Elaboration and Characterization of Active Films Containing Iron–Montmorillonite Nanocomposites for O2 Scavenging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of MMT-Fe
2.1.2. Processing of MMT-Fe/LLDPE Films
2.2. Methods
2.2.1. Structural and Morphological Analysis
2.2.2. Thermal Properties
2.2.3. O2 and Vapor Barrier Properties
2.2.4. Oxygen-Scavenging Capacity
2.2.5. Iron Content by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
2.2.6. Mössbauer Spectroscopy
2.3. Modeling of Oxygen Adsorption in the Active Nanocomposite
2.3.1. Modeling of O2 Adsorption for Iron Nanoparticles
2.3.2. Modeling of O2 Adsorption for an Active Nanocomposite
2.3.3. Numerical Simulations
2.4. Statistics
3. Results
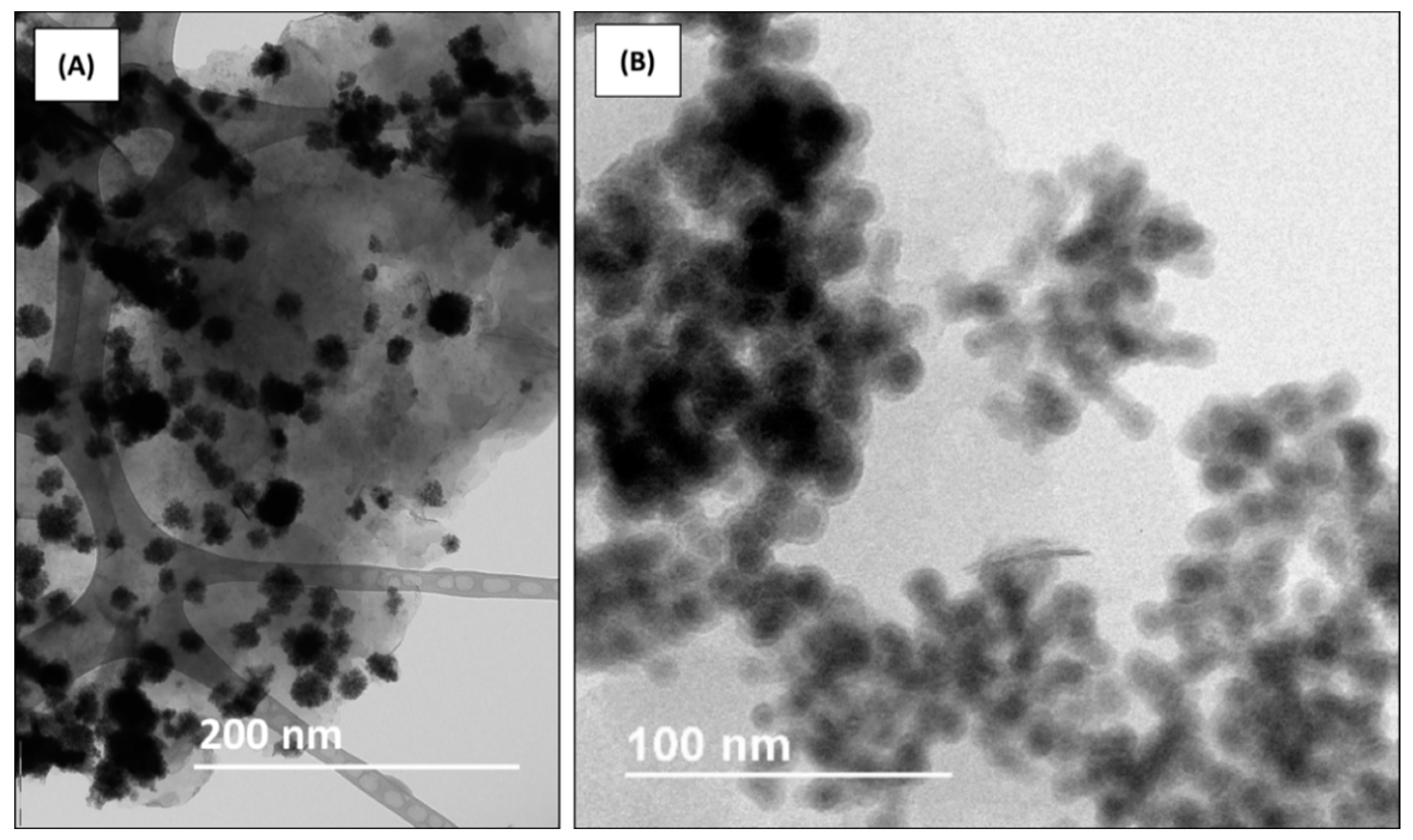
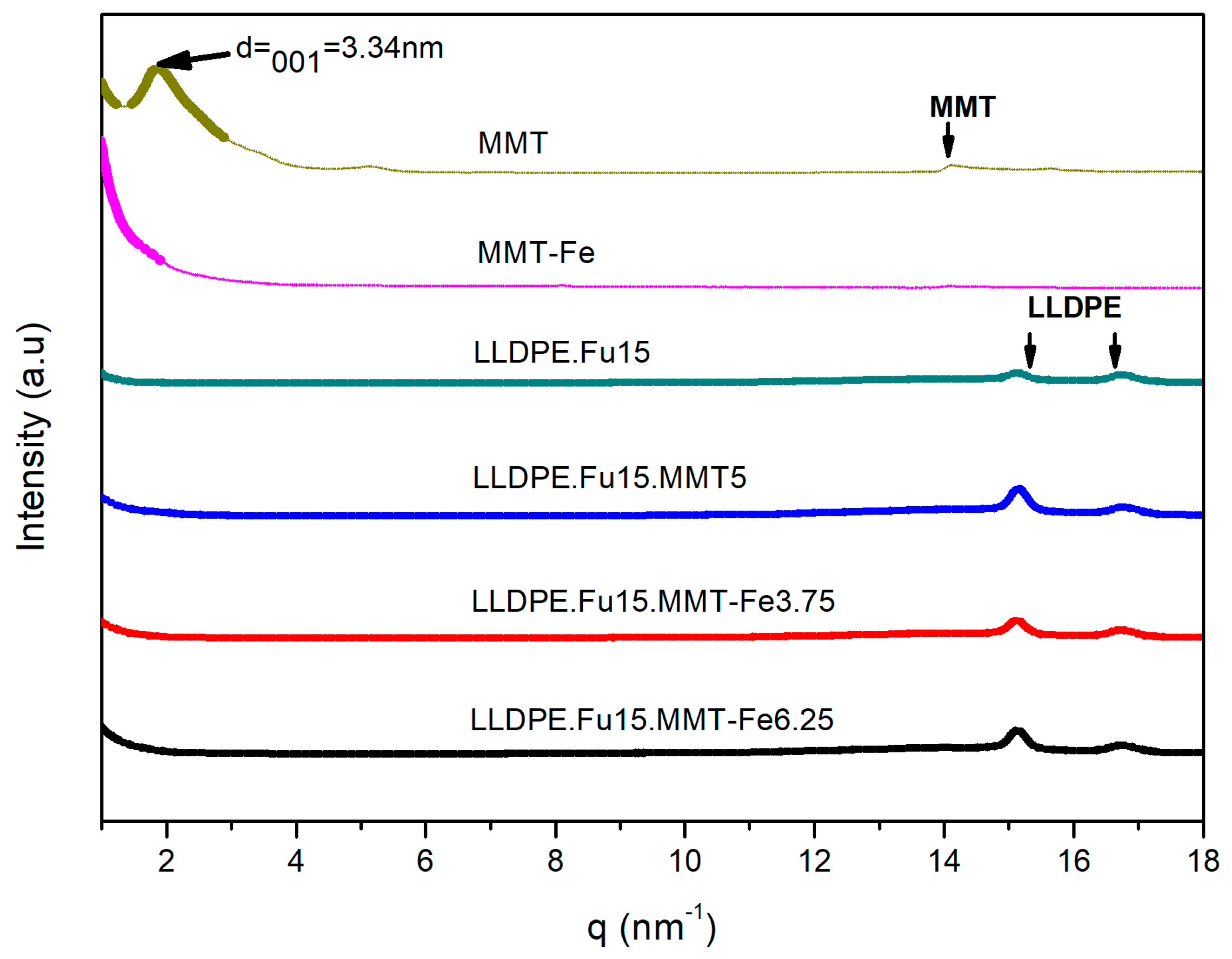
3.1. Structural and Morphological Characterization of the MMT-Fe and MMT-Fe/LLDPE Films
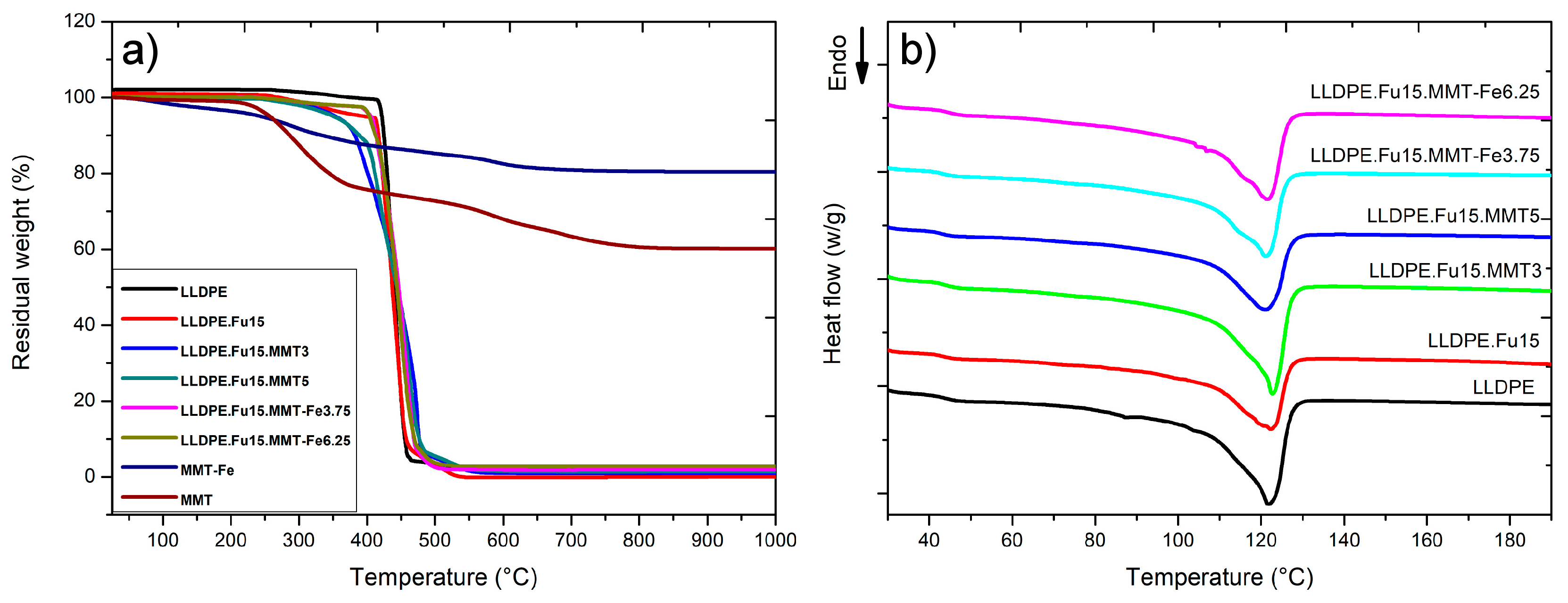
3.2. Effect of MMT-Fe Content on the Thermal Stability of LLDPE Nanocomposite Films
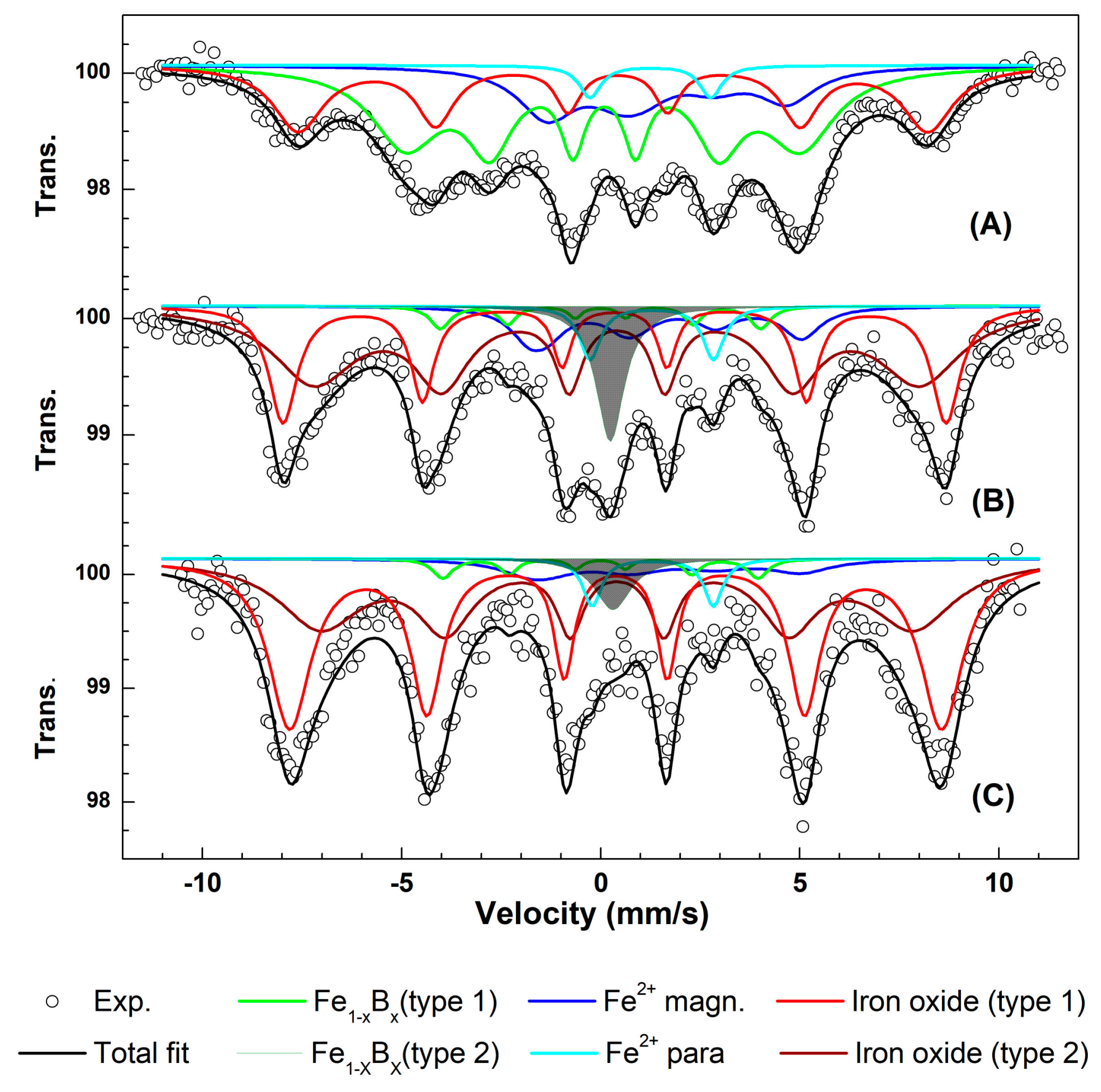
3.3. Mössbauer Spectroscopy Study of the Active Nanocomposite
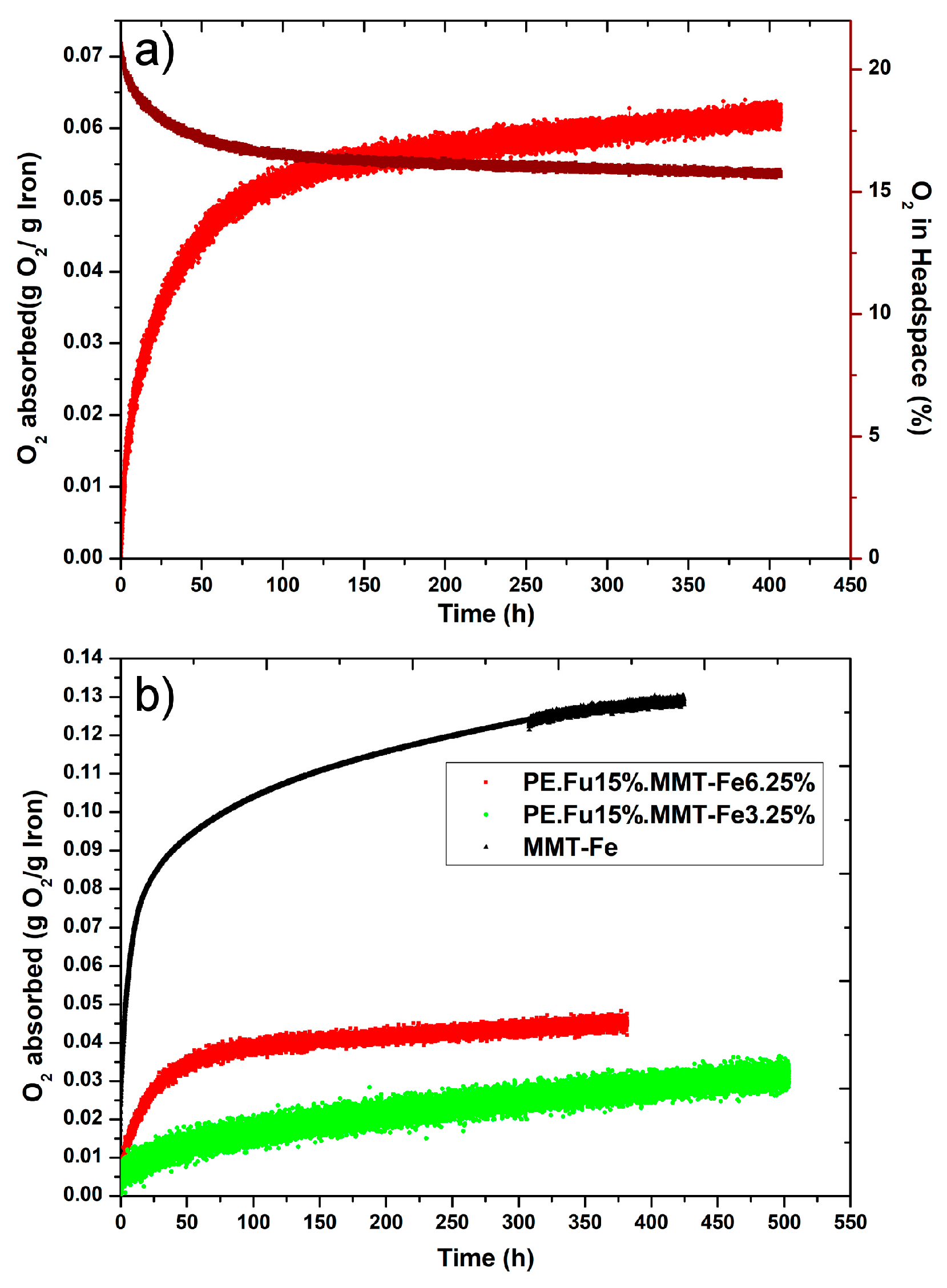
3.4. Oxygen-Scavenging Capabilities for MMT-Fe and Active Nanocomposites
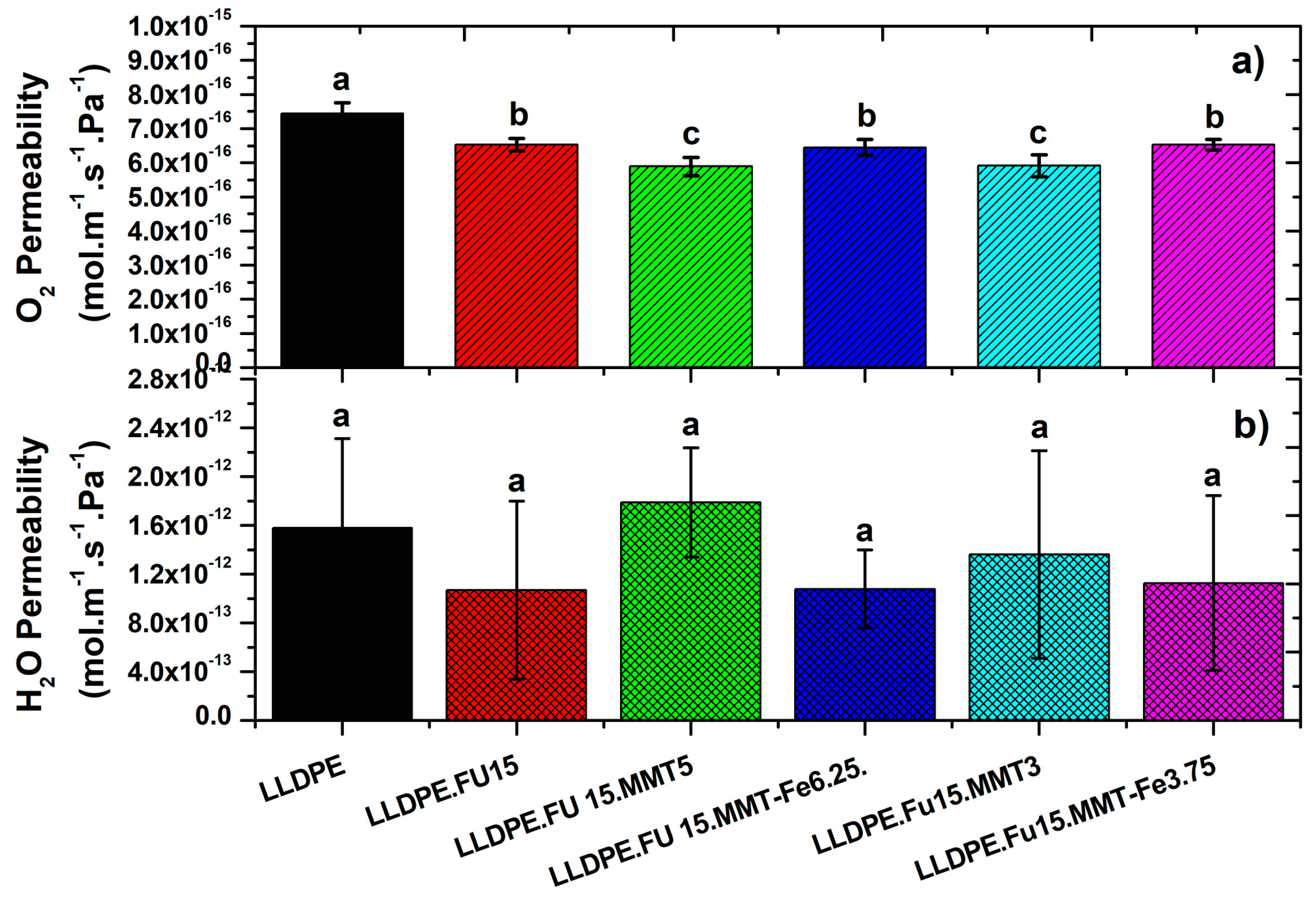
3.5. Oxygen and Water Vapor Barrier Properties of LLDPE/MMT-Fe Active Film Composite
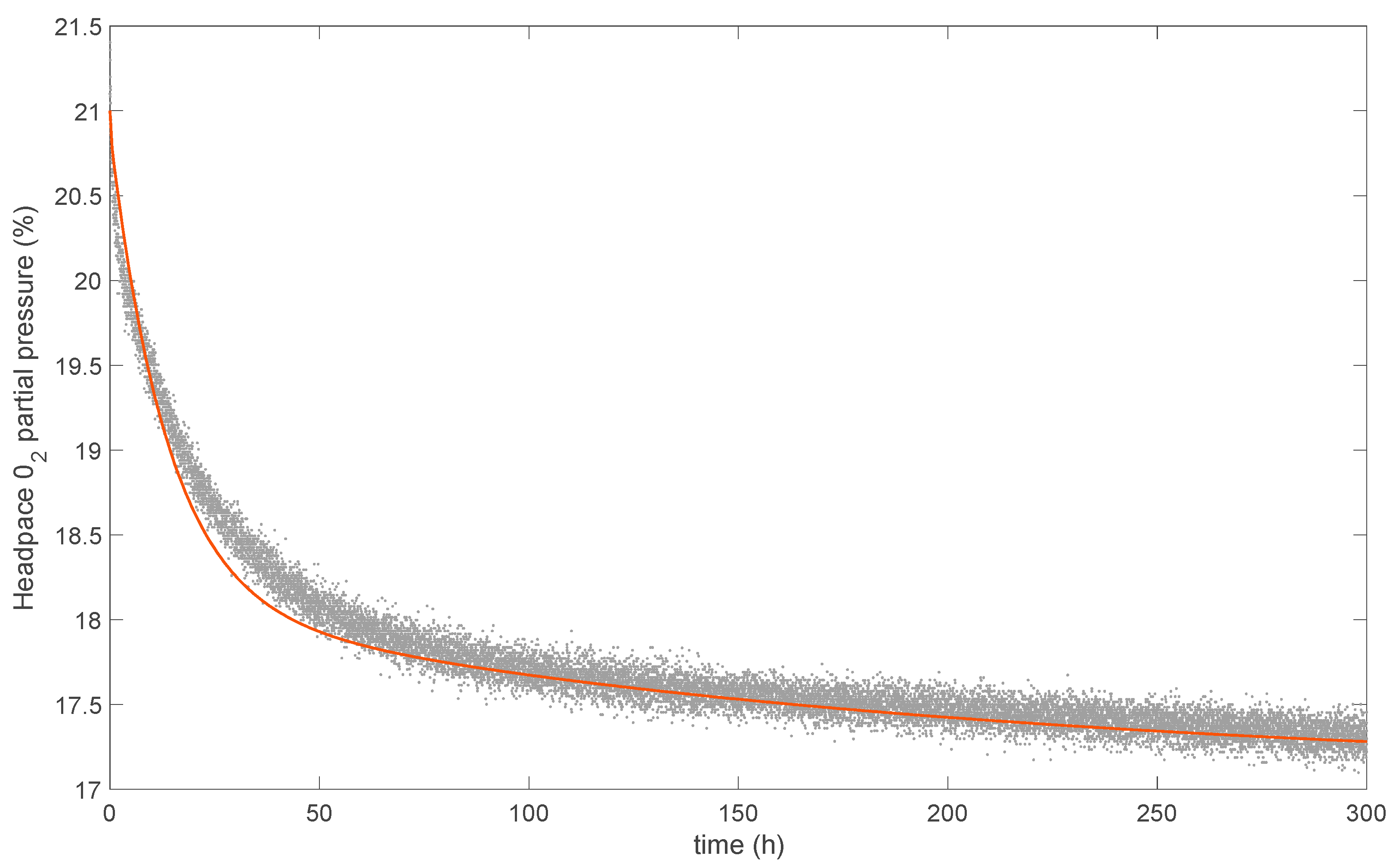
3.6. Modeling the Oxygen-Scavenging Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix B

References
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of polymer-based multilayer packaging: A Review. Recycling 2018, 3, 1. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Bharadwaj, R.K. Modeling the barrier properties of polymer-layered silicate nanocomposites. Macromolecules 2001, 34, 9189–9192. [Google Scholar] [CrossRef]
- Wolf, C.; Angellier-Coussy, H.; Gontard, N.; Doghieri, F.; Guillard, V. How the shape of fillers affects the barrier properties of polymer/non-porous particles nanocomposites: A review. J. Memb. Sci. 2018, 556, 394–418. [Google Scholar] [CrossRef]
- Foltynowicz, Z.; Bardenshtein, A.; Sängerlaub, S.; Antvorskov, H.; Kozak, W. Nanoscale, zero valent iron particles for application as oxygen scavenger in food packaging. Food Packag. Shelf Life 2017, 11, 74–83. [Google Scholar] [CrossRef]
- Pant, A.F.; Sängerlaub, S.; Müller, K. Gallic acid as an oxygen scavenger in bio-based multilayer packaging films. Materials 2017, 10, 489. [Google Scholar] [CrossRef]
- Busolo, M.A.; Lagaron, J.M. Oxygen scavenging polyolefin nanocomposite films containing an iron modified kaolinite of interest in active food packaging applications. Innov. Food Sci. Emerg. Technol. 2012, 16, 211–217. [Google Scholar] [CrossRef]
- Wang, C.; Luo, H.; Zhang, Z.; Wu, Y.; Zhang, J.; Chen, S. Removal of As(III) and As(V) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites. J. Hazard. Mater. 2014, 268, 124–131. [Google Scholar] [CrossRef]
- Shahwan, T.; Üzüm, C.; ̧Eroǧlu, A.E.; Lieberwirth, I. Synthesis and characterization of bentonite/iron nanoparticles and their application as adsorbent of cobalt ions. Appl. Clay Sci. 2010, 47, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.L.; Xi, Y.; He, H. Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J. Colloid Interface Sci. 2010, 341, 153–161. [Google Scholar] [CrossRef]
- Uzum, C.; Shahwan, T.; Eroglu, A.; Hallam, K.; Scott, T.; Lieberwirth, I. Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+ and Co2+ ions. Appl. Clay Sci. 2009, 43, 172–181. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Kombaya-Touckia-Linin, E.-M.; Gaucel, S.; Sougrati, M.T.; Khederlou, K.; Pen, N.; Stievano, L.; Gontard, N.; Guillard, V. Hybrid iron montmorillonite nano-particles as an oxygen scavenger. Chem. Eng. J. 2019, 357, 750–760. [Google Scholar] [CrossRef]
- Fan, M.; Yuan, P.; Zhu, J.; Chen, T.; Yuan, A.; He, H.; Chen, K.; Liu, D. Core-shell structured iron nanoparticles well dispersed on montmorillonite. J. Magn. Magn. Mater. 2009, 321, 3515–3519. [Google Scholar] [CrossRef]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar]
- Khalaj, M.; Ahmadi, H.; Lesankhosh, R. Trends in Food Science & Technology Study of physical and mechanical properties of polypropylene nanocomposites for food packaging application: Nano-clay modified with iron nanoparticles. Trends Food Sci. Technol. 2016, 51, 41–48. [Google Scholar]
- Nasiri, A.; Gontard, N.; Gastaldi, E.; Peyron, S. Contribution of nanoclay to the additive partitioning in polymers. Appl. Clay Sci. 2017, 146, 27–34. [Google Scholar] [CrossRef]
- Nasiri, A.; Peyron, S.; Gastaldi, E.; Gontard, N. Effect of nanoclay on the transfer properties of immanent additives in food packages. J. Mater. Sci. 2016, 51, 9732–9748. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Kim, H.; Kobayashi, S.; Abdurrahim, M.A.; Zhang, M.J.; Khusainova, A.; Hillmyer, M.A.; Abdala, A.A.; Macosko, C.W. Graphene/polyethylene nanocomposites: Effect of polyethylene functionalization and blending methods. Polymer 2011, 52, 1837–1846. [Google Scholar] [CrossRef]
- Turan, D.; Sängerlaub, S.; Stramm, C.; Gunes, G. Gas permeabilities of polyurethane films for fresh produce packaging: Response of O2 permeability to temperature and relative humidity. Polym. Test. 2017, 59, 237–244. [Google Scholar] [CrossRef]
- Müller, K.; Scheuerer, Z.; Florian, V.; Skutschik, T. Comparison of test methods for oxygen permeability: Optical method versus carrier gas method. Polym. Test. 2017, 63, 126–132. [Google Scholar] [CrossRef]
- Angellier-Coussy, H.; Gastaldi, E.; Gontard, N.; Guillard, V. Influence of processing temperature on the water vapour transport properties of wheat gluten based agromaterials. Ind. Crops Prod. 2011, 33, 457–461. [Google Scholar] [CrossRef]
- NF X31−147 Qualité des Sols-Sols, Sédiments—Mise en Solution Totale par Attaque Acide; AFNOR: Paris, France, 1996.
- NF ISO 14869−1 Qualité du Sol—Mise en Solution Pour la Détermination des Teneurs Elémentaires Totales-Partie 1: Mise en Solution par L’acide Fluorhydrique et L’acide Perchlorique; AFNOR: Paris, France, 2001.
- Grosse, G. PC-Mos, 2nd ed.; Technische Universität München: Munich, Germany, 1993. [Google Scholar]
- Cerclé, C.; Sarazin, P.; Favis, B.D. High performance polyethylene/thermoplastic starch blends through controlled emulsification phenomena. Carbohydr. Polym. 2013, 92, 138–148. [Google Scholar] [CrossRef]
- Wang, K.; Wang, C.; Li, J.; Su, J.; Zhang, Q.; Du, R.; Fu, Q. Effects of clay on phase morphology and mechanical properties in polyamide 6/EPDM-g-MA/organoclay ternary nanocomposites. Polymer 2007, 48, 2144–2154. [Google Scholar] [CrossRef]
- De Resende, V.G.; De Grave, E.; da Costa, G.M.; Janssens, J. Influence of the borohydride concentration on the composition of the amorphous Fe–B alloy produced by chemical reduction of synthetic, nano-sized iron-oxide particles. J. Alloy. Compd. 2007, 440, 236–247. [Google Scholar] [CrossRef]
- Shen, J.; Li, Z.; Yan, Q.; Chen, Y. Reactions of bivalent metal ions with borohydride in aqueous solution for the preparation of ultrafine amorphous alloy particles. J. Phys. Chem. 1993, 97, 8504–8511. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Tratnyek, P.G.; Amonette, J.E.; Pecher, K.; Wang, C.; Linehan, J.C.; Matson, D.W.; Penn, R.L.E.E.; Driessen, M.D. Characterization and properties of metallic iron nanoparticles: Spectroscopy, electrochemistry, and kinetics. Environ. Sci. Technol. 2005, 39, 1221–1230. [Google Scholar] [CrossRef]
- Kumar, N.; Auffan, M.; Gattacceca, J.; Rose, J.; Olivi, L.; Borschneck, D.; Kvapil, P.; Jublot, M.; Kaifas, D.; Malleret, L.; et al. Molecular insights of oxidation process of iron nanoparticles: Spectroscopic, magnetic, and microscopic evidence. Environ. Sci. Technol. 2014, 48, 13888–13894. [Google Scholar] [CrossRef] [PubMed]
- Miltz, J.; Perry, M. Evaluation of the performance of iron-based oxygen scavengers, with comments on their optimal applications. Packag. Technol. Sci. 2005, 18, 21–27. [Google Scholar] [CrossRef]
- Lehner, M.; Schlemmer, D.; Sängerlaub, S. Recycling of blends made of polypropylene and an iron-based oxygen scavenger—Influence of multiple extrusions on the polymer stability and the oxygen absorption capacity. Polym. Degrad. Stab. 2015, 122, 122–132. [Google Scholar] [CrossRef]
- Lagarón, J.-M.; Busolo, M.A. Active nanocomposites for food and beverage packaging. In Emerging Food Packaging Technologies; Yam, K.L., Lee, D.S., Eds.; Elsevier: Philadelphia, PA, USA, 2012; pp. 55–65. [Google Scholar]
- Amberg-Schwab, S.; Weber, U.; Burger, A.; Nique, S.; Xalter, R. Development of passive and active barrier coatings on the basis of inorganic–organic polymers. Monatshefte Chem. 2006, 137, 657–666. [Google Scholar] [CrossRef]
- Poley, L.H.; Ana, P.; Siqueira, L.; Silva, M.G.; Vargas, H. Photothermal characterization of low density polyethylene food packages. Polímeros Ciência Tecnol. 2004, 14, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Mrkić, S.; Galić, K.; Ivanković, M.; Hamin, S.; Ciković, N. Gas transport and thermal characterization of mono- and di-polyethylene films used for food packaging. J. Appl. Polym. Sci. 2006, 99, 1590–1599. [Google Scholar] [CrossRef]

| Sample * | Composition | |||
|---|---|---|---|---|
| LLDPE wt % | PE-g-MA wt % | MMT wt % | MMT-Fe wt % | |
| LLDPE | 100 | |||
| LLDPE.Fu15 | 85 | 15 | ||
| LLDPE.Fu15.MMT3 | 82 | 15 | 3 | |
| LLDPE.Fu5.MMT5 | 80 | 15 | 5 | |
| LLDPE.Fu15.MMT-Fe3.75 | 81.25 | 15 | 3.75 | |
| LLDPE.Fu15.MMT-Fe6.25 | 78.75 | 15 | 6.25 | |
| Sample | Heat of Fusion (J/g) | Tm (°C) | Crystallinity | Temp. Onset Degradation (°C) | Temp. Endset Degradation (°C) |
|---|---|---|---|---|---|
| LLDPE | 104 ± 10 | 122.1 ± 0.5 | 35.8 ± 3.6 | 416 ± 9 | 475 ± 4 |
| LLDPE.Fu15 | 104 ± 8 | 121.3 ± 1.2 | 35.7 ± 3.1 | 416 ± 2 | 457 ± 2 |
| LLDPE.Fu15.MMT3 | 104 ± 5 | 121.9 ± 0.7 | 34.8 ± 0.9 | 416 ± 22 | 478 ± 19 |
| LLDPE.Fu15.MMT5 | 99 ± 10 | 122.2 ± 1.0 | 31.3 ± 0.6 | 429 ± 9 | 480 ± 4 |
| LLDPE.Fu15.MMT-Fe3.75 | 107 ± 2 | 121.6 ± 0.4 | 36.3 ± 0.7 | 419 ± 5 | 468 ± 3 |
| LLDPE.Fu15.MMT-Fe6.25 | 102 ± 4 | 122.3 ± 1.2 | 34.5 ± 1.2 | 418 ± 7 | 466 ± 2 |
| Sample | Component | δ (mm/s) | Δ (mm/s) | Γ (mm/s) | BHF (T) | Area (%) |
|---|---|---|---|---|---|---|
| MMT-Fe | Fe1−xBx | 0.08 | −0.03 | 0.47 | 30.8 | 53 |
| Fe2+ para | 1.25 | 3.03 | 0.66 | - | 6 | |
| Fe2+ magn | 1.21 | −2.78 | 1.72 | 15.0 | 6 | |
| Fe3+ oxide | 0.37 | −0.12 | 0.60 | 49.0 | 35 | |
| Fresh LLDPE.Fu15.MMT-Fe6.25 | Fe1−xBx type1 | 0.00 | 0.00 | 0.60 | 25.00 | 3 |
| Fe1−xBx type2 | 0.24 | 0.00 | 0.86 | - | 8 | |
| Fe2+ para | 1.28 | 3.09 | 0.64 | - | 4 | |
| Fe2+ magn | 1.21 | −2.07 | 1.14 | 19.45 | 10 | |
| Fe3+ oxide type1 | 0.34 | 0.00 | 0.85 | 51.62 | 75 | |
| Fe3+ oxide type 2 | 0.41 | 0.00 | 2.5 | 47.17 | ||
| LLDPE.Fu15.MMT-6.25 after four months of oxidation | Fe1−xBx type1 | 0.00 | 0.00 | 0.60 | 24.55 | 3 |
| Fe1−xBx type2 | 0.30 | 0.00 | 1.11 | - | 3 | |
| Fe2+ para | 1.30 | 3.04 | 0.56 | - | 3 | |
| Fe2+ magn | 1.21 | −2 | 1.81 | 19.52 | 5 | |
| Fe3+ oxide type1 | 0.37 | 0.00 | 1.27 | 50.76 | 86 | |
| Fe3+ oxide type 2 | 0.41 | 0.00 | 2.5 | 46.16 |
| Film | O2 Adsorption Capacity mg O2/g of Absorber | Ref. |
|---|---|---|
| PE.Fu15.MMT-Fe6.25 film | 55 ± 2 | This work |
| PE.Fu15.MMT-Fe3.75 film | 31 ± 2 | This work |
| Polymer with iron powder and additives (“SHELFPLUS”); masterbatch | 26–48 | [36] |
| O2Block1 (NanoBioMatters S.L., Paterna, Spain); masterbatch | >10–25 | [37] |
| Cyclo-olefin bonded to a silicate backbone “ORMOCER1”; lacquer | 90 | [38] |
| Nanoscale iron in silicon matrix | >32 ± 80 | [7] |
| Polyolefin films containing 10 wt % of iron modified kaolinite | 5.7 | [9] |
| Nanoscale iron powder | >68 ± 2 | [7] |
| Nanoscale iron-MMT powder | 122 | [15] |
| Stoichiometric capacity of iron | 394 | [15] |
| Symbol | Definition | Unit | Value | Source |
|---|---|---|---|---|
| Thickness of the nanocomposite sample | 296 × 10−6 | This work | ||
| Surface area of a single side of the nanocomposite film sample | 0.1376 | This work | ||
| Volume of the headspace | 458 × 10−6 | This work | ||
| Mass fraction of iron in the sample | 0.22 | This work | ||
| Mass fraction of zerovalent iron | 0.11 | This work | ||
| Mass fraction of bivalent iron | 0.14 | This work | ||
| Molar mass of iron | 55.8 × 10−3 | |||
| Apparent density of the nanocomposite, taken as the corresponding value for the pure polymer (LLDPE) | 918 | [15] | ||
| oxidation | 2.4 × 10−6 | [15] | ||
| oxidation | 3.2 × 10−5 | [15] | ||
| oxidation | 0.19 | [15] | ||
| oxidation | 0.40 | [15] | ||
| Apparent O2 diffusivity in the nanocomposite, taken as the corresponding value for the pure polymer (LLDPE) | 1.68 × 10−11 | [39] | ||
| O2 solubility in the nanocomposite material | m−3 | 3.54 × 10−6 | [40] | |
| Biot number | dimensionless | 1 × 105 | This work | |
| Ideal gas constant | 8.314 | |||
| Temperature of the experiment | 293.15 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kombaya-Touckia-Linin, E.-M.; Gaucel, S.; Sougrati, M.T.; Stievano, L.; Gontard, N.; Guillard, V. Elaboration and Characterization of Active Films Containing Iron–Montmorillonite Nanocomposites for O2 Scavenging. Nanomaterials 2019, 9, 1193. https://doi.org/10.3390/nano9091193
Kombaya-Touckia-Linin E-M, Gaucel S, Sougrati MT, Stievano L, Gontard N, Guillard V. Elaboration and Characterization of Active Films Containing Iron–Montmorillonite Nanocomposites for O2 Scavenging. Nanomaterials. 2019; 9(9):1193. https://doi.org/10.3390/nano9091193
Chicago/Turabian StyleKombaya-Touckia-Linin, Erland-Modeste, Sébastien Gaucel, Moulay T. Sougrati, Lorenzo Stievano, Nathalie Gontard, and Valérie Guillard. 2019. "Elaboration and Characterization of Active Films Containing Iron–Montmorillonite Nanocomposites for O2 Scavenging" Nanomaterials 9, no. 9: 1193. https://doi.org/10.3390/nano9091193






