Synthesis and Properties of Ferrite-Based Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results
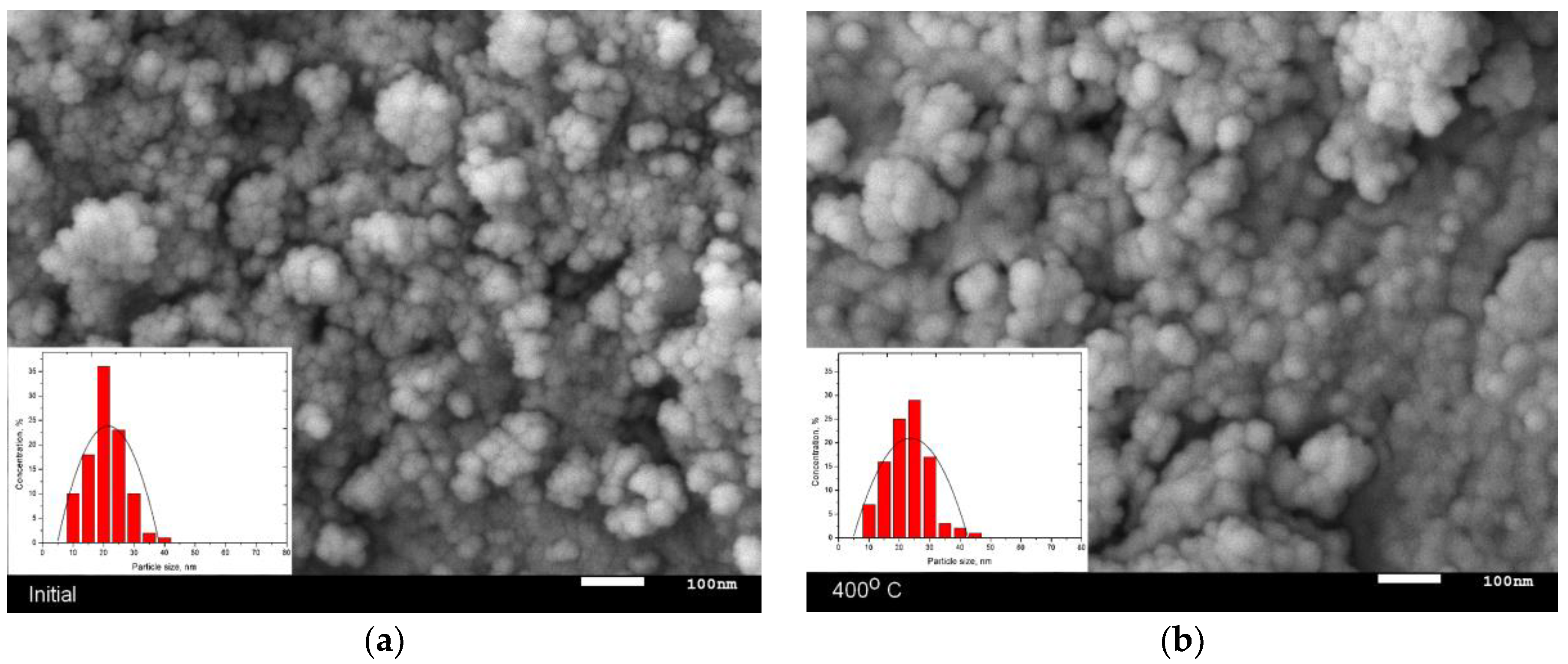
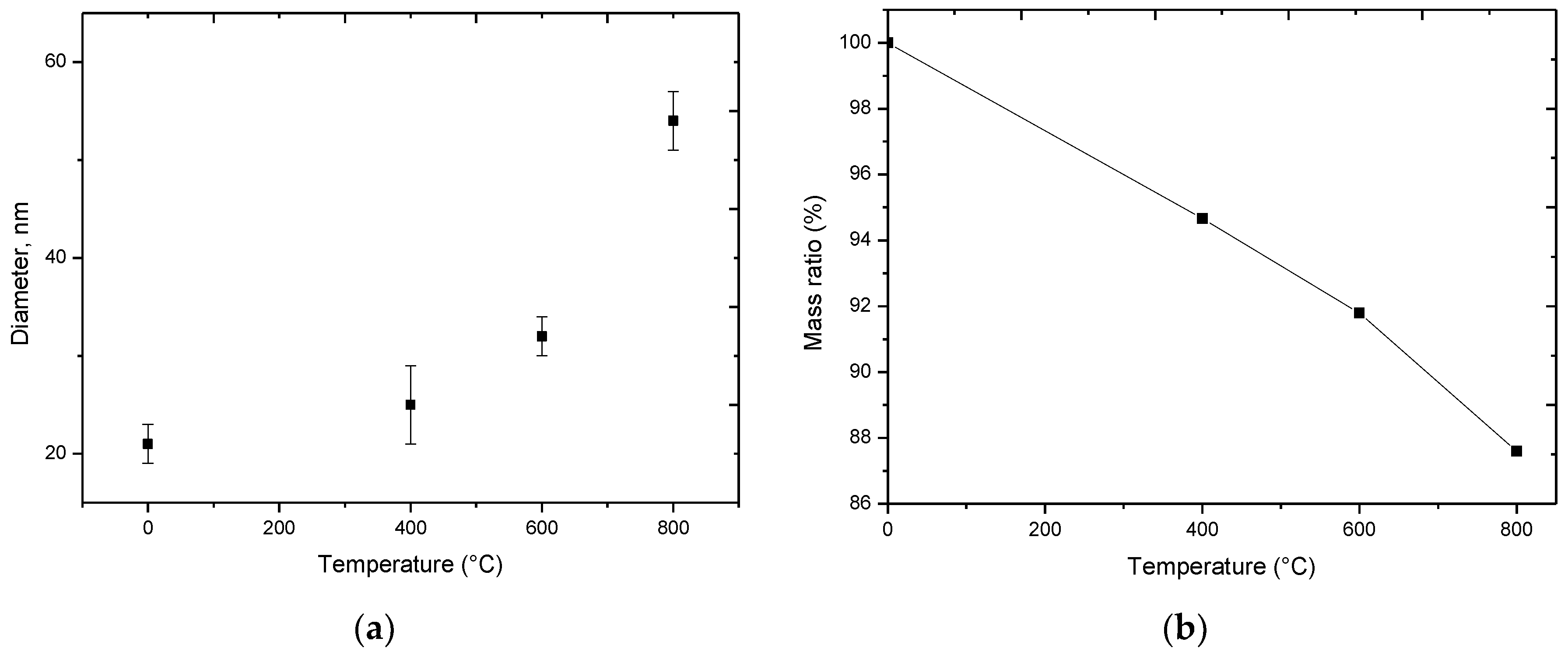
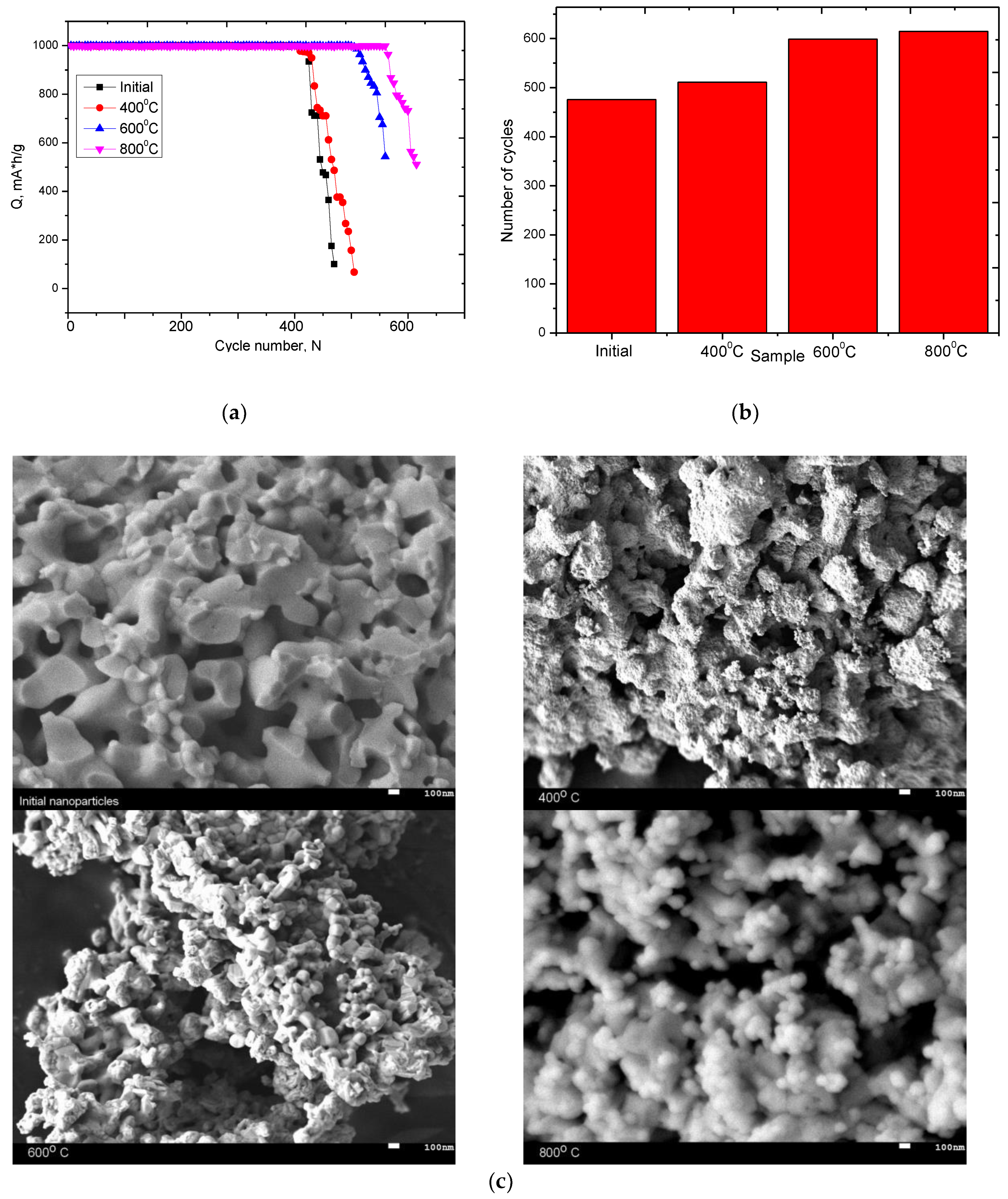
3.1. Changes in the Morphology and Elemental Composition of Nanoparticles as a Result of Thermal Annealing
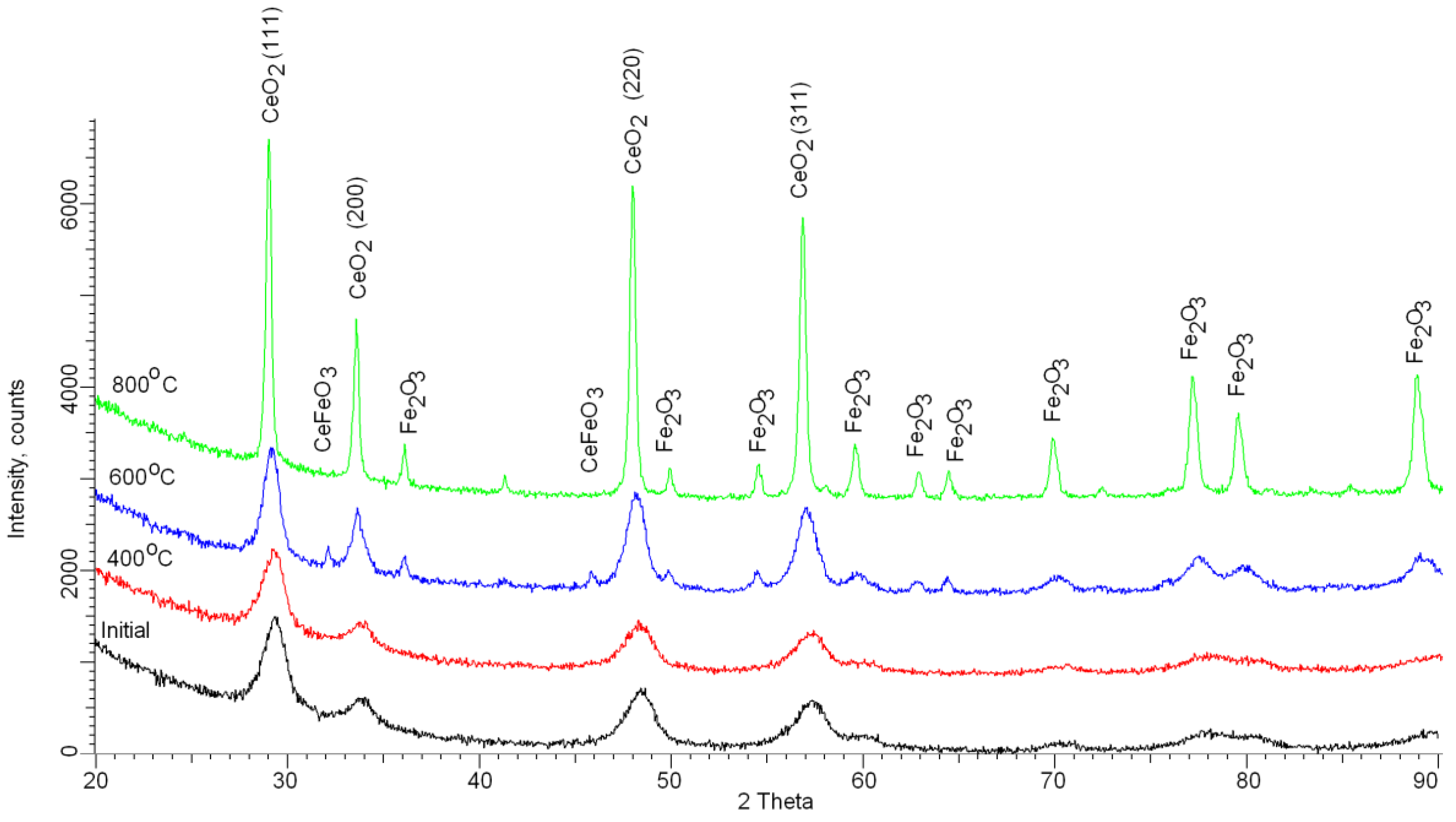
3.2. Studies of Phase Transformations in Nanoparticles as a Result of Thermal Annealing
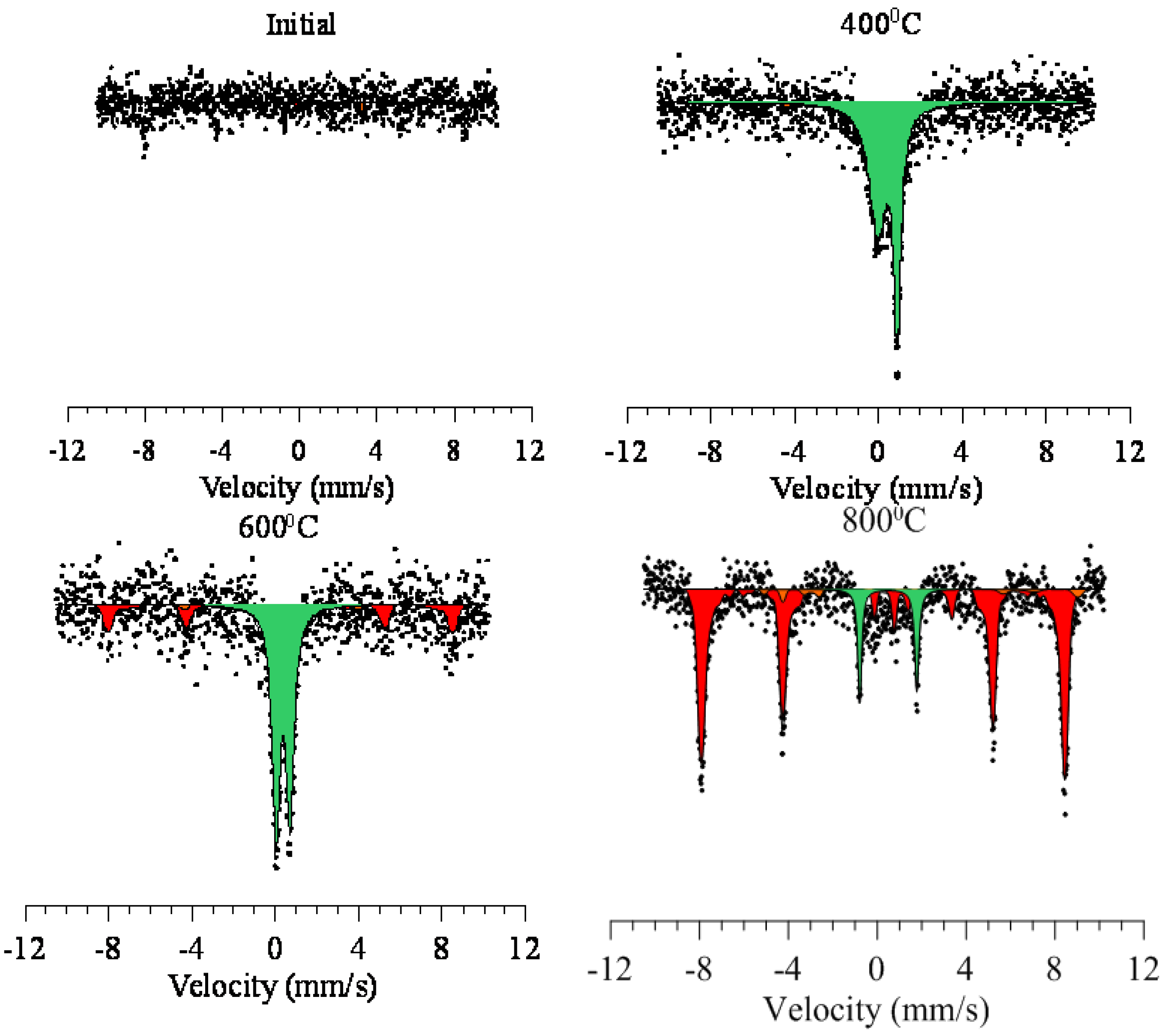
3.3. The Study of Changes in the Magnetic Texture of Nanoparticles as a Result of Thermal Annealing
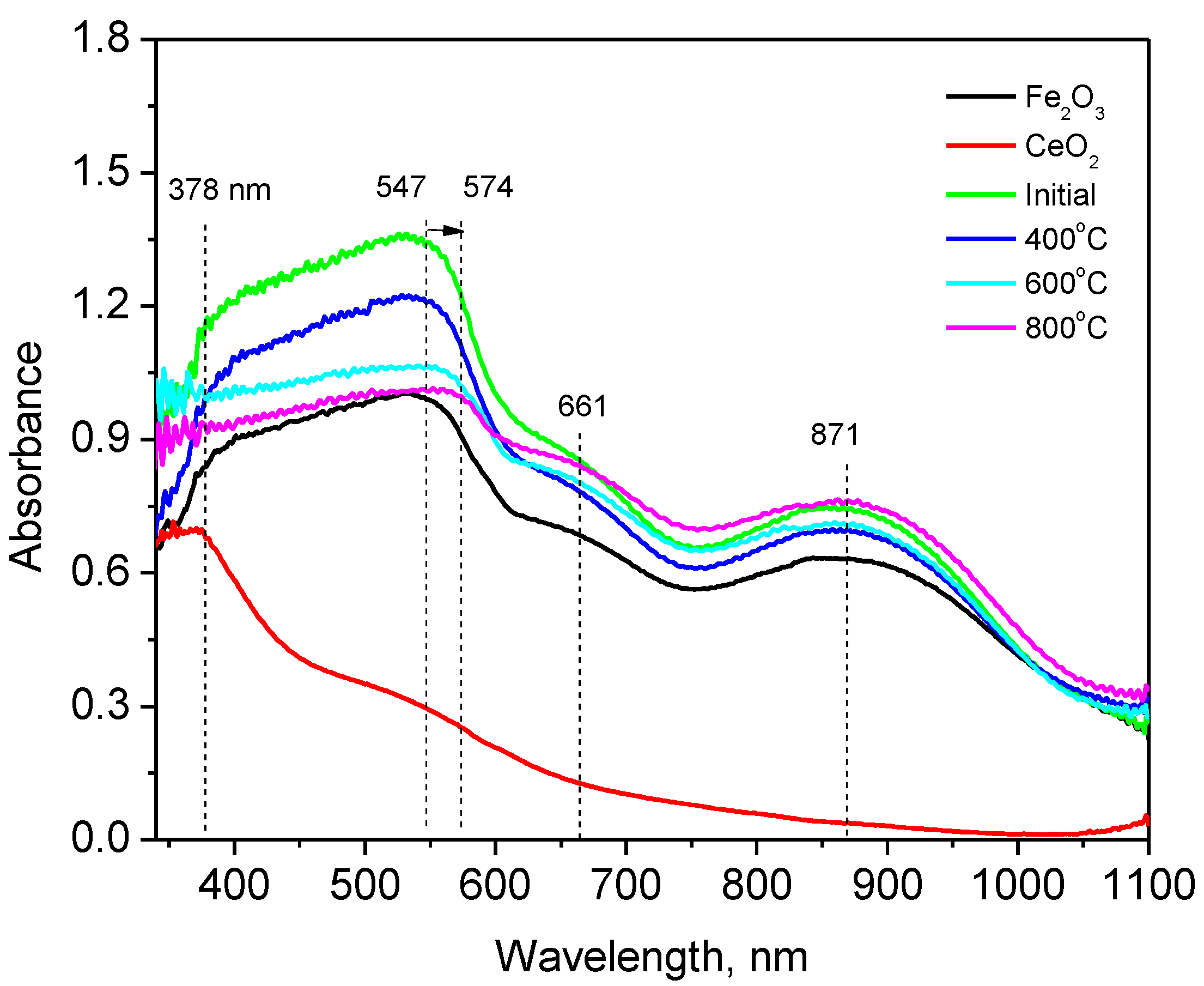
3.4. The Study of the Optical Properties of Nanoparticles
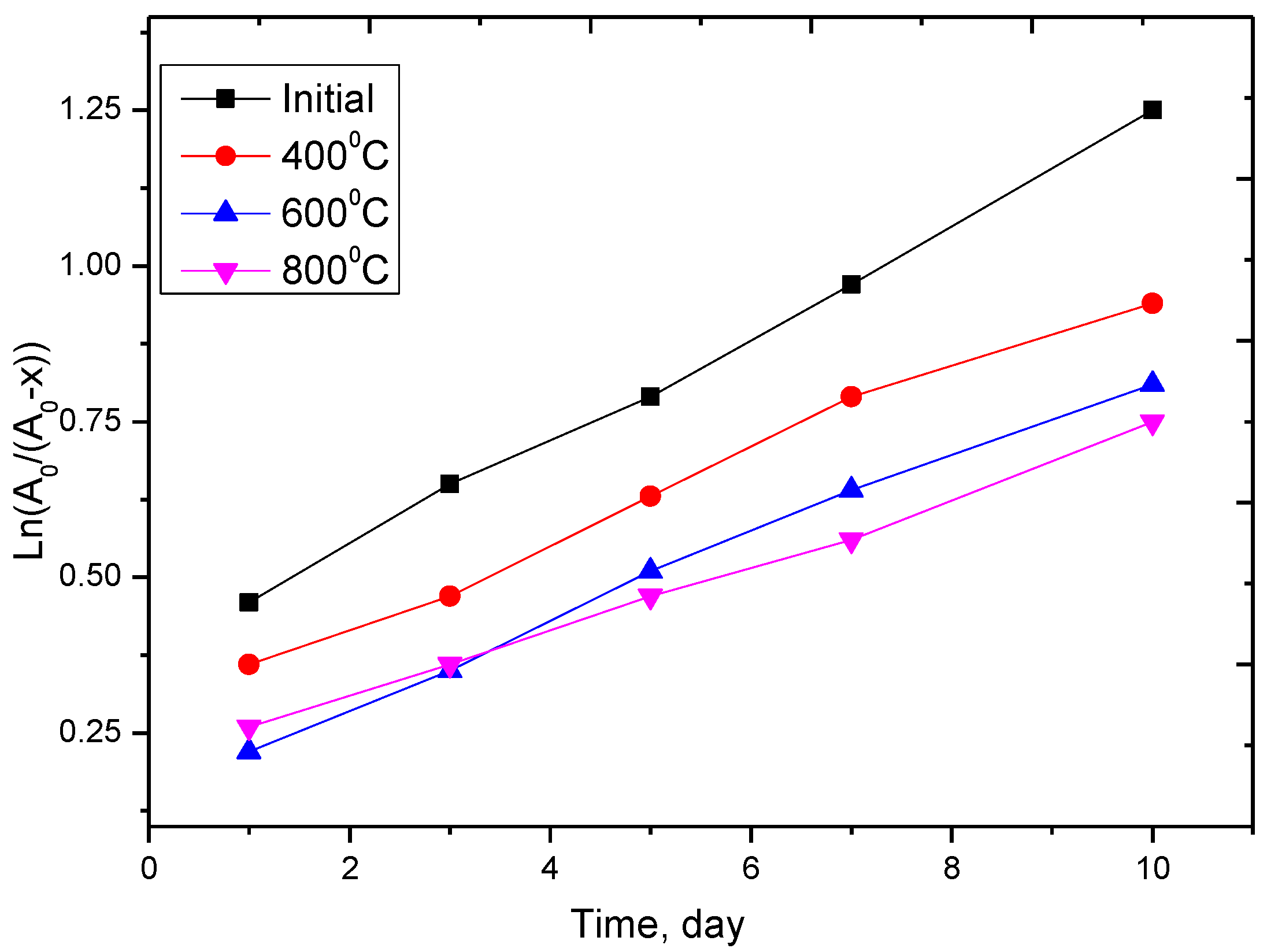
3.5. Investigation of the Corrosion Resistance
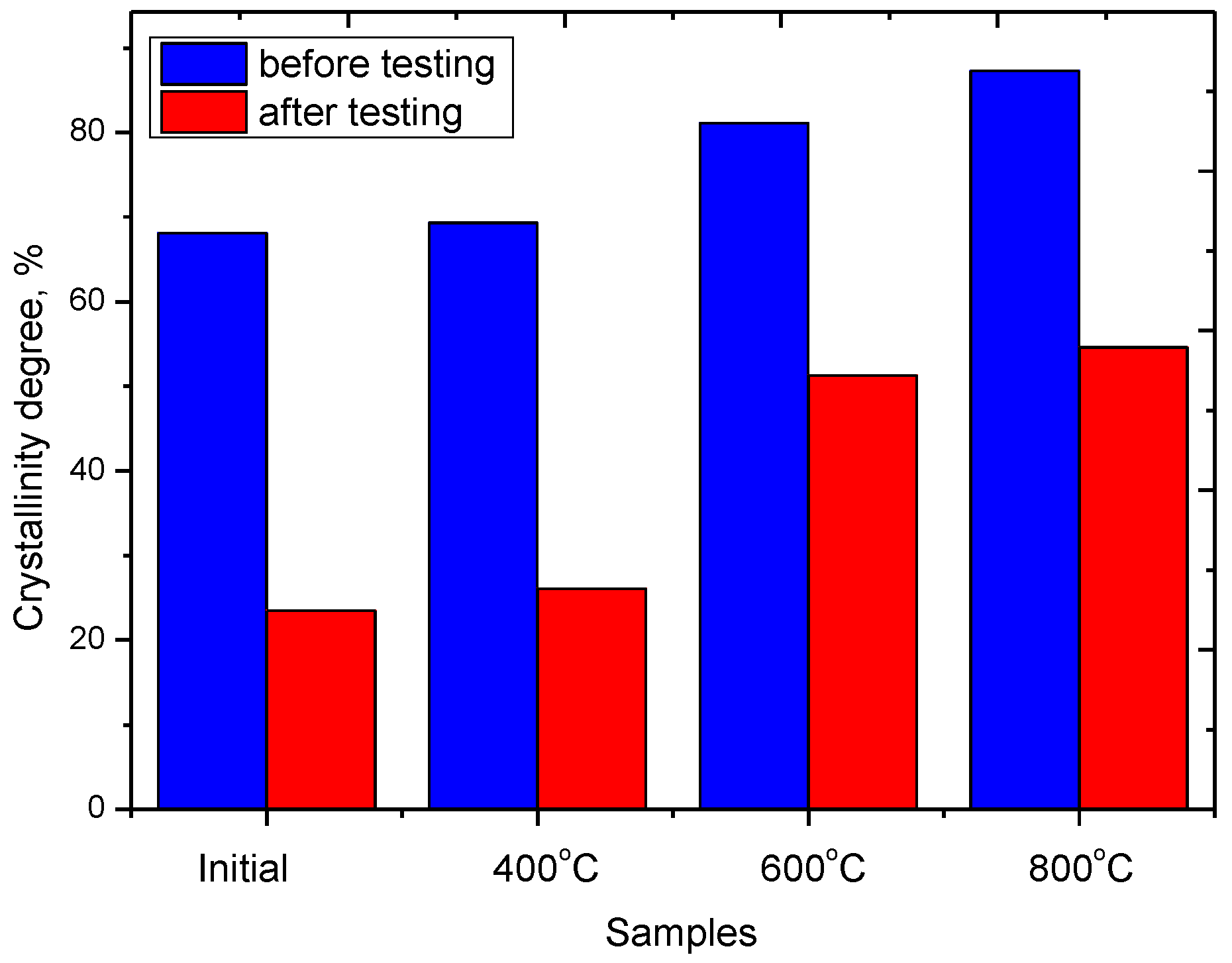
3.6. Resource Testing of Ceramics as Cathode Materials for Lithium-Ion Batteries
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fried, T.; Gabriel, S.; Markovich, G. Ordered two-dimensional arrays of ferrite nanoparticles. Adv. Mater. 2001, 13, 1158–1161. [Google Scholar] [CrossRef]
- Mathew, D.S.; Juang, R.S. An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 2007, 129, 51–65. [Google Scholar] [CrossRef]
- Kaniukov, E.Y.; Shumskaya, A.E.; Kutuzau, M.D.; Bundyukova, V.D.; Yakimchuk, D.V.; Borgekov, D.B.; Ibragimova, M.A.; Korolkov, I.V.; Kozlovskiy, A.L.; Zdorovets, M.V.; et al. Degradation mechanism and way of surface protection of nickel nanostructures. Mater. Chem. Phys. 2019, 223, 88–97. [Google Scholar] [CrossRef]
- Pilo, J.; Miranda, Á.; Trejo, A.; Carvajal, E.; Cruz-Irisson, M. Bidimensional perovskite systems for spintronic applications. J. Mol. Model. 2017, 23, 322. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S. Synthesis, photoelectrochemical properties and solar light-induced photocatalytic activity of bismuth ferrite nanoparticles. J. Nanopart. Res. 2018, 20. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Korolkov, I.V.; Ibragimova, M.A.; Zdorovets, M.V.; Kutuzau, M.D. Magnetic Nanostructured System for Biomedical Applications Based on FeNi Nanotubes. Nanotechnol. Russ. 2018, 13, 331–336. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Z.; Li, G.; Zhao, H. Enhanced visible-light-driven photocatalytic bacterial inactivation by ultrathin carbon-coated magnetic cobalt ferrite nanoparticles. Environ. Sci. Technol. 2018, 52, 4774–4784. [Google Scholar] [CrossRef]
- Alberti, S.; Villa, S.; Singh, G.; Seland, F. Systematic Study on TiO2 Crystallization via Hydrothermal Synthesis in the Presence of Different Ferrite Nanoparticles as Nucleation Seeds. J. Nanosci. Nanotechnol. 2019, 19, 4994–4999. [Google Scholar] [CrossRef]
- Cruz, I.F.; Cristina, F.; Pereira, C.; Pereira, A.M. Multifunctional Ferrite Nanoparticles: From Current Trends Toward the Future. Magn. Nanostruct. Mater. 2018, 59–116. [Google Scholar] [CrossRef]
- Srinivasan, S.Y.; Paknikar, K.M.; Bodas, D.; Gajbhiye, V. Applications of cobalt ferrite nanoparticles in biomedical nanotechnology. Nanomedicine 2018, 13, 1221–1238. [Google Scholar] [CrossRef]
- Campo, G.; Pineider, F.; Fantechi, E.; Innocenti, C.; Caneschi, A.; Fernández, C.J. Addressing the Influence of Localized Plasmon Resonance on the Magneto-Optical Properties of Cobalt Ferrite Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 4946–4953. [Google Scholar] [CrossRef] [PubMed]
- Kutuzau, M.D.; Kaniukov, E.Y.; Elena, E.; Bundyukova, V.D.; Kalkabay, G.R. The behavior of Ni nanotubes under the influence of environments with different acidities. CrystEngComm 2018, 20, 3258–3266. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Lin, C.; Zhang, H. Cobalt ferrite nanoparticles supported on drinking water treatment residuals: An efficient magnetic heterogeneous catalyst to activate peroxymonosulfate for the degradation of atrazine. Chem. Eng. J. 2019, 367, 208–218. [Google Scholar] [CrossRef]
- Das, H.; Aleksander, L.; Geng, Y.; Wu, W. Bulk magnetoelectricity in the hexagonal manganites and ferrites. Nat. Commun. 2014, 5, 2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X. Effect of Gd and La doping on the structure, optical and magnetic properties of NiZnCo ferrites. Ceram. Int. 2019, 45, 6236–6242. [Google Scholar] [CrossRef]
- Sinha, K.; Zhou, L.; Wang, H. Tuning the Néel temperature of hexagonal ferrites by structural distortion. Phys. Rev. Lett. 2018, 121, 237203. [Google Scholar] [CrossRef]
- Liao, Y.; Xu, F.; Zhang, D. Effect of ZnO–B2O3–SiO2 glass additive on magnetic properties of low-sintering Li0.43Zn0.27Ti0.13Fe2.17O4 ferrites. J. Mater. Sci. Mater. Electron. 2016, 27, 811–817. [Google Scholar] [CrossRef]
- Prasad, B.B.V.S.; Vara, K.; Ramesh, V.; Srinivas, A. Physical, structural, morphological, magnetic and electrical properties of Co0.5−xNixZn0.5Fe2O4 nanocrystalline ferrites. Ceram. Int. 2019, 45, 4549–4563. [Google Scholar] [CrossRef]
- Bahiraei, H.; Ong, K. Microstructural and electromagnetic study of low temperature fired nano crystalline MgCuZn ferrite with Bi2O3 addition. Ceram. Int. 2017, 43, 4780–4784. [Google Scholar] [CrossRef]
- Xu, F.; Liao, Y. Synthesis of highly uniform and compact lithium zinc ferrite ceramics via an efficient low temperature approach. Inorg. Chem. 2017, 56, 4512–4520. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, H.; Xie, F. Dispersion of LiZnTiBi ferrite particles into PMDS film for miniaturized flexible antenna application. Ceram. Int. 2019, 45, 8914–8918. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Kiyoharu, T.; Akira, M.; Mikio, H. Effect of sintering additives on relative density and Li-ion conductivity of Nb-doped Li7La3ZrO12 solid electrolyte. J. Am. Ceram. Soc. 2017, 100, 276–285. [Google Scholar] [CrossRef]
- Kagita, S.; Ananjan, B.; Shiban, K. Characterization of LTCC-Based Ferrite Tape in ${X} $-band and Its Application to Electrically Tunable Phase Shifter and Notch Filter. IEEE Trans. Magn. 2017, 53, 1–8. [Google Scholar] [CrossRef]
- Xie, F.; Jia, L.; Zheng, Z.; Zhang, H. Influences of Li2O–B2O3–SiO2 Glass Addition on Microstructural and Magnetic Properties of LiZnTi Ferrites. IEEE Trans. Magn. 2015, 51. [Google Scholar] [CrossRef]
- Sun, R.; Li, X. Hexagonal SrFe12O19 ferrite with high saturation magnetization. Ceram. Int. 2018, 44, 13551–13555. [Google Scholar] [CrossRef]
- Elayakumar, K. Structural, morphological, enhanced magnetic properties and antibacterial bio-medical activity of rare earth element (REE) cerium (Ce3+) doped CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2019, 476, 157–165. [Google Scholar] [CrossRef]
- Akhtar, M.N. Systematic study of Ce3+ on the structural and magnetic properties of Cu nanosized ferrites for potential applications. J. Rare Earths 2018, 36, 156–164. [Google Scholar] [CrossRef]
- Li, L.; Wang, R.; Tu, X.; Hua, L. Effects of Ce substitution on the structural and electromagnetic properties of NiZn ferrite. J. Magn. Magn. Mater. 2019, 475, 1–4. [Google Scholar] [CrossRef]
- Srinivasamurthy, K.M.; Hua, L.; Kumar, P.M.; Nagaraj, B.S. Synthesis and study of structural, microstructural and dielectric properties of Ce3+ doped Co-Ni ferrites for automotive applications. AIP Conf. Proc. 2018, 1953. [Google Scholar] [CrossRef]
- Sonia, M.; Lumina, M.; Vinosel, V.M. Effect of lattice strain on structural, magnetic and dielectric properties of sol–gel synthesized nanocrystalline Ce3+ substituted nickel ferrite. J. Mater. Sci. Mater. Electron. 2018, 29, 15006–15021. [Google Scholar] [CrossRef]
- Dehghan, A.S. Efficient Removal of Methylene Blue from Aqueous Solution by Adsorption on Cerium Vanadate Nanoparticles. Pollution 2019, 5, 339–349. [Google Scholar]
- Wu, Z.; Zhang, R. Study on preparation and magnetic properties of Sr1−xGdxFe12−xCuxO19 (0.00 ≤ x ≤ 0.20) strontium ferrite prepared by solid phase method. Ferroelectrics 2018, 523, 82–88. [Google Scholar] [CrossRef]
- El-Hafiz, D.R.; Abd, G.E.; Ahmed, E.; Metwally, E. Recent enhancement of ammonia borane hydrolysis using spinel-type metal ferrites nano-catalysts. Mater. Chem. Phys. 2018, 217, 562–569. [Google Scholar] [CrossRef]
- Hanini, A.; Souad, A.; Kamel, K.; Gavard, J. Ferrite Nanoparticles for Cancer Hyperthermia Therapy. In Handbook of Nanomaterials for Industrial Applications; Elsevier, Institute of Technology: Newark, NJ, USA, 2018; pp. 638–661. [Google Scholar]
- Zhuravlev, V.; Volya, I.; Dmitry, V. Mechanochemical Synthesis of Hexagonal Ferrites BaFe12O19. Key Eng. Mater. Trans. Technol. Publ. 2018, 781, 119–124. [Google Scholar] [CrossRef]
- Lashkaryani, E.B. Activation of peroxymonosulfate into amoxicillin degradation using cobalt ferrite nanoparticles anchored on graphene (CoFe2O4@Gr). Toxin Rev. 2019, 1–10. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Kenzhina, I.; Zdorovets, M. Synthesis, phase composition and magnetic properties of double perovskites of A (FeM)O4−x type (A = Ce; M = Ti). Ceram. Int. 2019, 45, 8669–8676. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L. Study of phase transformations, structural, corrosion properties and cytotoxicity of magnetite-based nanoparticles. Vacuum 2019, 163, 236–247. [Google Scholar] [CrossRef]
- Kang, Y.S. Synthesis and characterization of nanometer-size Fe3O4 and γ-Fe2O3 particles. Chem. Mater. 1996, 8, 2209–2211. [Google Scholar] [CrossRef]
- Sreeja, V.; Joy, P.A. Microwave–hydrothermal synthesis of γ-Fe2O3 nanoparticles and their magnetic properties. Mater. Res. Bull. 2007, 42, 1570–1576. [Google Scholar] [CrossRef]
- Kozlovskiy, A. Mossbauer research of Fe/Co nanotubes based on track membranes. Nuclear Instrum. Methods Phys. Res. Section B Beam Int. Mater. Atoms 2016, 381, 103–109. [Google Scholar] [CrossRef]
- Matsnev, M.E.; Rusakov, V.S. SpectrRelax: An application for Mössbauer spectra modeling and fitting. AIP Conf. Proc. 2012, 1489. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Shlimas, D.; Kenzhina, N. Study of the use of ionizing radiation to improve the efficiency of performance of nickel nanostructures as anodes of lithium-ion batteries. Mater. Res. Express 2019, 6, 055026. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Kenzhina, I.; Zdorovets, M.V. Effect of irradiation with C2+ and O2+ ions on the structural and conductive characteristics of copper nanostructures. Mater. Res. Express 2019, 6, 075072. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Kenzhina, I.; Zdorovets, M.V. Optical and structural properties of AlN ceramics irradiated with heavy ions. Opt. Mater. 2019, 91, 130–137. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Shlimas, I.; Dukenbayev, K. Structure and corrosion properties of thin TiO2 films obtained by magnetron sputtering. Vacuum 2019, 164, 224–232. [Google Scholar] [CrossRef]





| Sample | Atomic Ratio, % | ||
|---|---|---|---|
| Ce | Fe | O | |
| Initial | 59.3 ± 1.3 | 16.1 ± 1.1 | 24.6 ± 1.5 |
| 400 °C | 54.6 ± 1.5 | 20.3 ± 1.2 | 21.1 ± 1.4 |
| 600 °C | 59.8 ± 1.6 | 20.4 ± 1.4 | 19.8 ± 1.7 |
| 800 °C | 59.6 ± 1.5 | 22.6 ± 1.2 | 17.8 ± 1.3 |
| Phase | Type of Structure | Space Group | Phase Content, % | |||
|---|---|---|---|---|---|---|
| Initial | 400 °C | 600 °C | 800 °C | |||
| CeO2—Cerianite | Cubic | Fm-3m (225) | 100 | 100 | 66.6 | 65.1 |
| CeFeO3 | Orthorhombic | Pbnm (62) | - | - | 23.7 | - |
| Fe2O3—Hematite | Rhombo.H.axes | R-3c (167) | - | - | 9.7 | 34.9 |
| Phase | Lattice Parameter, Å | |||
|---|---|---|---|---|
| Initial | 400 °C | 600 °C | 800 °C | |
| CeO2—Cerianite | a = 5.2761 | a = 5.2803 | a = 5.2917 | a = 5.3197 |
| CeFeO3 | - | - | a = 5.6151, b = 5.7473, c = 7.8581 | - |
| Fe2O3—Hematite | - | - | a = 4.9756, c = 13.7846 | a = 4.9432, c = 13.7552 |
| Sample | Crystalline Size, nm | Crystallinity Degree, % | Dislocation Density, 1015 |
|---|---|---|---|
| Initial | 6.7 ± 0.8 | 68.1 ± 2.2 | 22.27 |
| 400 °C | 7.5 ± 1.2 | 69.3 ± 3.1 | 17.78 |
| 600 °C | 10.9 ± 1.4 | 81.1 ± 3.6 | 8.42 |
| 800 °C | 28.2 ± 2.1 | 87.3 ± 3.4 | 1.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadyrzhanov, K.K.; Egizbek, K.; Kozlovskiy, A.L.; Zdorovets, M.V. Synthesis and Properties of Ferrite-Based Nanoparticles. Nanomaterials 2019, 9, 1079. https://doi.org/10.3390/nano9081079
Kadyrzhanov KK, Egizbek K, Kozlovskiy AL, Zdorovets MV. Synthesis and Properties of Ferrite-Based Nanoparticles. Nanomaterials. 2019; 9(8):1079. https://doi.org/10.3390/nano9081079
Chicago/Turabian StyleKadyrzhanov, Kayrat K., Kamila Egizbek, Artem L. Kozlovskiy, and Maxim V. Zdorovets. 2019. "Synthesis and Properties of Ferrite-Based Nanoparticles" Nanomaterials 9, no. 8: 1079. https://doi.org/10.3390/nano9081079





