, J.; Jung, Y. High-performance one-body core/shell nanowire supercapacitor enabled by conformal growth of capacitive 2D WS2 layers. ACS Nano. 2016, 10, 10726–10735. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, T.; Chen, Q.; Li, P.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Wei, B.; Zhu, H. Flexible all solid-state supercapacitors based on chemical vapor deposition derived graphene fibers. Phys. Chem. Chem. Phys. 2013, 15, 17752–17757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.M.; Li, F.; Cheng, H.M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Li, L.; Wu, Z.; Yuan, S.; Zhang, X.B. Advances and challenges for flexible energy storage and conversion devices and systems. Energy Environ. Sci. 2014, 7, 2101–2122. [Google Scholar] [CrossRef]
- Sun, G.Z.; Liu, J.Q.; Zhang, X.; Wang, X.W.; Li, H.; Yu, Y.; Huang, W.; Zhang, H.; Chen, P. Fabrication of ultralong hybrid microfibers from nanosheets of reduced graphene oxide and transition-metal dichalcogenides and their application as supercapacitors. Angew. Chem. Int. Ed. 2014, 53, 12576–12580. [Google Scholar]




| Year | Composition | Shell Numbers | Methodology | Refs. |
|---|---|---|---|---|
| 2007 | SiO2 | 6–10 | Soft templating | [135] |
| 2007 | Cu2O | 1–4 | Soft templating | [140] |
| 2009 | SiO2 | 6–10 | Soft templating | [133] |
| 2009 | VOOH | 1–3 | Kirkendall effect | [151] |
| 2010 | SiO2 | 1–4 | Soft templating | [141] |
| 2010 | Carbon | 3–9 | Soft templating | [142] |
| 2011 | SiO2 | 6–10 | Soft templating | [134] |
| 2011 | Cu2O | 1–4 | Ostwald ripening | [153] |
| 2011 | PdAuAg | 1–3 | Galvanic Exchange and Kirkendall | [156] |
| 2012 | WO3 | 1–4 | Hard templating | [131] |
| 2012 | SiO2 | 6–10 | Soft templating | [136] |
| 2012 | Cu2S | 1–4 | Ion exchange | [154] |
| 2013 | Co3O4 | 1–4 | Hard templating | [129] |
| 2013 | α-Fe2O3 | 1–4 | Hard templating | [132] |
| 2014 | SiO2 | 2–7 | Soft templating | [137] |
| 2014 | TiO2 | 1–3 | Hard templating | [123] |
| 2014 | CeO2 | 1–4 | Ostwald ripening | [152] |
| 2015 | PMO | 1–3 | Soft@hard | [155] |
| 2016 | CoFe2O3 | 1–3 | Soft templating | [139] |
| 2017 | VPANI | 6–7 | Soft templating | [138] |
| 2017 | NiCo2O4 | 1–3 | Hard templating | [130] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Q.; Gao, T.; Hu, T.; Zhou, G. Synthesis and Electrochemical Energy Storage Applications of Micro/Nanostructured Spherical Materials. Nanomaterials 2019, 9, 1207. https://doi.org/10.3390/nano9091207
Gong Q, Gao T, Hu T, Zhou G. Synthesis and Electrochemical Energy Storage Applications of Micro/Nanostructured Spherical Materials. Nanomaterials. 2019; 9(9):1207. https://doi.org/10.3390/nano9091207
Chicago/Turabian StyleGong, Qinghua, Tingting Gao, Tingting Hu, and Guowei Zhou. 2019. "Synthesis and Electrochemical Energy Storage Applications of Micro/Nanostructured Spherical Materials" Nanomaterials 9, no. 9: 1207. https://doi.org/10.3390/nano9091207