Enhancing the Electrochemical Performance of SbTe Bimetallic Anodes for High-Performance Sodium-Ion Batteries: Roles of the Binder and Carbon Support Matrix
Abstract
1. Introduction
2. Experimental
2.1. Preparing the SbTe-C Bimetallic Compounds
2.2. Material Characterization
2.3. Electrochemical Experiments
3. Results and Discussion
3.1. Characterizing Structures and Morphologies
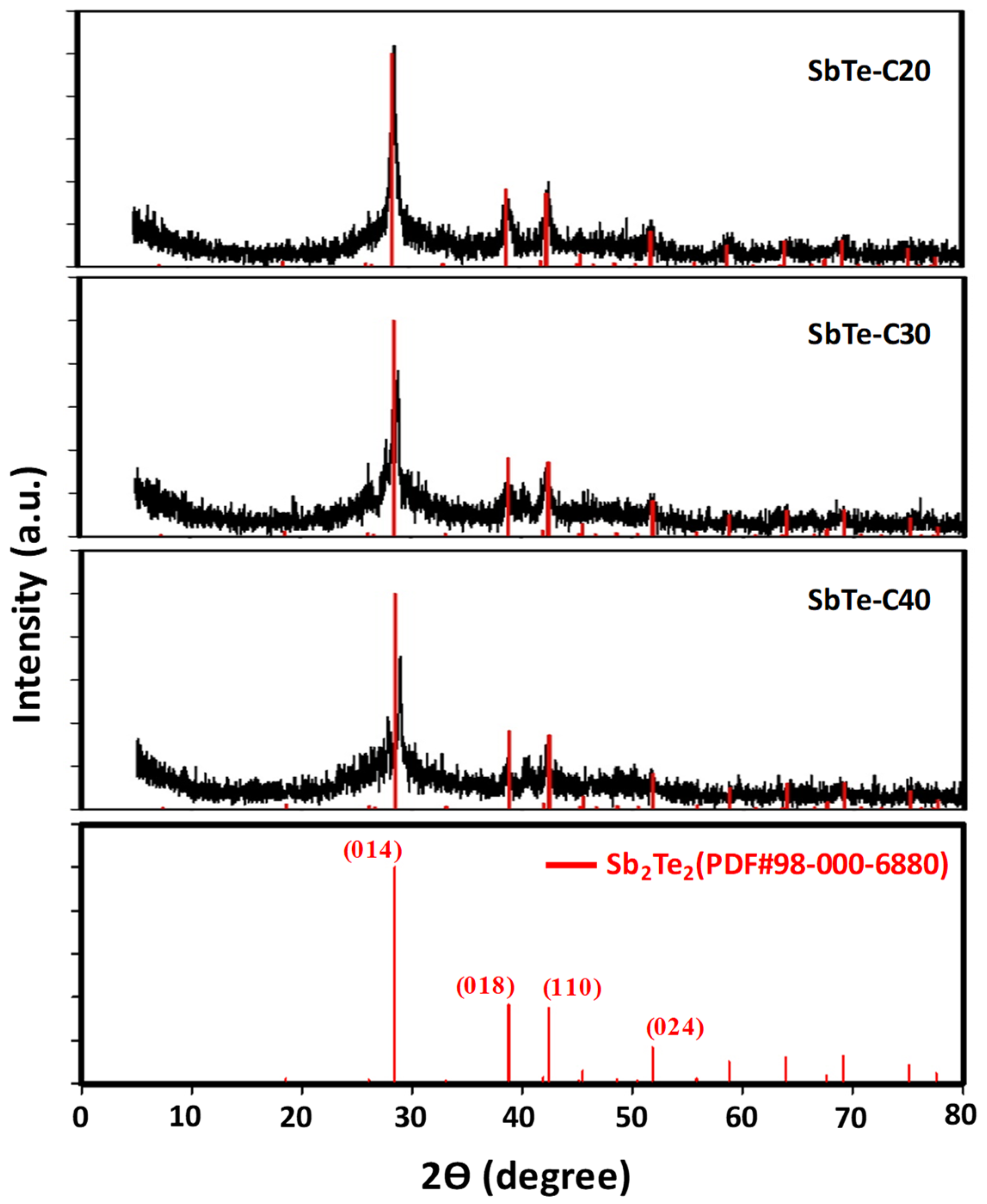
3.1.1. XRD
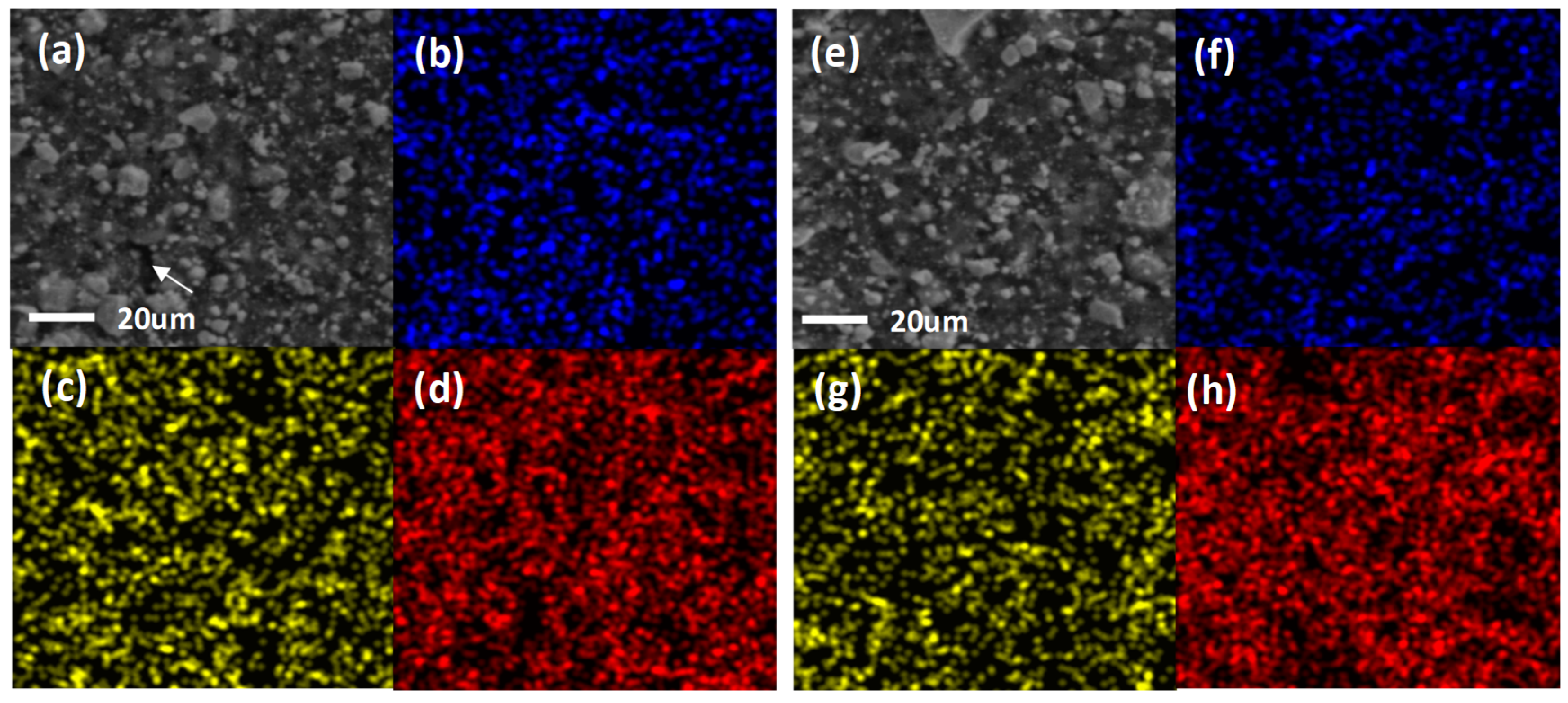
3.1.2. SEM and EDS
3.2. Electrochemical Performance
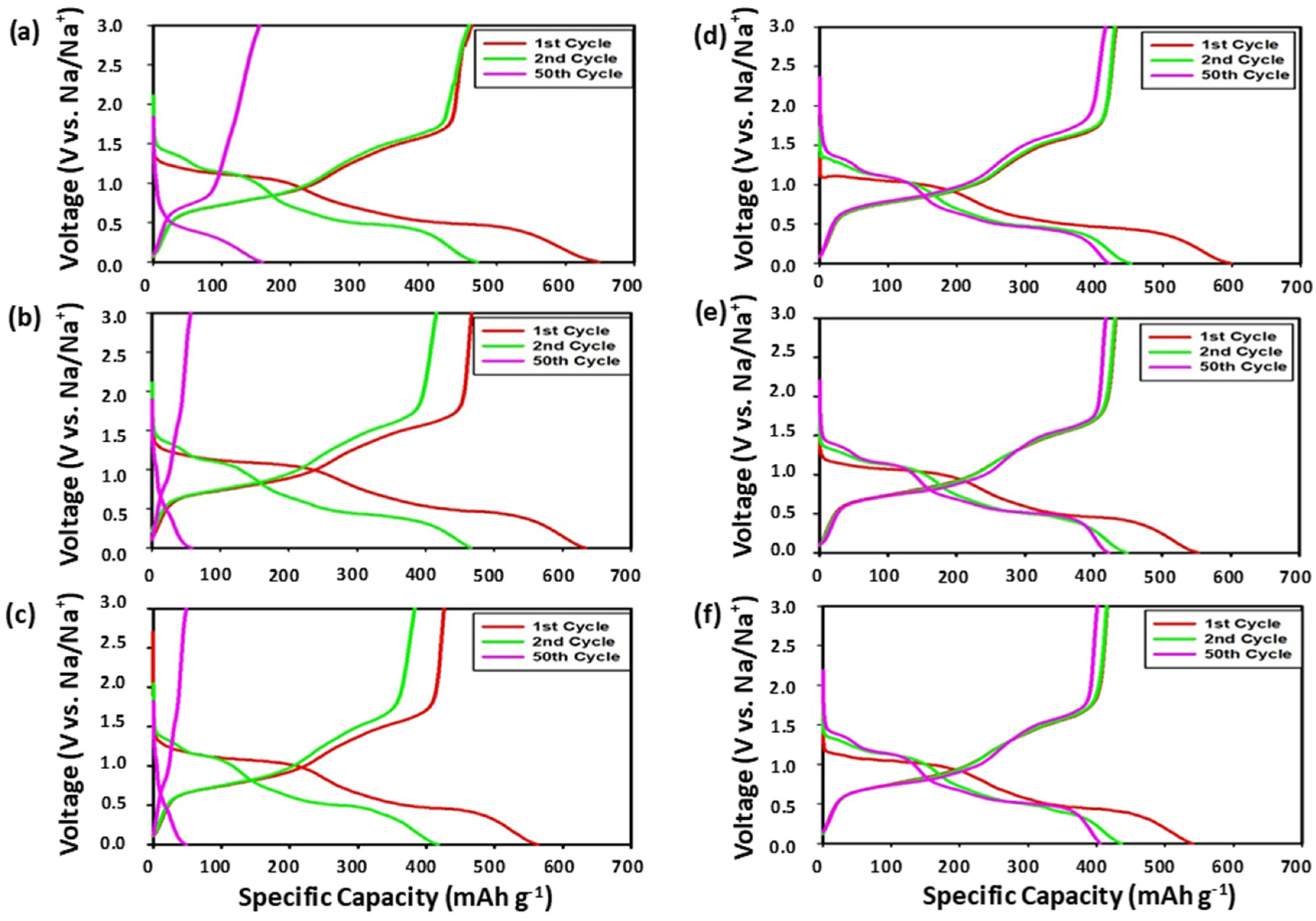
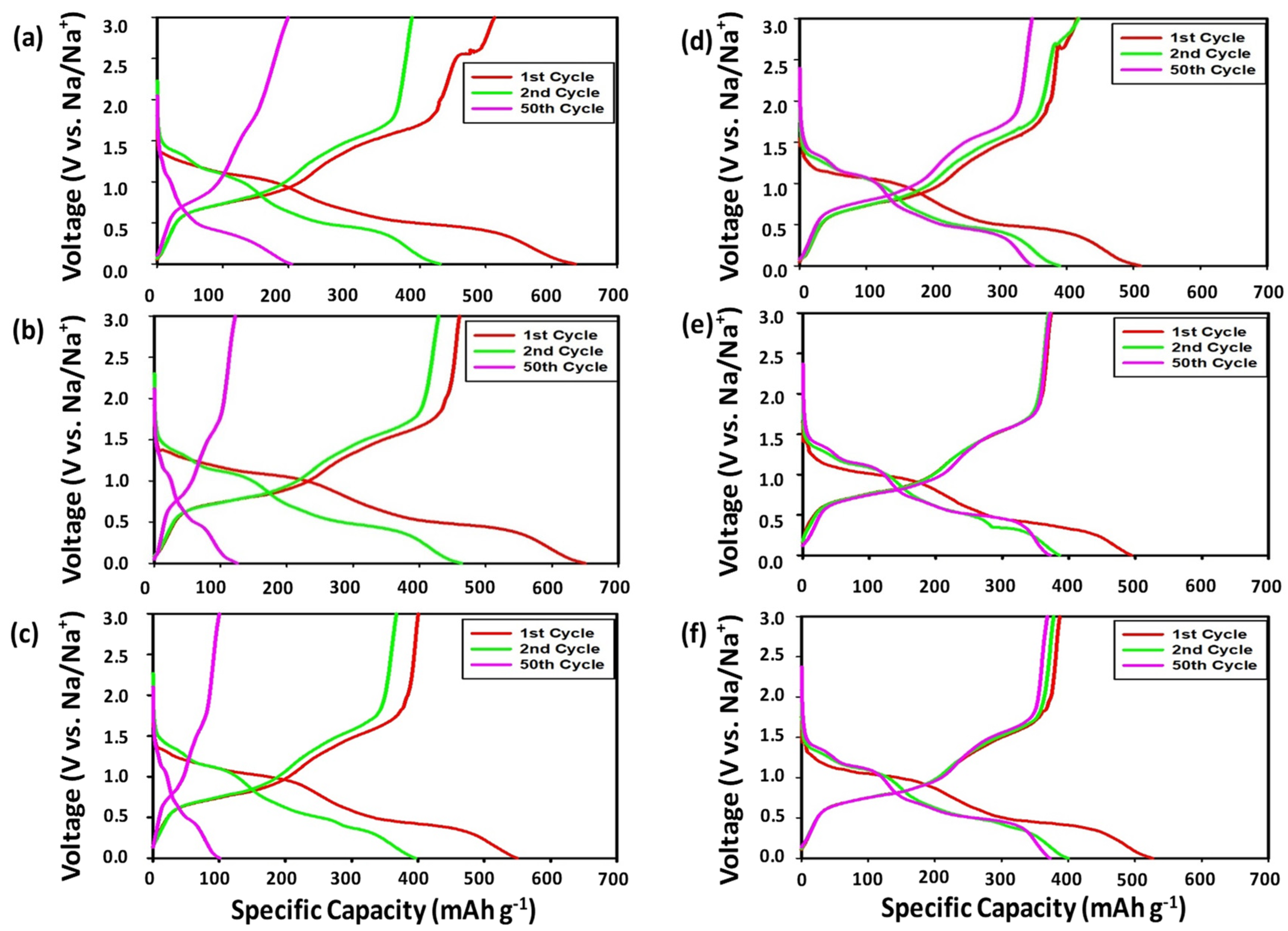
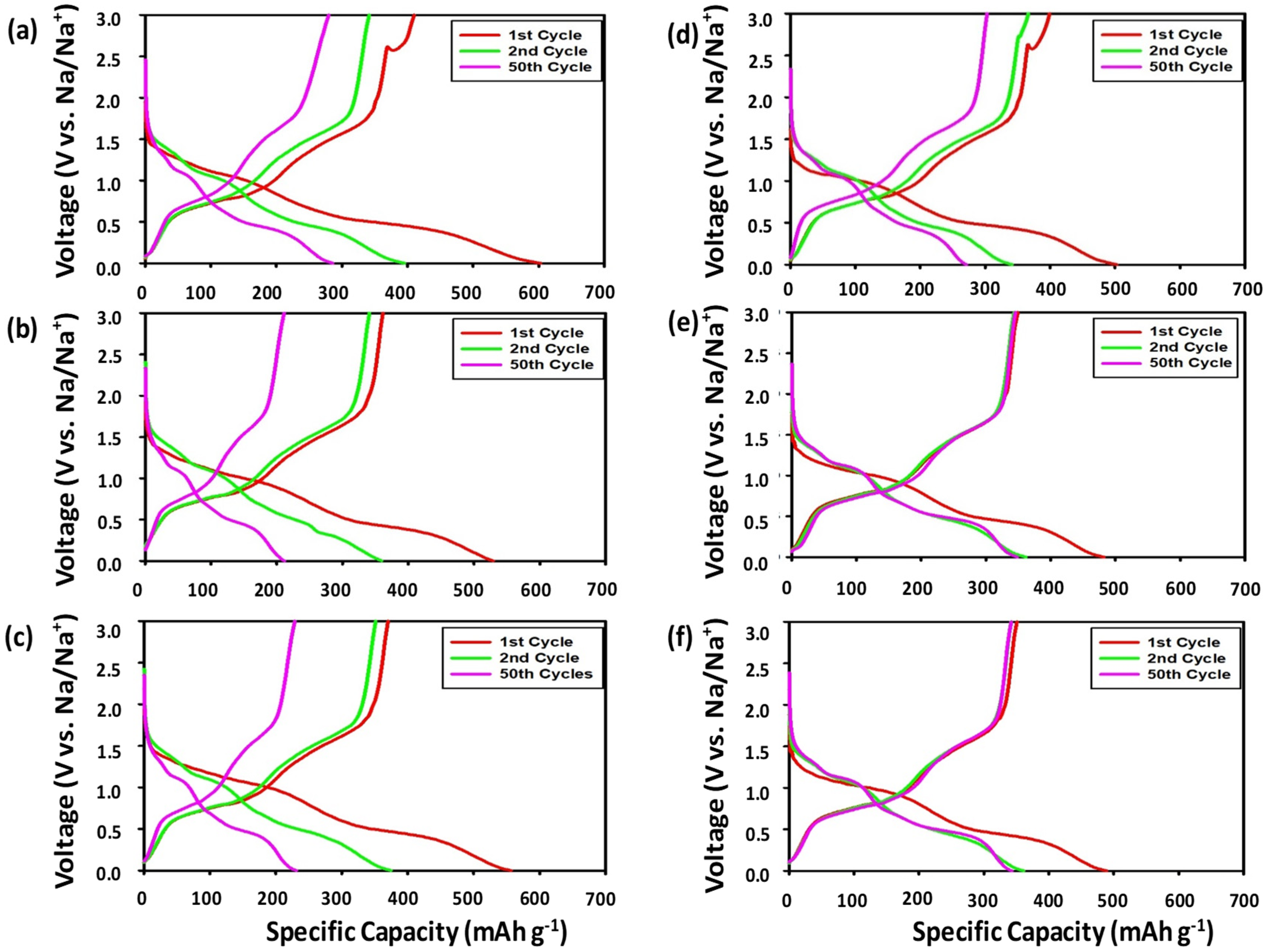
3.2.1. Voltage Profiles
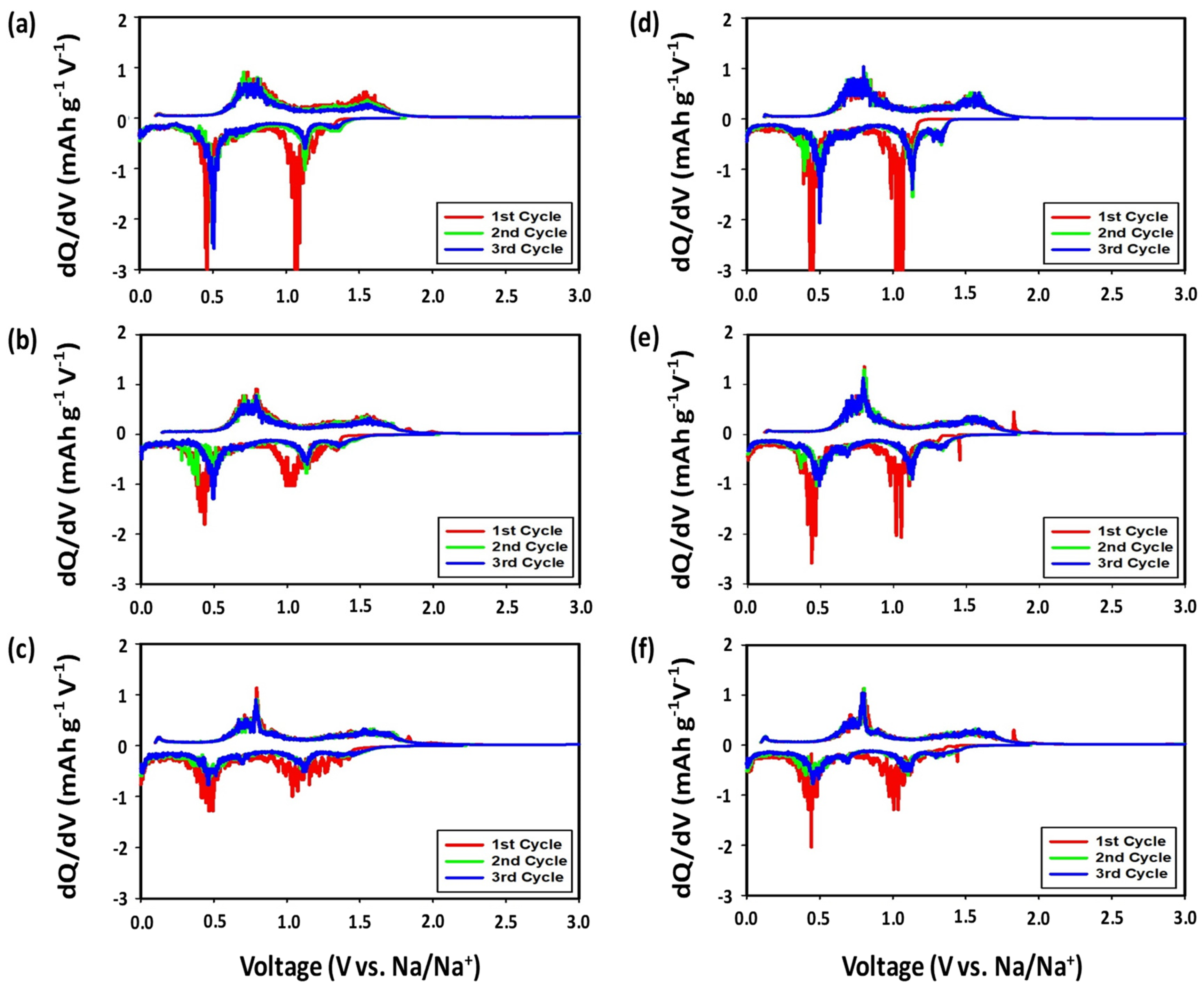
3.2.2. Differential Capacities
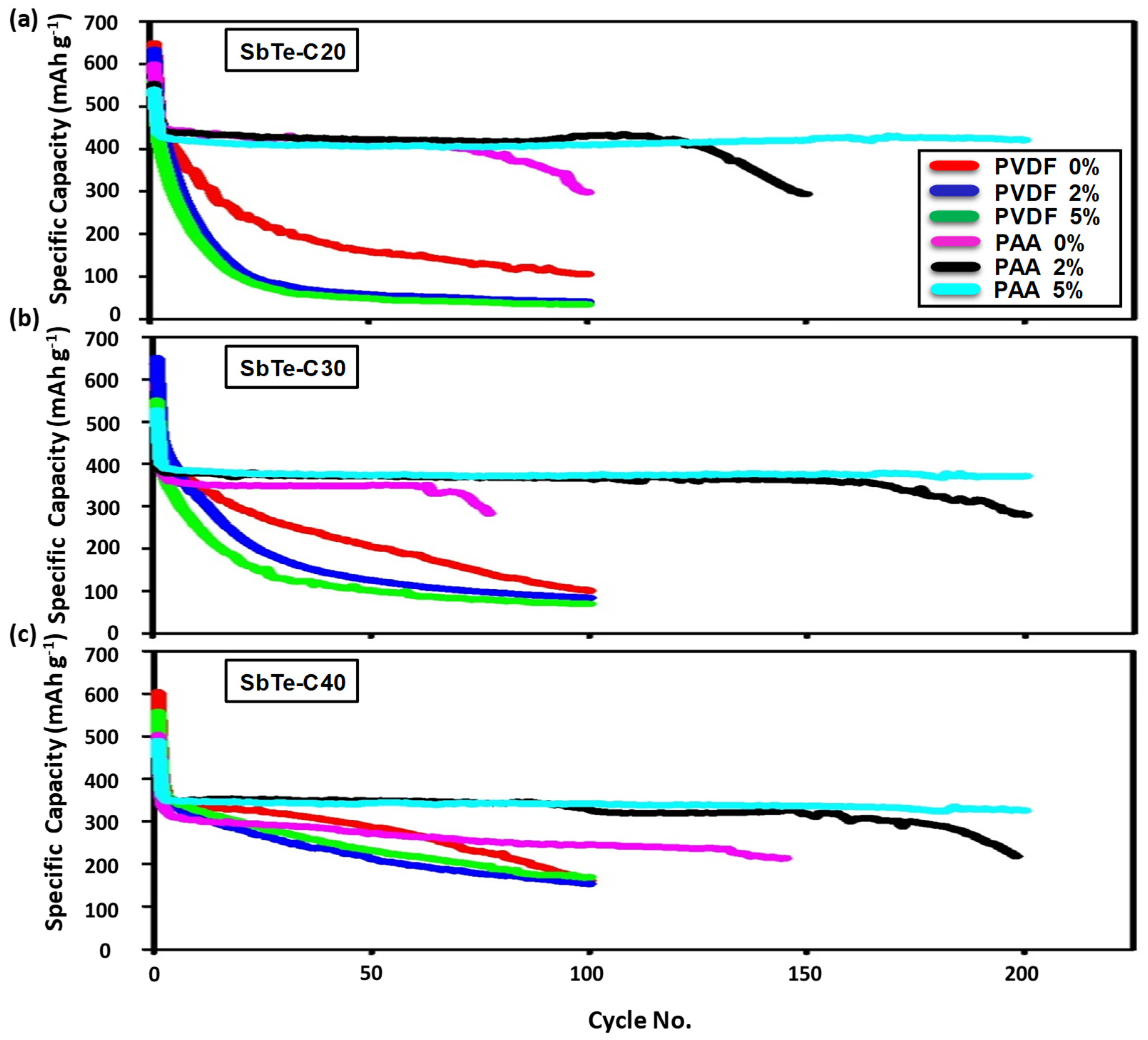
3.2.3. Cycling Performance
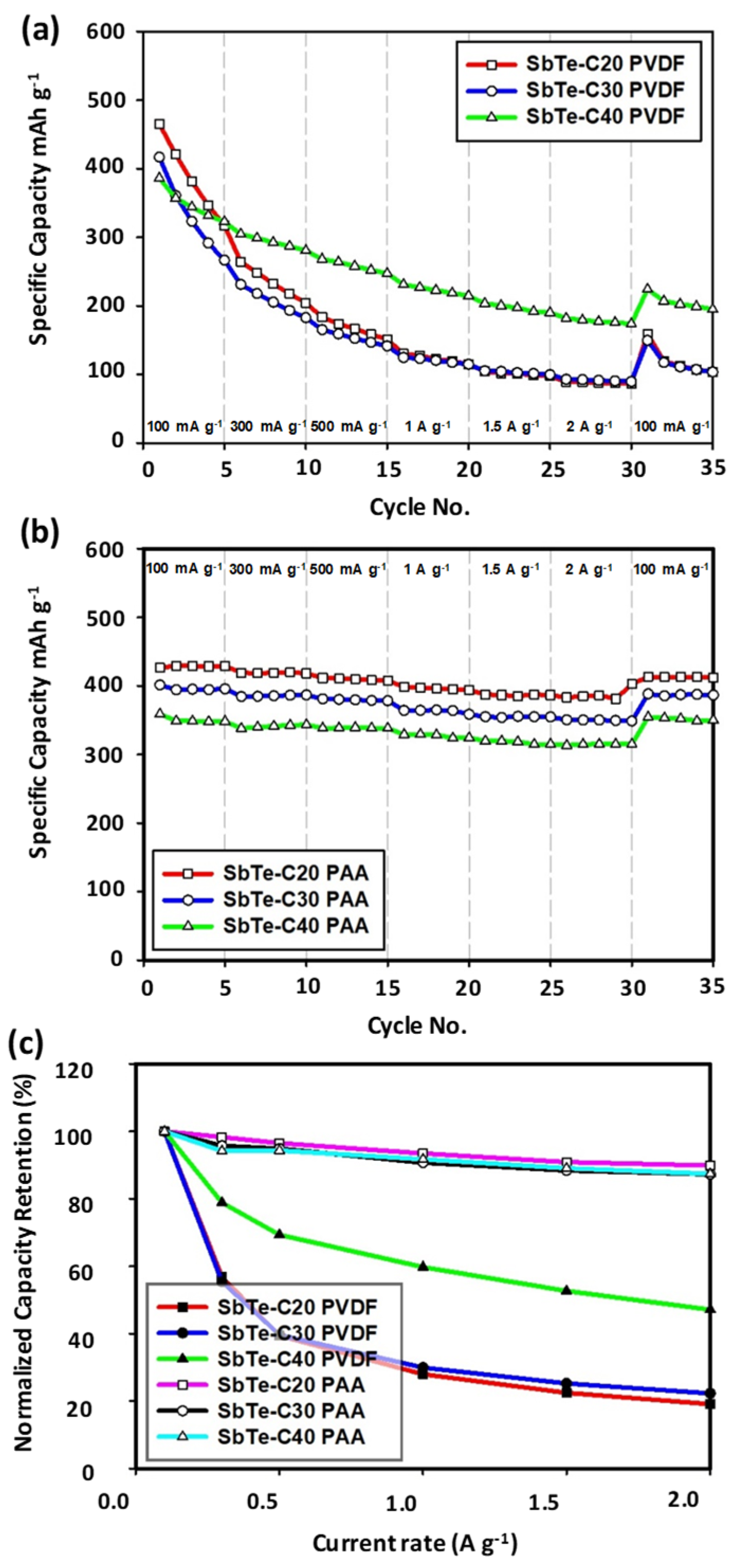
3.2.4. Rate Capabilities and Capacity Retentions
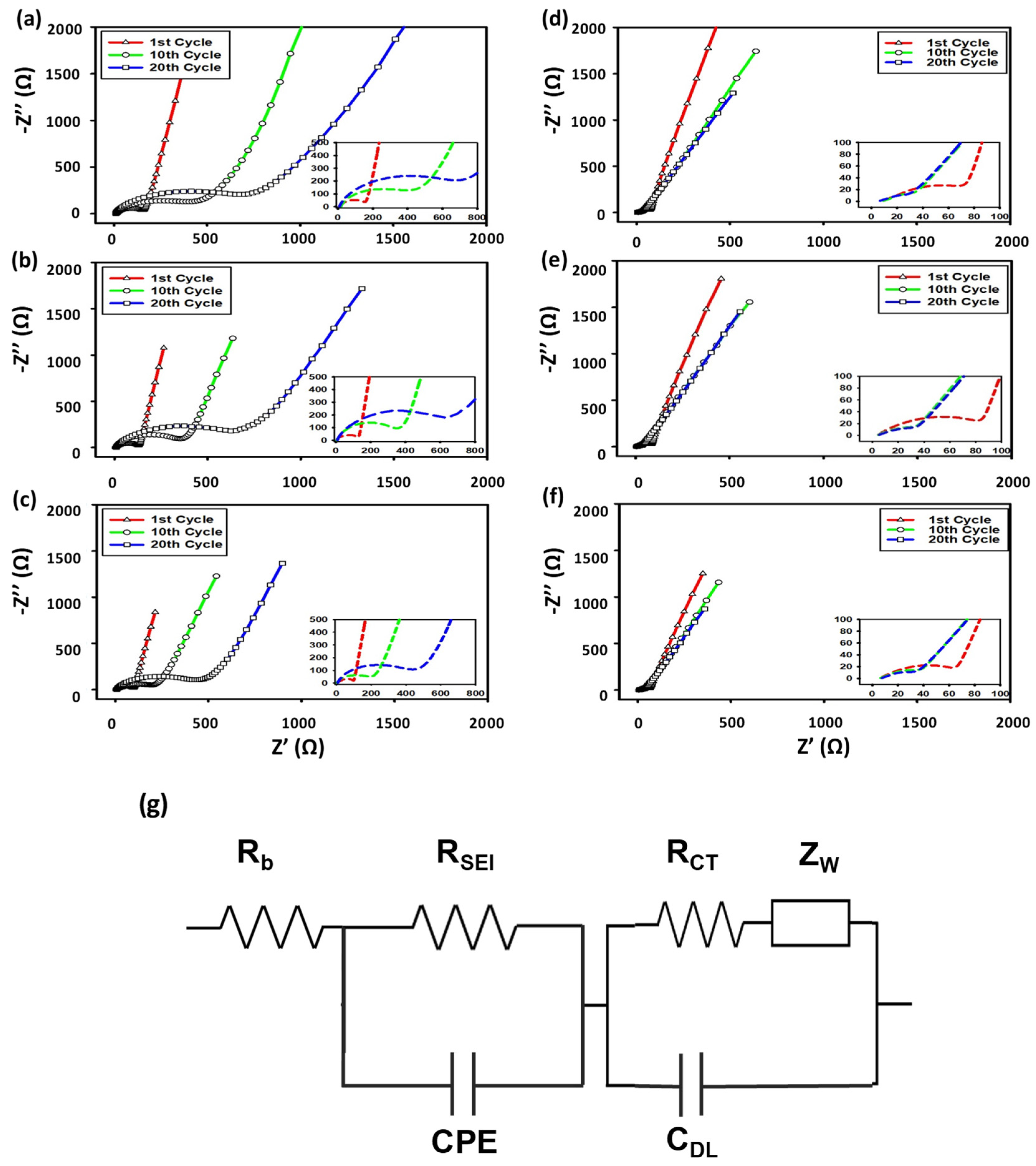
3.2.5. Electrochemical Impedance Spectroscopy (EIS)
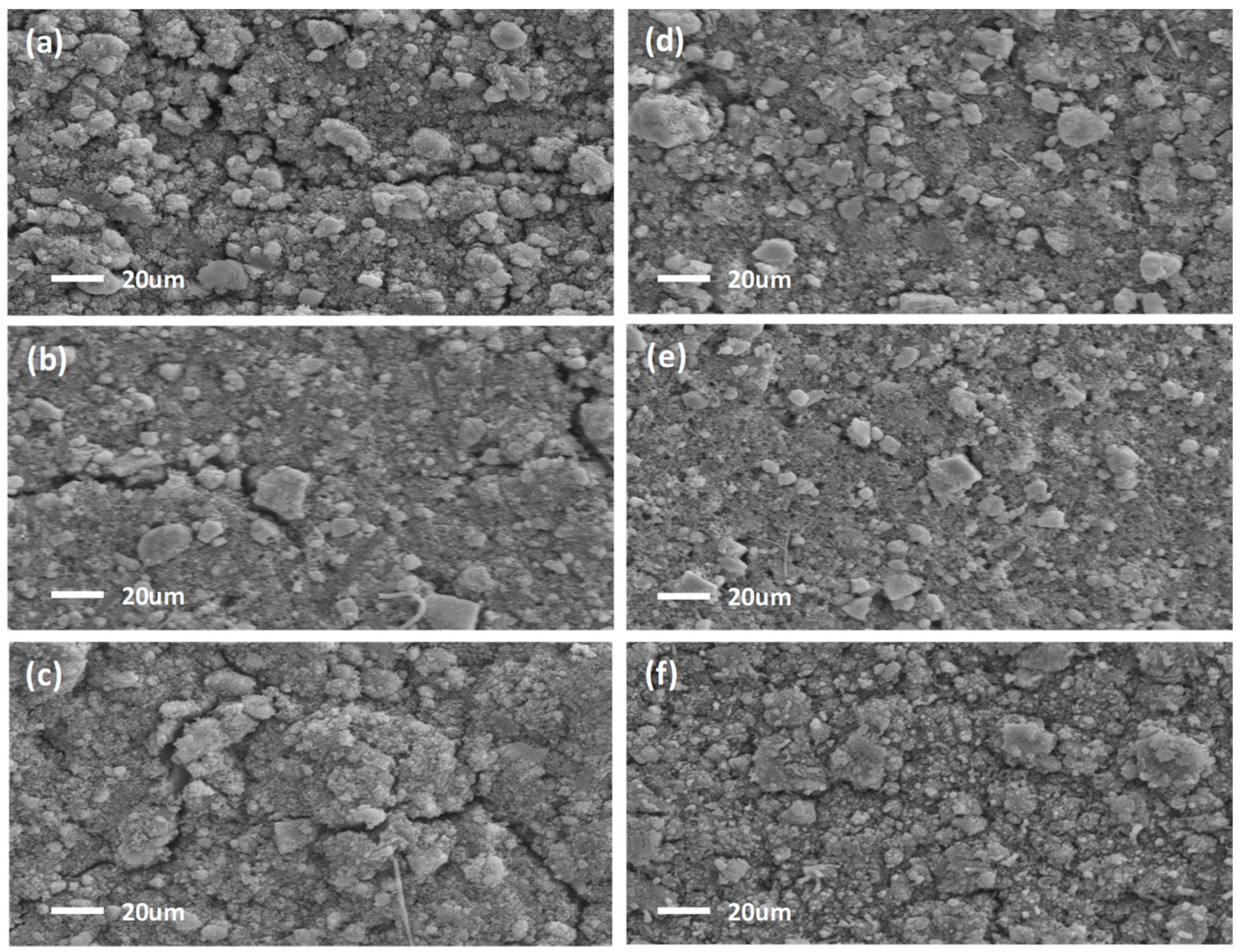
3.3. Ex Situ SEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhou, T.; Zheng, Y.; Gao, H.; Liu, K.H.; Guo, Z. Two-dimensional nanostructures for sodium-ion battery anodes. J. Mater. Chem. A 2018, 6, 3284–3303. [Google Scholar] [CrossRef]
- Lao, M.; Zhang, Y.; Luo, W.; Yan, Q.; Sun, W.; Dou, S.X. Alloy-based anode materials toward advanced sodium-ion batteries. Adv. Mater. 2017, 29, 1700622. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, S.; Zhou, H. Exploration of Advanced Electrode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2018, 9, 1800212. [Google Scholar] [CrossRef]
- Wang, J.; Luo, C.; Gao, T.; Langrock, A.; Mignerey, A.C.; Wang, C. An Advanced MoS2/Carbon Anode for High-Performance Sodium-Ion Batteries. Small 2015, 11, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, Y.G. Highly disordered carbon as a superior anode material for room-temperature sodium-ion batteries. ChemElectroChem 2014, 1, 83–86. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, D.K. Conversion-Alloying Anode Materials for Na-ion Batteries: Recent Progress, Challenges, and Perspective for the Future. J. Korean Ceram. Soc. 2018, 55, 307–324. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, Y.; Li, Y.; Jiang, L.; Rong, X.; Hu, Y.S.; Li, H.; Chen, L. Novel Methods for Sodium-Ion Battery Materials. Small Methods 2017, 1, 1600063. [Google Scholar] [CrossRef]
- Nagulapati, V.M.; Yoon, Y.H.; Kim, D.S.; Kim, H.; Lee, W.S.; Lee, J.H.; Kim, K.H.; Hur, J.; Kim, I.T.; Lee, S.G. Effect of binders and additives to tailor the electrochemical performance of Sb2Te3–TiC alloy anodes for high-performance sodium-ion batteries. J. Ind. Eng. Chem. 2019, 76, 419–428. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Luo, Y.; Qiu, W.; Liu, P.; Tong, Y. A review of carbon materials and their composites with alloy metals for sodium ion battery anodes. Carbon 2016, 98, 162–178. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Wang, S.; Miao, Z.; Liu, H.K.; Chou, S. Structural design of anode materials for sodium-ion batteries. J. Mater. Chem. A 2018, 6, 6183–6205. [Google Scholar] [CrossRef]
- Chou, S.-L.; Pan, Y.; Wang, J.-Z.; Liu, H.-K.; Dou, S.-X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Ha, C.-W.; Choi, H.-Y.; Lee, S.-M. Carbon embedded SnSb composite tailored by carbothermal reduction process as high performance anode for sodium-ion batteries. J. Ind. Eng. Chem. 2018, 60, 451–457. [Google Scholar] [CrossRef]
- Son, S.Y.; Hur, J.; Kim, K.H.; Son, H.B.; Lee, S.G.; Kim, I.T. SnTe–TiC–C composites as high-performance anodes for Li-ion batteries. J. Power Sources 2017, 365, 372–379. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Kim, D.S.; Kim, M.; Park, M.S.; Lee, Y.-C.; Kim, K.H.; Kim, I.T.; Hur, J.; Lee, S.G. Investigation of electrochemical performance on carbon supported tin-selenium bimetallic anodes in lithium-ion batteries. Electrochim. Acta 2018, 266, 193–201. [Google Scholar] [CrossRef]
- Luo, W.; Shen, F.; Bommier, C.; Zhu, H.; Ji, X.; Hu, L. Na-ion battery anodes: materials and electrochemistry. Acc. Chem. Res. 2016, 49, 231–240. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.; Wu, L.; Cao, Y.; Ai, X.; Yang, H. High capacity Na-storage and superior cyclability of nanocomposite Sb/C anode for Na-ion batteries. Chem. Commun. 2012, 48, 7070–7072. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Z.; Wang, J.; Li, W.; Gu, L.; Yu, Y. Sb nanoparticles encapsulated in a reticular amorphous carbon network for enhanced sodium storage. Small 2015, 11, 5381–5387. [Google Scholar] [CrossRef]
- Kalisvaart, W.P.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Sb–Si Alloys and Multilayers for Sodium-Ion Battery Anodes. ACS Appl. Energy Mater. 2019, 2, 2205–2213. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Y.-X.; Guo, Y.-G. High-capacity te anode confined in microporous carbon for long-life Na-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 27838–27844. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, J.; Ni, Y.; Zhao, Z.; Bao, J.; Chen, S. Facile synthesis and in situ transmission electron microscopy investigation of a highly stable Sb2Te3/C nanocomposite for sodium-ion batteries. Energy Storage Mater. 2017, 9, 214–220. [Google Scholar]
- Grishanov, D.A.; Mikhaylov, A.A.; Medvedev, A.G.; Gun, J.; Nagasubramanian, A.; Madhavi, S.; Lev, O.; Prikhodchenko, P.V. Synthesis of high volumetric capacity graphene oxide-supported tellurantimony Na-and Li-ion battery anodes by hydrogen peroxide sol gel processing. J. Colloid Interface Sci. 2018, 512, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mun, Y.S.; Yoon, Y.; Hur, J.; Park, M.S.; Bae, J.; Kim, J.H.; Yoon, Y.S.; Yoo, I.S.; Lee, S.G.; Kim, I.T. Copper–antimony–red phosphorus composites as promising anode materials for sodium-ion batteries. J. Power Sources 2017, 362, 115–122. [Google Scholar] [CrossRef]
- Ramireddy, T.; Rahman, M.M.; Xing, T.; Chen, Y.; Glushenkov, A.M. Stable anode performance of an Sb–carbon nanocomposite in lithium-ion batteries and the effect of ball milling mode in the course of its preparation. J. Mater. Chem. A 2014, 2, 4282–4291. [Google Scholar] [CrossRef]
- Hou, H.; Yang, Y.; Zhu, Y.; Jing, M.; Pan, C.; Fang, L.; Song, W.; Yang, X.; Ji, X. An electrochemical study of Sb/acetylene black composite as anode for sodium-ion batteries. Electrochim. Acta 2014, 146, 328–334. [Google Scholar] [CrossRef]
- Parikh, P.; Sina, M.; Banerjee, A.; Wang, X.; D’Souza, M.S.; Doux, J.-M.; Wu, E.A.; Trieu, O.Y.; Gong, Y.; Zhou, Q. Role of Polyacrylic acid (PAA) binder on the solid electrolyte interphase in silicon anodes. Chem. Mater. 2019, 31, 2535–2544. [Google Scholar] [CrossRef]
- Ma, Z.; Lyu, Y.; Yang, H.; Li, Q.; Guo, B.; Nie, A. Systematic investigation of the Binder’s role in the electrochemical performance of tin sulfide electrodes in SIBs. J. Power Sources 2018, 401, 195–203. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, J.; Cui, G. Small things make big deal: Powerful binders of lithium batteries and post-lithium batteries. Energy Storage Mater. 2019, 20, 146–175. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, T.; Lai, Y.; Jia, M.; Li, J. A comparative study of different binders and their effects on electrochemical properties of LiMn2O4 cathode in lithium ion batteries. J. Power Sources 2014, 247, 1–8. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, W.; Duan, J.; Hong, K.; Xue, L.; Huang, Y. Effects of binders on electrochemical performance of nitrogen-doped carbon nanotube anode in sodium-ion battery. Electrochim. Acta 2015, 174, 970–977. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Choi, Y.; Kwon, S.-H.; Bae, J.-S.; Jeong, E.D. Cracking resistance and electrochemical performance of silicon anode on binders with different mechanical characteristics. J. Ind. Eng. Chem. 2019, 74, 216–222. [Google Scholar] [CrossRef]
- Vogt, L.O.; El Kazzi, M.; Jämstorp Berg, E.; Pérez Villar, S.A.; Novák, P.; Villevieille, C. Understanding the interaction of the carbonates and binder in Na-ion batteries: A combined bulk and surface study. Chem. Mater. 2015, 27, 1210–1216. [Google Scholar] [CrossRef]
- Choi, H.; Baeck, J.H.; Kim, T.-H.; Song, J.Y.; Shin, S.; Cho, H.; Ko, D.-H.; Kim, J.-S.; Jeong, K.H.; Cho, M.-H. Synthesis of self-ordered Sb2 Te2 films with atomically aligned Te layers and the effect of phonon scattering modulation. J. Mater. Chem. C 2013, 1, 7043–7053. [Google Scholar] [CrossRef]
- Komaba, S.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Ito, A.; Ohsawa, Y. Fluorinated ethylene carbonate as electrolyte additive for rechargeable Na batteries. ACS Appl. Mater. Interfaces 2011, 3, 4165–4168. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.-H.; Choi, J.-H.; Park, C.-M. Highly Reversible Na-Ion Reaction in Nanostructured Sb2Te3-C Composites as Na-Ion Battery Anodes. J. Electrochem. Soc. 2017, 164, A2056–A2064. [Google Scholar] [CrossRef]
- Kim, I.T.; Kim, S.-O.; Manthiram, A. Effect of TiC addition on SnSb–C composite anodes for sodium-ion batteries. J. Power Sources 2014, 269, 848–854. [Google Scholar] [CrossRef][Green Version]
- Li, K.; Zhang, J.; Lin, D.; Wang, D.-W.; Li, B.; Lv, W.; Sun, S.; He, Y.-B.; Kang, F.; Yang, Q.-H. Evolution of the electrochemical interface in sodium ion batteries with ether electrolytes. Nat. Commmun. 2019, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Piriya, V.A.; Shende, R.C.; Seshadhri, G.M.; Ravindar, D.; Biswas, S.; Loganathan, S.; Balasubramanian, T.; Rambabu, K.; Kamaraj, M.; Ramaprabhu, S. Synergistic Role of Electrolyte and Binder for Enhanced Electrochemical Storage for Sodium-Ion Battery. ACS Omega 2018, 3, 9945–9955. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagulapati, V.M.; Kim, D.S.; Oh, J.; Lee, J.H.; Hur, J.; Kim, I.T.; Lee, S.G. Enhancing the Electrochemical Performance of SbTe Bimetallic Anodes for High-Performance Sodium-Ion Batteries: Roles of the Binder and Carbon Support Matrix. Nanomaterials 2019, 9, 1134. https://doi.org/10.3390/nano9081134
Nagulapati VM, Kim DS, Oh J, Lee JH, Hur J, Kim IT, Lee SG. Enhancing the Electrochemical Performance of SbTe Bimetallic Anodes for High-Performance Sodium-Ion Batteries: Roles of the Binder and Carbon Support Matrix. Nanomaterials. 2019; 9(8):1134. https://doi.org/10.3390/nano9081134
Chicago/Turabian StyleNagulapati, Vijay Mohan, Doo Soo Kim, Jinwoo Oh, Jin Hong Lee, Jaehyun Hur, Il Tae Kim, and Seung Geol Lee. 2019. "Enhancing the Electrochemical Performance of SbTe Bimetallic Anodes for High-Performance Sodium-Ion Batteries: Roles of the Binder and Carbon Support Matrix" Nanomaterials 9, no. 8: 1134. https://doi.org/10.3390/nano9081134
APA StyleNagulapati, V. M., Kim, D. S., Oh, J., Lee, J. H., Hur, J., Kim, I. T., & Lee, S. G. (2019). Enhancing the Electrochemical Performance of SbTe Bimetallic Anodes for High-Performance Sodium-Ion Batteries: Roles of the Binder and Carbon Support Matrix. Nanomaterials, 9(8), 1134. https://doi.org/10.3390/nano9081134







