Highly Efficient Wideband Microwave Absorbers Based on Zero-Valent Fe@γ-Fe2O3 and Fe/Co/Ni Carbon-Protected Alloy Nanoparticles Supported on Reduced Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ferromagnetic Nanoparticles Supported on Graphene Oxide
2.3. Characterization
3. Results
3.1. Optimization of the Citric Acid to Ethylene Glycol (CA:EG) and Metal Precursor (CA:M) Ratios
3.2. Characterization of the Chemical Composition of the ZVI Nanoparticles Deposited onto rGO
3.3. Synthesis and Characterization of Fe/Co/Ni Alloy NPs Deposited onto rGO
3.4. Characterization of the Nanocomposites Magnetic Properties and Microwave Absorption Behavior
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thomassin, J.-M.; Jérôme, C.; Pardoen, T.; Bailly, C.; Huynen, I.; Detrembleur, C. Polymer/carbon based composites as electromagnetic interference (EMI) shielding materials. Mater. Sci. Eng. R. 2013, 74, 211–232. [Google Scholar] [CrossRef]
- Scientific Committee on Emerging and Newly Identified Health Risks, European Commission, Opinion on Potential Health Effects of Exposure to Electromagnetic Fields (EMF). 2015. Available online: https://ec.europa.eu/health/sites/health/files/scientific_committees/emerging/docs/scenihr_o_041.pdf (accessed on 20 July 2019).
- Hemming, L.H. Architectural Electromagnetic Shielding Handbook: A Design and Specification Guide; IEEE Press: Piscataway, NJ, USA, 2000. [Google Scholar]
- Kong, L.B.; Liu, L.; Yang, Z.; Li, S.; Zhang, T. Magnetic Nanomaterials: Fundamentals, Synthesis and Applications; Hou, Y., Sellmyer, D.J., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2017; Chapter 15; pp. 473–514. [Google Scholar]
- Timonen, J.V.I.; Ras, R.H.A.; Ikkala, O.; Oksanen, M.; Seppälä, E.; Chalapat, K.; Li, J.; Paraoanu, G.S. Trends in Nanophysics: Theory, Experiment and, Technology; Aldea, A., Bârsan, V., Eds.; Springer Science: London, UK, 2010; Chapter 11; pp. 257–285. [Google Scholar]
- Zhao, X.; Zhang, Z.; Wang, L.; Xi, K.; Cao, Q.; Wang, D.; Yang, Y.; Du, Y. Excellent microwave absorption property of Graphene-coated Fe nanocomposites. Sci. Rep. 2013, 3, 3421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Lee, J.; Lee, K.; Park, B.; Jung, B.M.; Lee, S.B. Magnetic and dispersible FeCoNi-graphene film produced without heat treatment for electromagnetic wave absorption. Chem. Eng. J. 2019, 361, 1182–1189. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, F.; Zhang, S.; Yuan, H.; Zhu, C.; Zhang, X.; Chen, Y. Hollow N-doped carbon polyhedron containing CoNi alloy nanoparticles embedded within few-layer N-doped graphene as high-performance electromagnetic wave absorbing material. ACS Appl. Mater. Inter. 2018, 10, 24920–24929. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zong, Y.; Sun, Y.; Zhang, Y.; Yang, X.; Long, G.; Wang, Y.; Li, X.; Zheng, X. Optimization of porous FeNi3/N-GN composites with superior microwave absorption performance. Chem. Eng. J. 2018, 345, 441–451. [Google Scholar] [CrossRef]
- Fang, J.; Zha, W.; Kang, M.; Lu, S.; Cui, L.; Li, S. Microwave absorption response of nickel/graphene nanocomposites prepared by electrodeposition. J. Mater. Sci. 2013, 48, 8060–8067. [Google Scholar] [CrossRef]
- Kim, B.-J.; Bae, K.-M.; Sil Lee, Y.S.; Ana, K.-H.; Park, S.-J. EMI shielding behaviors of Ni-coated MWCNTs-filled epoxy matrix nanocomposites. Surf. Coat. Tech. 2014, 42, 125–131. [Google Scholar] [CrossRef]
- Xiang, J.; Li, J.; Zhang, X.; Ye, Q.; Xu, J.; Shen, X. Magnetic carbon nanofibers containing uniformly dispersed Fe/Co/Ni nanoparticles as stable and high-performance electromagnetic wave absorbers. J. Mater. Chem. A 2014, 2, 16905–16914. [Google Scholar] [CrossRef]
- Mederos-Henry, F.; Pichon, B.; Tchuitio Yagang, Y.; Delcorte, A.; Bailly, C.; Huynen, I.; Hermans, S. Decoration of nanocarbon solids with magnetite nanoparticles: towards microwave metamaterial absorbers. J. Mater. Chem. C 2016, 3, 3290–3303. [Google Scholar] [CrossRef]
- Danlée, Y.; Huynen, I.; Bailly, C. Thin smart multilayer microwave absorber based on hybrid structure of polymer and carbon nanotubes. Appl. Phys. Lett. 2012, 100, 213105. [Google Scholar] [CrossRef]
- Danlée, Y.; Huynen, I.; Bailly, C. Thin and flexible multilayer polymer composite structures for effective control of microwave electromagnetic absorption. Comp. Sci. Tech. 2014, 100, 182–188. [Google Scholar] [CrossRef]
- Mesfin, H.M.; Baudouin, A.C.; Hermans, S.; Delcorte, A.; Huynen, I.; Bailly, C. Frequency selective microwave absorption induced by controlled orientation of graphene-like nanoplatelets in thin polymer films. Appl. Phys. Lett. 2014, 105, 103105. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, W.; Sun, D.; Wang, Y.; Li, F. One-pot synthesis of urchinlike Ni nanoparticles/RGO composites with extraordinary electromagnetic absorption properties. Appl. Surf. Sci. 2014, 314, 523–529. [Google Scholar] [CrossRef]
- Carreño, N.L.V.; Escote, M.T.; Valentini, A.; McCafferty, L.; Stolojan, V.; Beliatis, M.; Mills, C.A.; Rhodes, R.; Smith, C.T.G.; Silva, S.R.P. Adsorbent 2D and 3D carbon matrices with protected magnetic iron nanoparticles. Nanoscale 2015, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Pechini, M.P. Sprague Electric Co, assignee. Patent US 3330697 A, 11 July 1967. [Google Scholar]
- Galceran, M.; Pujol, M.C.; Aguiló, M.; Díaz, F. Sol-gel modified Pechini method for obtaining nanocrystalline KRE(WO4)2 (RE = Gd and Yb). J. Sol-Gel Sci. Technol. 2007, 42, 79–88. [Google Scholar] [CrossRef]
- Yu, K.; Shi, Z.-X.; Qian, S.-M. Structure and performance of YBa2Cu3O7 prepared by sol-gel methods. Gongneng Cailiao/J. Funct. Mater. 2004, z1, 898–902. [Google Scholar]
- Amano, M.E.; Betancourt, I.; Arellano-Jimenez, M.J.; Sánchez-Llamazares, J.L.; Sánchez-Valdés, C.F. Magnetocaloric response of submicron (LaAg)MnO3 manganite obtained by Pechini method. J. Sol-Gel Sci. Technol 2015, 78, 1–7. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, L.; Li, Y.; Huang, Y.; Cheng, H.; Seo, H.J. A visible-light-driven photocatalytic activity of vanadate garnet AgCa2Ni2V3O12 nanoparticles. J. Nanopart. Res. 2015, 17, 405. [Google Scholar] [CrossRef]
- Hajizadeh-Oghaz, M.; Razavi, R.S.; Barekat, M.; Naderi, M.; Malekzadeh, S.; Rezazadeh, M. Synthesis and characterization of Y2O3 nanoparticles by sol–gel process for transparent ceramics applications. J. Sol-Gel Sci. Technol. 2016, 78, 682–691. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Ye, E.; Liu, B.; Fan, W.Y. Preparation of Graphite-Coated Iron Nanoparticles Using Pulsed Laser Decomposition of Fe3(CO)12 and PPh3 in Hexane. Chem. Mater. 2007, 19, 3845–3849. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides. Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley-VCH: Darmstadt, Germany, 2003. [Google Scholar]
- Wang, C.; Kang, F.-Y.; Gu, J.-L. Synthesis and microwave absorbing properties of FeCoNi alloy particles/graphite flaky composites. J. Inorg. Mater. 2010, 25, 408–410. [Google Scholar] [CrossRef]
- Quan, B.; Liang, X.; Ji, G.; Zhang, Y.; Xu, G.; Du, Y. Cross-Linking-Derived Synthesis of Porous CoxNiy/C Nanocomposites for Excellent Electromagnetic Behaviors. ACS Appl. Mater. Inter. 2017, 9, 38814–38823. [Google Scholar] [CrossRef] [PubMed]
- Arief, I.; Biswas, S.; Bose, S. FeCo-Anchored Reduced Graphene Oxide Framework-Based Soft Composites Containing Carbon Nanotubes as Highly Efficient Microwave Absorbers with Excellent Heat Dissipation Ability. ACS Appl. Mater. Inter. 2017, 9, 19202–19214. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Liu, X.; Sun, Y.P.; Jin, C.; Lv, Y. Enhanced microwave absorption of flower-like FeNi@C nanocomposites by dual dielectric relaxation and multiple magnetic resonance. RSC Adv. 2014, 4, 22710–22715. [Google Scholar] [CrossRef]
- Mederos-Henry, F.; Hermans, S.; Huynen, I. Coplanar waveguide method for microwave and ferromagnetic resonance characterization of nanocarbon powders decorated with magnetic nanoparticles. Microw. Opt. Technol. Lett. 2017, 59, 2330–2335. [Google Scholar] [CrossRef]
- Mederos-Henry, F.; Hermans, S.; Huynen, I. Microwave Characterization of Metal-Decorated Carbon Nanopowders Using a Single Transmission Line. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef]
- Petrykin, V.; Kakihana, M. Chemistry and Applications of Polymeric Gel Precursors. In Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications; Sakka, S., Ed.; Kluwer Academic Publishers: London, UK, 2005; Chapter 4; pp. 77–104. [Google Scholar]
- Mashreghi, A.; Davoudi, F. The effect of ethylene glycol/citric acid molar ratio in the initial precursor of TiO2 nanoparticle paste synthesized by a polymerizable complex method on the photovoltaic properties of dye-sensitized solar cells. Mater. Sci. Semicond. Process. 2015, 30, 618–624. [Google Scholar] [CrossRef]
- Li, X.; Agarwal, V.; Liu, M.; Rees, W.S. Investigation of the mechanism of sol-gel formation in the Sr(NO3)2/citric acid/ethylene glycol system by solution state 87Sr nuclear magnetic resonance spectroscopy. J. Mater. Res. 2000, 15, 2393–2399. [Google Scholar] [CrossRef]
- Kassaee, M.Z.; Motamedi, E.; Majdi, M. Magnetic Fe3O4-graphene oxide/polystyrene: Fabrication and characterization of a promising nanocomposite. Chem. Eng. J. 2011, 172, 540–549. [Google Scholar] [CrossRef]
- Tuz Johra, F.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Ray, R.; Das, S.; Patra, M.; Thakur, M. Iron nanoparticles from an electrochemical route. Nanosci. Methods 2012, 1, 1–8. [Google Scholar] [CrossRef]
- Zakharova, I.N.; Shipilin, M.A.; Alekseev, V.P.; Shipilin, A.M. Mössbauer study of maghemite nanoparticles. Tech. Phys. Lett. 2012, 38, 55–58. [Google Scholar] [CrossRef]
- Wei, B.; Shima, M.; Pati, R.; Nayak, S.K.; Singh, D.J.; Ma, R.; Li, Y.; Bando, Y.; Nasu, S.; Ajayan, P.M. Room-Temperature Ferromagnetism in Doped Face-Centered Cubic Fe Nanoparticles. Small 2006, 2, 804–809. [Google Scholar] [CrossRef]
- Vargas, J.M.; Socolovsky, L.M.; Goya, G.F.; Knobel, M.; Zanchet, D. Structural, magnetic, and Mossbauer characterization of size-controlled iron-iron oxide nanoparticles obtained by chemical methods. IEEE Trans. Magn. 2003, 39, 2681–2683. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Gibb, T.C. Mössbauer Spectroscopy; Springer Netherlands: Dordrecht, the Netherlands, 1971. [Google Scholar]
- Grosvenor, A.P.; Kobe, B.A.; McIntyre, M.S. Examination of the oxidation of iron by oxygen using X-ray photoelectron spectroscopy and QUASESTM. Surf. Sci. 2004, 565, 151–162. [Google Scholar] [CrossRef]
- Wise, H.; Oudar, J. Material Concepts in Surface Reactivity and Catalysis; Dover Publications INC: Mineola, NY, USA, 1990. [Google Scholar]
- Zero-valent iron Powder Diffraction File (PDF) 00-006-0696 (2017) and γ-Fe2O3 PDF 00-039-1346 (2017); International Centre for Diffraction Data (ICDD): Newtown Square, PA, USA, 2017.
- Balaban, A.T.; Klein, D.J. Local interconversions between graphite and diamond structures. Carbon 1997, 35, 247–251. [Google Scholar] [CrossRef]
- ASM Handbook Volume 3: Alloy. Phase Diagrams; Okamoto, H.; Schlesinger, M.E.; Mueller, E.M. (Eds.) ASM International: Materials Park, OH, USA, 1992. [Google Scholar]
- Wohlfarth, E.P. Ferromagnetic Materials: A Handbook on the Properties of Magnetically Ordered Substances, Vol. 2; North Holland Pub. Co.: Amsterdam, The Netherlands, 1999. [Google Scholar]
- FeCo Powder Diffraction File (PDF) 00-049-1568 (2017) and FeNi PDF 04-009-3507 (2017) and FeCoNi PDF 04-016-6385; International Centre for Diffraction Data (ICDD): Newtown Square, PA, USA, 2017.
- de Mayo, B.; Forester, D.W.; Spooner, S. Structure and magnetic properties of nanocrystalline (Fe1−xCox)90Zr7B2Cu1 (0 ⩽ x ⩽ 0.6). J. Appl. Phys. 1970, 41, 1319. [Google Scholar]
- Narayanasamy, A.; Nagarajan, T.; Muthukumarasamy, P.; Radhakrishnan, T.N. Hyperfine field distribution in disordered binary alloys. J. Phys. F Met. Phys. 1979, 9, 2261. [Google Scholar] [CrossRef]
- Kodama, D.; Shinoda, K.; Sato, K.; Konno, Y.; Joseyphus, R.J.; Motomiya, K.; Takahashi, H.; Matsumoto, T.; Sato, Y.; Tohji, K.; et al. Chemical Synthesis of sub-micrometer- to nanometer-sized magnetic FeCo dice. Adv. Mater. 2006, 18, 3154. [Google Scholar] [CrossRef]
- Djekoun, A.; Bouzabata, B.; Otmani, A.; Greneche, J.M. X-ray diffraction and Mössbauer studies of nanocrystalline FeNi alloys prepared by mechanical alloying. Catal. Today 2004, 89, 319–323. [Google Scholar] [CrossRef]
- Guittoum, A.; Layadi, A.; Bourzami, A.; Tafat, H.; Souami, N.; Boutarfaia, S.; Lacour, D. X-ray diffraction, microstructure, Mössbauer and magnetization studies of nanostructured Fe50Ni50 alloy prepared by mechanical alloying. J. Magn. Magn. Mater. 2008, 320, 1385–1392. [Google Scholar] [CrossRef]
- Ok, H.N.; Han, M.S. Mössbauer studies on the superparamagnetic behavior of 69–31 at.% FeNi fine particles. J. Appl. Phys. 1973, 44, 1932–1933. [Google Scholar] [CrossRef]
- dos Santos, C.M.; Nogueira Martins, A.F.; Costa, B.C.; Soares Ribeiro, T.; Pinheiro Braga, T.; Soares, J.M.; Sasaki, J.M. Synthesis of FeNi Alloy Nanomaterials by proteic sol-gel method: crystallographic, morphological, and magnetic Properties. J. Nanomater. 2016, 2016, 1637091. [Google Scholar] [CrossRef]
- Shinjo, T.; Itoh, F.; Takaki, H.; Nakamura, Y.; Shikazono, N. Fe57 Mössbauer effect in Fe2B, FeB and Fe3C. J. Phys. Soc. Jpn. 1964, 19, 1252. [Google Scholar] [CrossRef]
- Shinga, M.; Maeda, Y.; Nakamura, Y. Mössbauer Effect of invar type Fe-Ni-C and Fe-Ni-Mn alloys in the critical concentration. J. Phys. Soc. Jpn. 1974, 37, 363–370. [Google Scholar] [CrossRef]
- Douvalis, A.P.; Zboril, R.; Bourlinos, A.B.; Tucek, J.; Spyridi, S.; Bakas, T. A facile synthetic route toward air-stable magnetic nanoalloys with Fe-Ni/Fe-Co core and iron oxide shell. J. Nanopart. Res. 2012, 14, 1130. [Google Scholar] [CrossRef]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.-M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. C 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Mazaleyrat, F.; Ammar, M.; Lobue, M.; Bonnet, J.P.; Audebert, P.; Wang, G.Y.; Champion, Y.; Hÿtch, M.; Snoeck, E. Silica coated nanoparticles: synthesis, magnetic properties and spin structure. Alloy. Compd. 2009, 483, 473–478. [Google Scholar] [CrossRef]
- Guimarães, A.P. Principles of Nanomagnetism; Springer-Verlag: Berlin, Germany, 2009. [Google Scholar]
- Liu, X.; Pichon, B.P.; Ulhaq, C.; Lefèvre, C.; Grenèche, J.-M.; Bégin, D.; Bégin-Colin, S. Systematic Study of Exchange Coupling in Core–Shell Fe3−δO4@CoO Nanoparticles. Chem. Mater. 2015, 27, 4073–4081. [Google Scholar] [CrossRef]
- Kura, H.; Takahashi, M.; Ogawa, T. Synthesis of Monodisperse Iron Nanoparticles with a High Saturation Magnetization Using an Fe(CO)x−Oleylamine Reacted Precursor. J. Phys. Chem. C 2010, 114, 5835–5838. [Google Scholar] [CrossRef]
- Serna, C.J.; Morales, M.P. Surface and Colloid Science; Matijevic, E., Borkovec, M., Eds.; Springer US: New York, NY, USA, 2004; Chapter 2; pp. 27–81. [Google Scholar]
- Culity, B.D.; Graham, C.D. Introduction to Magnetic Materials; IEEE Press: Piscataway, NJ, USA, 2009. [Google Scholar]
- Kodama, R.H. Magnetic Nanoparticles. J. Magn. Magn. Mater. 1999, 200, 359–372. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [PubMed]
- Marusak, K.E.; Johnston-Peck, A.C.; Wu, W.-C.; Anderson, B.D.; Tracy, J.B. Size and Composition Control of CoNi Nanoparticles and Their Conversion into Phosphides. Chem. Mater. 2017, 29, 2739–2747. [Google Scholar] [CrossRef]
- Kittel, C. On the theory of ferromagnetic resonance absorption. Phys. Rev. 1948, 73, 155–161. [Google Scholar] [CrossRef]
- Charilaou, M.; Sahu, K.K.; Faivre, D.; Fischer, A.; García-Rubio, I.; Gehring, A.U. Magnetic anisotropy of non-interacting collinear nanocrystal-chains. Appl. Phys. Lett. 2011, 99, 182504. [Google Scholar] [CrossRef]
- Wen, C.P. Coplanar Waveguide: a surface strip transmission line suitable for nonreciprocal gyromagnetic device applications. IEEE T. Microw. Theory 1969, 17, 1087–1090. [Google Scholar] [CrossRef]
- Saib, A.; Huynen, I. Transmission lines on periodic bandgap metamaterials: from microwaves to optics applications. J. Opt. A Pure Appl. Op. 2005, 7, S124–S132. [Google Scholar] [CrossRef]
- Saib, A.; Huynen, I.; Vanhoenacker-Janvier, D. Design of a stopband filter based on a Magnetic Photonic Bandgap Material. In Proceedings of the 33th European Microwave Conference, Munich, Germany, 6–10 October 2003; pp. 809–812. [Google Scholar]
- Qin, F.; Peng, H.-X. Ferromagnetic microwires enabled multifunctional composite materials. Prog. Mater. Sci. 2013, 58, 183–259. [Google Scholar] [CrossRef]
- Li, B.; Zhong, W.-H. Polymer Nanocomposites for Dielectrics; Zhong, K., Li, B., Eds.; Pan Stanford Publishing Pte. Ltd: Singapore, 2017; Chapter 8; pp. 171–192. [Google Scholar]
- Quan, B.; Liang, X.; Ji, G.; Ma, J.; Ouyang, P.; Gong, H.; Xu, G.; Du, Y. Strong Electromagnetic Wave Response Derived from the Construction of Dielectric/Magnetic Media Heterostructure and Multiple Interfaces. ACS Appl. Mater. Inter. 2017, 9, 9964–9974. [Google Scholar] [CrossRef] [PubMed]
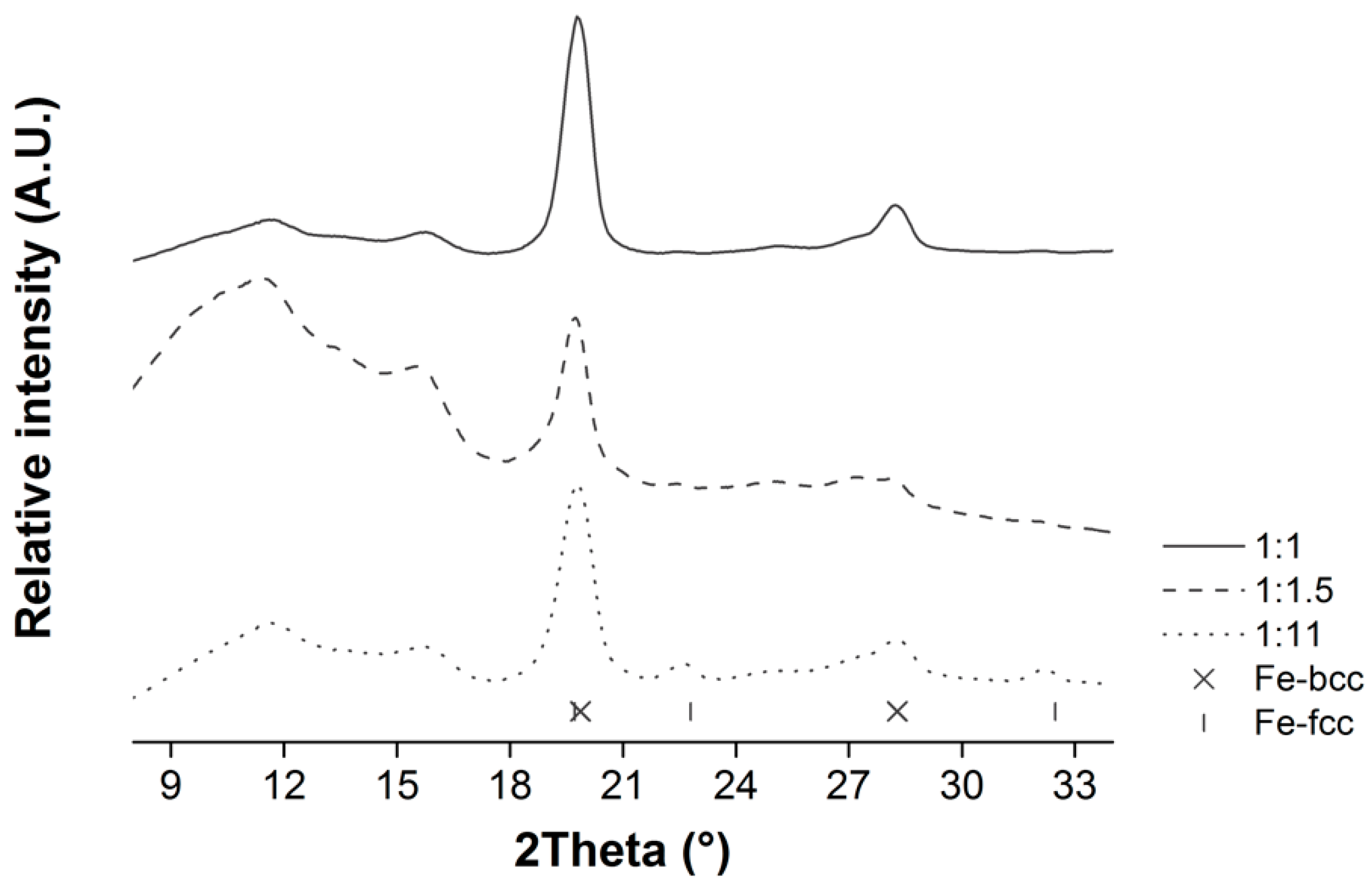




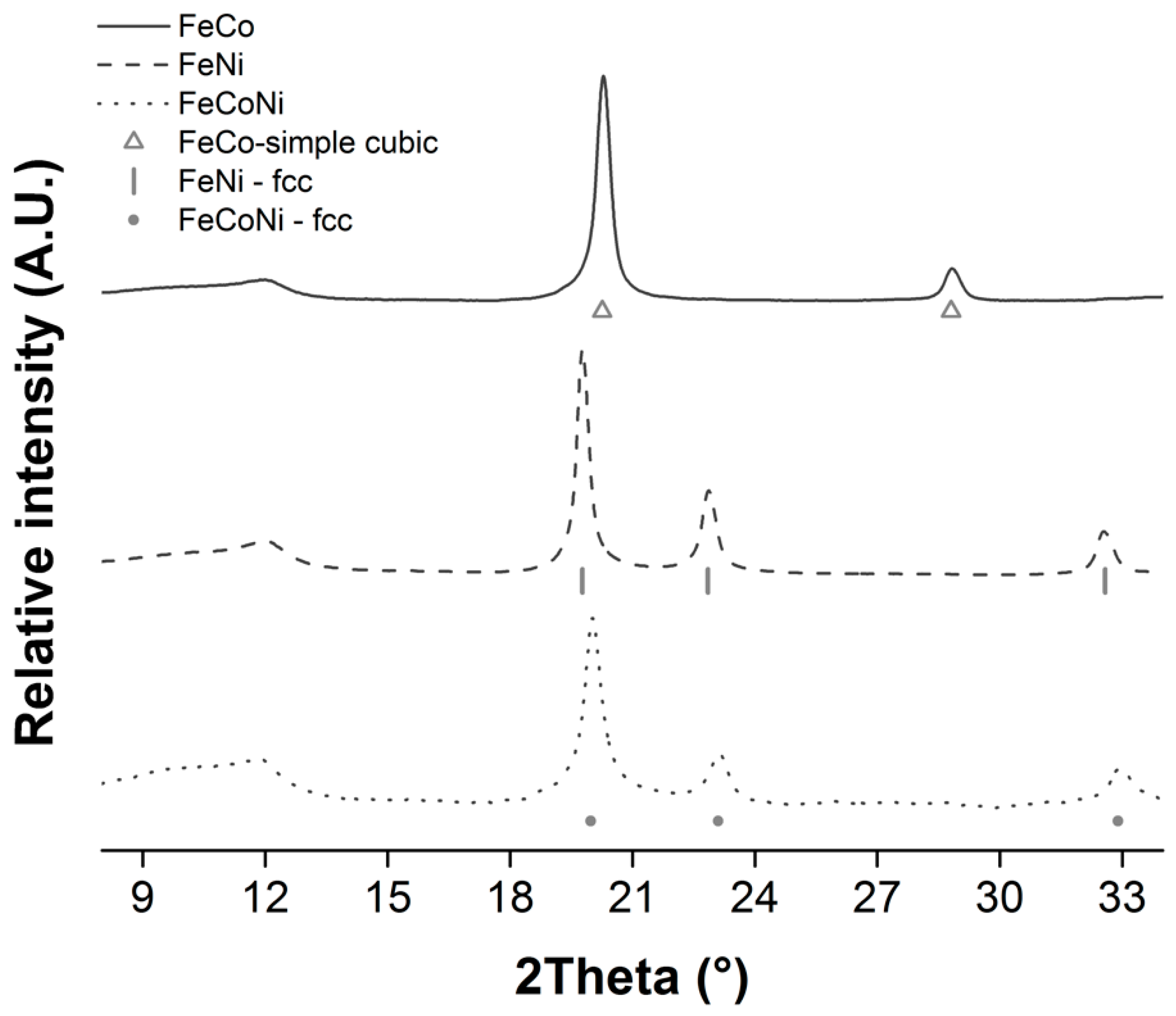

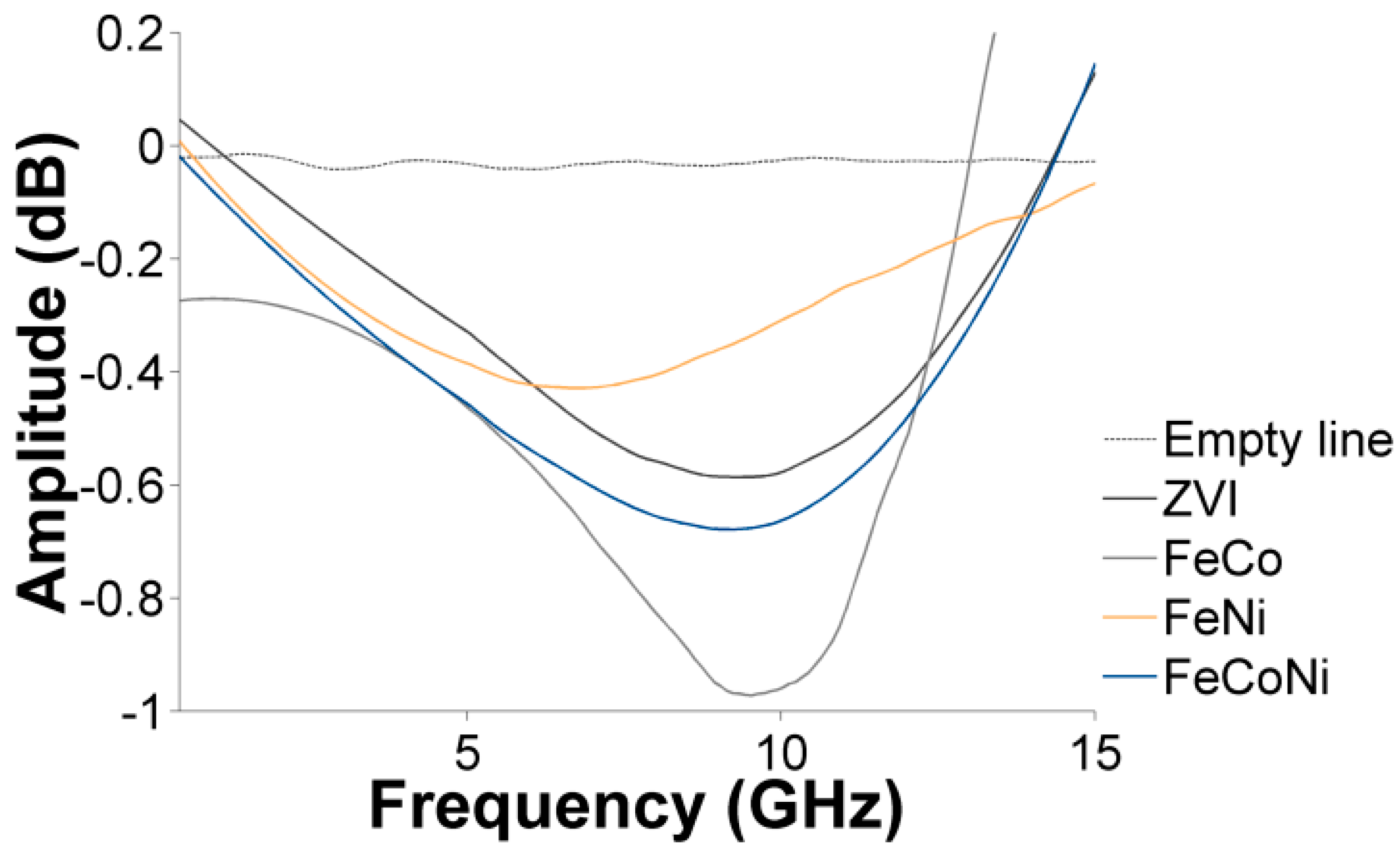
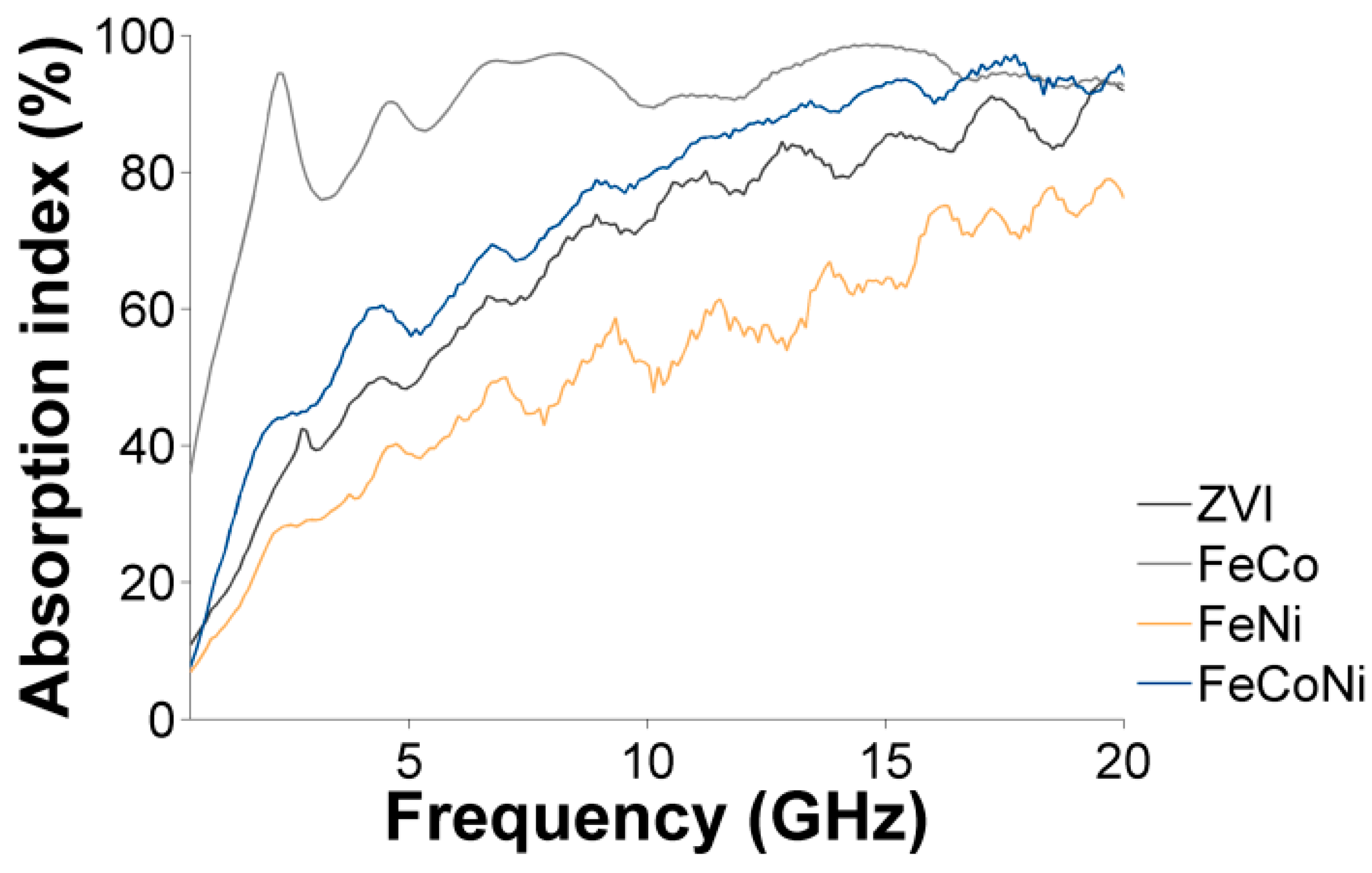
| CA:M Ratio | ||
|---|---|---|
| CA:EG Ratio | 3:1 | 6:1 |
| 1:1 | 16.8 ± 6.5 nm | 14.8 ± 4.7 nm |
| 1:1.5 | 17.9 ± 8.1 nm | 14.3 ± 7.2 nm |
| 1:11 | 18.3 ± 9.6 nm | 19.7 ± 10.4 nm |
| Nanocomposite | T | δ | ε, ΔEQ | Bhf | Г/2 | Relative Area | Sites |
|---|---|---|---|---|---|---|---|
| [K] | [mm/s] | [mm/s] | [Tesla] | [mm/s] | [%] | ||
| ZVI@rGO | 77 | 0.05(1) | – | – | 0.18 * | 4 | γ-Fe (fcc) |
| 0.11(1) | 0 | 34.1 | 0.21(1) | 86 | α-Fe (bcc) | ||
| 0.43(1) | 0 | 39 | 0.28 * | 10 | γ-Fe2O3 | ||
| FeCo@rGO | 77 | 0.12(1) | 0 | 34.6 | 0.22(1) | 100 | FeCo alloy |
| FeNi@rGO | 77 | 0.16(1) | 0.35(1) | – | 0.15 * | 6 | superparamagnetic phase |
| 0.027(1) 0.06(2) | −0.018(1) 0.01(1) | 34.1 31.5 | 0.20(1) 0.29(1) | 24 70 | FeNi (fcc) alloy | ||
| FeCoNi@rGO | 77 | 0.35(1) | −0.07(1) | 21.2 | 0.40(1) | 30 | Fe carbide |
| 0.15(1) | −0.05(1) | 32 | 0.17(1) | 70 | FeCoNi (fcc) alloy |
| EDX | ICP | |||||
|---|---|---|---|---|---|---|
| Alloy NPs | % Fe | % Co | % Ni | % Fe | % Co | % Ni |
| FeCo | 47 ± 4 | 53 ± 6 | – | 49 | 51 | – |
| FeNi | 48 ± 3 | – | 52 ± 3 | 52 | – | 48 |
| FeCoNi | 30 ± 8 | 36 ± 5 | 34 ± 6 | 32 | 34 | 34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mederos-Henry, F.; Mahin, J.; Pichon, B.P.; Dîrtu, M.M.; Garcia, Y.; Delcorte, A.; Bailly, C.; Huynen, I.; Hermans, S. Highly Efficient Wideband Microwave Absorbers Based on Zero-Valent Fe@γ-Fe2O3 and Fe/Co/Ni Carbon-Protected Alloy Nanoparticles Supported on Reduced Graphene Oxide. Nanomaterials 2019, 9, 1196. https://doi.org/10.3390/nano9091196
Mederos-Henry F, Mahin J, Pichon BP, Dîrtu MM, Garcia Y, Delcorte A, Bailly C, Huynen I, Hermans S. Highly Efficient Wideband Microwave Absorbers Based on Zero-Valent Fe@γ-Fe2O3 and Fe/Co/Ni Carbon-Protected Alloy Nanoparticles Supported on Reduced Graphene Oxide. Nanomaterials. 2019; 9(9):1196. https://doi.org/10.3390/nano9091196
Chicago/Turabian StyleMederos-Henry, Francisco, Julien Mahin, Benoit P. Pichon, Marinela M. Dîrtu, Yann Garcia, Arnaud Delcorte, Christian Bailly, Isabelle Huynen, and Sophie Hermans. 2019. "Highly Efficient Wideband Microwave Absorbers Based on Zero-Valent Fe@γ-Fe2O3 and Fe/Co/Ni Carbon-Protected Alloy Nanoparticles Supported on Reduced Graphene Oxide" Nanomaterials 9, no. 9: 1196. https://doi.org/10.3390/nano9091196








