Influence of the Silver Nanoparticles (AgNPs) Formation Conditions onto Titanium Dioxide (TiO2) Nanotubes Based Electrodes on Their Impedimetric Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TiO2 Nanotubes Fabrication
2.3. Thermal Modification
2.4. Modification of TNT with Silver Nanoparticles
2.4.1. Cyclic Voltammetry Method (CV)
2.4.2. Chronoamperometry Method (CA)
2.4.3. Sputter Deposition (SD)
2.5. Electrochemical Measurements
3. Results and Discussion
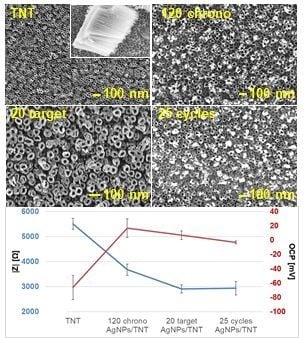
3.1. Characterization of Reference Platforms: TNT and Ag/TNT
3.2. Characterisation of AgNPs/TNT Platforms with Silver Nanoparticles Obtained by Cyclic Voltammetry
3.3. Characterization of AgNPs/TNT Platforms in which Silver Nanoparticles Were Obtained by Chronoamperometry
3.4. Characterisation of AgNPs/TNT Platforms in which Silver Nanoparticles Were Obtained by Sputter Deposition
4. Conclusions
- Produced silver spherical and near-spherical nanoparticles were concentrated mostly in the upper part of nanotubes around their rings and, to a lesser extent, also filled the space between the nanotubes and permeated into them. With the increase in duration of deposition, an increase in the spread of the size of nanoparticles, and an increase in the most frequent value of these sizes was observed. In the case of cyclic voltammetry, there occurred a linear growth of % silver weight content in the structure along with the increased number of cycles to 25, after which the layer became saturated. In the case of chronoamperometry and sputter deposition exponential growth of % wt. of Ag in time was noted.
- Generally, the addition of silver to the structure increased the OCP value, which in turn increased the corrosion resistance of these structures. In the case of electrodeposition it can be noticed that a shorter duration of nanoparticles deposition/lower number of cycles results in creating platforms whose OCP has positive values. This probably occurs because of a greater number of unreduced silver ions in non-stabilized structures rich in agglomerates.
- Nanoparticles caused a decrease in the impedance module (up to 50% lower when compared to TNT) and hence increased conductivity of the created electrodes. The highest conductivity among all samples without agglomerates was noted for the electrode after 25 cycles of AgNPs deposition by cyclic voltammetry (25 cycles_AgNPs/TNT).
Author Contributions
Funding
Conflicts of Interest
References
- Dervisevic, M.; Cevik, E.; Durmus, Z.; Senel, M. Electrochemical sensing platforms based on the different carbon derivative incorporated interface. Mater. Sci. Eng. C 2016, 58, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Shoja, Y.; Rafati, A.A.; Ghodsi, J. Glassy carbon electrode modified with horse radish peroxidase/organic nucleophilic-functionalized carbon nanotube composite for enhanced electrocatalytic oxidation and efficient voltammetric sensing of levodopa. Mater. Sci. Eng. C 2016, 58, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Q.; Chen, S.; Xu, F.; Chen, S.; Jia, J.; Tan, H.; Hou, H.; Song, Y. Electrochemical sensing and biosensing platform based on biomass-derived macroporous carbon materials. Anal. Chem. 2014, 86, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Shadjou, N.; de la Guardia, M.; Eskandani, M.; Sheikhzadeh, P. Mesoporous silica-based materials for use in biosensors. Trends Anal. Chem. 2012, 33, 117–129. [Google Scholar] [CrossRef]
- Gerard, M.; Chaubey, A.; Malhotra, B.D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Wang, X.; Uchiyama, S. Polymers for biosensors construction. In State of the Art in Biosensors – General Aspects; Rinken, T., Ed.; InTech: London, UK, 2013; pp. 67–86. [Google Scholar]
- Bianchi, R.C.; da Silva, E.R.; Dall‘Antonia, L.H.; Ferreira, F.F.; Alves, W.A. A nonenzymatic biosensor based on gold electrodes modified with peptide self-assemblies for detecting ammonia and urea oxidation. Langmuir 2014, 30, 11464–11473. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Li, G.; Liang, J.; Su, J.; Zhang, Y.; Chen, H.; Huang, Y.; Sui, W.; Zhao, Y. Non-enzymatic electrochemical biosensor based on Pt NPs/RGO-CS-Fc nano-hybrids for the detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2016, 82, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sandulescu, R.; Tertis, M.; Cristea, C.; Bodoki, E. New materials for the construction of electrochemical biosensors. In Biosensors - Micro and Nanoscale Applications; Rinken, T., Ed.; InTech: London, UK, 2015; pp. 1–36. [Google Scholar]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: a review. Front. Chem. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium dioxide nanomaterials for sensor applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Ngece, R.F.; West, N.; Ndangili, P.M.; Olowu, R.A. A silver Nanoparticle/Poly (8-Anilino-1-Naphthalene Sulphonic Acid) bioelectrochemical biosensor system for the analytical determination of ethambutol. Int. J. Electrochem. Sci. 2011, 6, 1820–1834. [Google Scholar]
- Chang, G.H.; Luo, Y.L.; Lu, W.B.; Liao, F.; Sun, X. Hydrothermal synthesis of ultra-highly concentrated; well-stable Ag nanoparticles and their application for enzymeless hydrogen peroxide detection. J. Nanoparticle. Res. 2011, 13, 2689–2695. [Google Scholar] [CrossRef]
- Khan, M.J.; Husain, Q.; Ansari, S.A. Polyaniline-assisted silver nanoparticles: a novel support for the immobilization of α-amylase. Appl. Microbiol. Biotechnol. 2013, 97, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Narang, J.; Chauhan, N.; Jain, P.; Pundir, C.S. Silver nanoparticles/multiwalled carbon nanotube/polyaniline film for amperometric glutathione biosensor. Int. J. Biol. Macromol. 2012, 50, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Xu, G.; Liu, H.; Lv, J.; Zheng, Z.; Wu, Y. Glucose biosensors based on Ag nanoparticles modified TiO2 nanotube arrays. J. Solid State Electrochem. 2014, 18, 163–171. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, B.; Du, J.; Liu, G.; Guo, Y.; Xiao, D. Electrophoresis deposition of Ag nanoparticles on TiO2 nanotube arrays electrode for hydrogen peroxide sensing. Talanta 2013, 112, 129–135. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nanostructured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Roguska, A.; Belcarz, A.; Piersiak, T.; Pisarek, M. Evaluation of the antibacterial activity of Ag-loaded TiO2 nanotubes. Eur. J. Inorg. Chem. 2012, 5199–5206. [Google Scholar] [CrossRef]
- Paramasivam, I.; Macak, J.M.; Schmuki, P. Photocatalytic activity of TiO2 nanotube layers loaded with Ag and Au nanoparticles. Electrochem. Commun. 2008, 10, 71–75. [Google Scholar] [CrossRef]
- Syrek, K.; Grudzień, J.; Sennik-Kubiec, A.; Brudzisz, A.; Sulka, G.D. Anodic titanium oxide layers modified with gold, silver, and copper nanoparticles. J. Nano 2019, 1–10. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Liu, D.; Chi, L. Ag and CdS nanoparticles co-sensitized TiO2 nanotubes for enhancing visible photoelectrochemical performance. Electrochim. Acta 2012, 83, 140–145. [Google Scholar] [CrossRef]
- Gudikandula, K.; Maringanti, S.C. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Starowicz, M.; Stypuła, B.; Banaś, J.; Kasprzyk, D. Electrochemical synthesis of silver nanoparticles. Electrochem. Commun. 2006, 8, 227–230. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.Y.; Takeda, Y.; Kondow, T.; Sawabe, H. Formation and size control of silver nanoparticles by laser ablation in aqueous solution. J. Phys. Chem. B 2000, 104, 9111–9117. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Sharonova, A.A.; Chernousova, S.; Prymak, O.; Loza, K.; Tkachev, M.S.; Shulepov, I.A.; Epple, M.; Surmenev, R.A. Incorporation of silver nanoparticles into magnetron-sputtered calcium phosphate layers on titanium as anantibacterial coating. Colloids Surf. B 2017, 156, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Oluwafemi, O.S.; Mochochoko, T.; Leo, A.J.; Mohan, S.; Jumbam, D.N.; Songca, S.P. Microwave irradiation synthesis of silver nanoparticles using cellulose from Eichhornia crassipes plant shoot. Mater. Lett. 2016, 185, 576–579. [Google Scholar] [CrossRef]
- Vinoth, V.; Wu, J.J.; Asiri, A.M.; Anandan, S. Sonochemical synthesis of silver nanoparticles anchored reduced graphene oxide nanosheets for selective and sensitive detection of glutathione. Ultrason. Sonochem. 2017, 39, 363–373. [Google Scholar] [CrossRef]
- Paramasivam, I.; Macak, J.M.; Ghicov, A.; Schmuki, P. Enhanced photochromism of Ag loaded selforganized TiO2 nanotube layers. Chem. Phys. Lett. 2007, 445, 233–237. [Google Scholar] [CrossRef]
- Baran, E.; Yazici, B. Effect of different nano-structured Ag doped TiO2-NTs fabricated by electrodeposition on the electrocatalytic hydrogen production. Int. J. Hydrogen Energy 2016, 41, 2498–2511. [Google Scholar] [CrossRef]
- Brugnera, M.F.; Miyata, M.; Leite, C.Q.F.; Zanoni, M.V.B. Silver ion release from electrodes of nanotubes of TiO2 impregnated with Ag nanoparticles applied in photoelectrocatalytic disinfection. J. Photochem. Photobiol. A Chem. 2014, 278, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Hao, S.; Chu, W. Facile fabrication of well-dispersed silver nanoparticles loading on TiO2 nanotubearrays by electrodeposition. Mater. Lett. 2012, 80, 66–68. [Google Scholar] [CrossRef]
- Li, J.; Yang, L.; Luo, S.; Chen, B.; Li, J.; Lin, H.; Cai, Q.; Yao, S. Polycyclic Aromatic Hydrocarbon Detection by Electrochemiluminescence Generating Ag/TiO2. Anal. Chem. 2010, 82, 7357–7361. [Google Scholar] [CrossRef]
- Roguska, A.; Kudelski, A.; Pisarek, M.; Opara, M.; Janik-Czachor, M. Raman investigations of SERS activity of Ag nanoclusters on a TiO2-nanotubes/Ti substrate. Vibrat. Spectr. 2011, 55, 38–43. [Google Scholar] [CrossRef]
- Salari, M.; Aboutalebi, S.H.; Chidembo, A.T.; Nevirkovets, I.P.; Konstantinov, K.; Liu, H.K. Enhancement of the electrochemical capacitance of TiO2 nanotube arrays through controlled phase transformation of anatase to rutile. Phys. Chem. Chem. Phys. 2012, 14, 4770–4779. [Google Scholar] [CrossRef]
- Arkusz, K.; Paradowska, E.; Nycz, M.; Krasicka-Cydzik, E. Influence of Thermal Modification and Morphology of TiO2 nanotubes on their electrochemical properties for biosensors applications. J. Nanosci. Nanotechnol. 2018, 18, 3713–3721. [Google Scholar] [CrossRef]
- Arkusz, K.; Nycz, M.; Paradowska, E.; Krasicka-Cydzik, E. Electrochemical detection method for interleukin-6 on titania nanotube platforms. Eng. Biomater. 2014, 17, 21–29. [Google Scholar]
- Leu, W.L.; Wang, N.; Gao, P.; Li, C.Y.; Zhao, H.S.; Zhang, Z.T. Effects of anodic titanium dioxide nanotubes of different diameters on macrophage secretion and expression of cytokines and chemokines. Cell Proliferat. 2015, 48, 95–104. [Google Scholar] [CrossRef]
- Liu, D.; Xiao, P.; Zhang, Y.; Garcia, B.B. TiO2 nanotube arrays annealed in N2 for efficient lithium-ion intercalation. J. Phys. Chem. C 2018, 112, 11175–11180. [Google Scholar] [CrossRef]
- Salari, M.; Konstantinov, K.; Liu, H.K. Enhancement of the capacitance in TiO2 nanotubes through controlled introduction of oxygen vacancies. J. Mater. Chem. 2011, 21, 5128–5133. [Google Scholar] [CrossRef]
- Ghicov, A.; Tsuchiya, H.; Macak, J.M.; Schmuki, P. Annealing effects on the photoresponse of TiO2 nanotubes. Phys. Stat. Sol. A 2006, 203, 28–30. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Huang, K.; Dong, F.; Zhuang, S. Electrochemical properties of freestanding TiO2 nanotube membranes annealed in Ar for lithium anode material. J. Solid. State Electr. 2012, 16, 723–729. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, Y.; Zheng, D.; Song, R.; Zhang, Y.; Jiang, P.; Vogler, E.A.; Lin, C. Effect of construction of TiO2 nanotubes on platelet behaviors: Structure-property relationships. Acta Biomater. 2017, 51, 505–512. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Cui, Z.D.; Zhu, S.L.; Liu, Y.; Yang, X.Y. Silver nanoparticles supported on TiO2 nanotubes as active catalysts for ethanol oxidation. J. Catal. 2011, 278, 276–287. [Google Scholar] [CrossRef]
- Bai, Y.; Bai, Y.; Wang, C.; Gao, J.; Ma, W. Fabrication and characterization of gold nanoparticle-loaded TiO2 nanotube arrays for medical implants. J. Mater. Sci. Mater. Med. 2016, 27, 31. [Google Scholar] [CrossRef]
- Kim, H.S.; Chun, M.H.; Suh, J.S.; Jun, B.-H.; Rho, W.-Y. Dual functionalized freestanding TiO2 nanotube arrays coated with Ag nanoparticles and carbon materials for dye-sensitized solar cells. App. Sci. 2017, 7, 576. [Google Scholar] [CrossRef]
- Azadbakht, A.; Abbasi, A.R.; Derikvand, Z.; Karimi, Z.; Roushani, M. Surface-renewable AgNPs/CNT/rGO nanocomposites as bifunctional impedimetric sensors. Nano-Micro Lett. 2017, 9, 1–11. [Google Scholar] [CrossRef]
- Raghav, R.; Srivastava, S. Core–shell gold–silver nanoparticles based impedimetric immunosensor for cancer antigen CA125. Sens. Actuators B 2015, 220, 557–564. [Google Scholar] [CrossRef]
- Huang, K.J.; Liu, Y.J.; Wang, H.B.; Wang, Y.Y. A sensitive electrochemical DNA biosensor based on silver nanoparticles - polydopamine@graphene composite. Electrochim. Acta 2014, 118, 130–137. [Google Scholar] [CrossRef]
- Gonzalez-Campos, J.B.; Prokhorov, E.; Luna-Barcenas, G.; Sanchez, I.C.; Lara-Romero, J.; Mendoza-Duarte, M.E.; Villasenor, F.; Guevara-Olvera, L. Chitosan/silver nanoparticles composite: molecular relaxations investigation by dynamic mechanical analysis and impedance spectroscopy. J. Polym. Sci. B Polym. Phys. 2010, 48, 739–748. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Borzoo, M. Green synthesis of silver nanoparticles using onion extract and their application for the preparation of a modified electrode for determination of ascorbic acid. J. Food Drug Anal. 2016, 24, 796–803. [Google Scholar] [CrossRef]
- Chawla, S.; Rawal, R.; Kumar, D.; Pundir, C.S. Amperometric determination of total phenolic content in wine by laccase immobilized onto silver nanoparticles/zinc oxide nanoparticles modified gold electrode. Analyt. Biochem. 2012, 430, 16–23. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, G.; Zhang, M.; Xu, G.; Lin, J.; Luo, X. Aptamer based label free thrombin assay based on the use of silver nanoparticles incorporated into self-polymerized dopamine. Microchim. Acta 2018, 185, 1–7. [Google Scholar] [CrossRef]
- Sha, H.; Zheng, W.; Shi, F.; Wang, X.; Sun, W. Direct electrochemistry of hemoglobin on electrodeposited three-dimensional interconnected graphene-silver nanocomposite modified electrode. Int. J. Electrochem. Sci. 2016, 11, 9656–9665. [Google Scholar] [CrossRef]
- Chen, K.; Feng, X.; Hu, R.; Li, Y. Effect of Ag nanoparticle size on the photoelectrochemical properties of Ag decorated TiO2 nanotube arrays. J. Alloys Compd. 2013, 554, 72–79. [Google Scholar] [CrossRef]










| Sample | Description |
|---|---|
| x cycles_AgNPs/TNT | TNT with AgNPs deposited using CV in 1 mM AgNO3; where x – number of cycles: x = 5, 10, 15, 20, 25, 30. |
| x chrono_AgNPs/TNT | TNT with AgNPs deposited using CA in 1 mM AgNO3; where x – time of deposition: x = 30, 60, 120, 180, 240, 300. |
| x target_AgNPs/TNT | TNT with AgNPs deposited using SD; where x – time of deposition: x = 10, 20, 40, 60. |
| Ag/TNT | TNT with silver micro layer deposited using CV in 50 mM AgNO3 for 25 cycles. |
| AgNPs/TNT | Ag/TNT | ||||||
|---|---|---|---|---|---|---|---|
| Number of cycles | 5 | 10 | 15 | 20 | 25 | 30 | 25 |
| Size of AgNPs | 5–80 a nm | 5–50 a nm | 5–40 a nm | 5–40 nm | 5–40 nm | 5–70 a nm | layer a |
| OCP [mV] versus Ag/AgCl | −18.0 ± 24 | 8.11 ± 15 | 27.0 ± 23 | −18.9 ± 9 | −2.6 ± 2 | −16.7 ± 13 | 26.9 ± 18 |
| AgNPs/TNT | ||||||
|---|---|---|---|---|---|---|
| Time [s] | 30 | 60 | 120 | 180 | 240 | 300 |
| Size of AgNPs | 5–30a nm | 5–25 nm | 5–35 nm | 5–45 nm | 10–50 nm | 10–70 a nm |
| OCP [mV] versus Ag/AgCl | 4.00 ± 19 | 21.6 ± 23 | 17.2 ± 13 | −18.9 ± 20 | −13.6 ± 12 | −26 ± 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nycz, M.; Arkusz, K.; Pijanowska, D.G. Influence of the Silver Nanoparticles (AgNPs) Formation Conditions onto Titanium Dioxide (TiO2) Nanotubes Based Electrodes on Their Impedimetric Response. Nanomaterials 2019, 9, 1072. https://doi.org/10.3390/nano9081072
Nycz M, Arkusz K, Pijanowska DG. Influence of the Silver Nanoparticles (AgNPs) Formation Conditions onto Titanium Dioxide (TiO2) Nanotubes Based Electrodes on Their Impedimetric Response. Nanomaterials. 2019; 9(8):1072. https://doi.org/10.3390/nano9081072
Chicago/Turabian StyleNycz, Marta, Katarzyna Arkusz, and Dorota Genowefa Pijanowska. 2019. "Influence of the Silver Nanoparticles (AgNPs) Formation Conditions onto Titanium Dioxide (TiO2) Nanotubes Based Electrodes on Their Impedimetric Response" Nanomaterials 9, no. 8: 1072. https://doi.org/10.3390/nano9081072
APA StyleNycz, M., Arkusz, K., & Pijanowska, D. G. (2019). Influence of the Silver Nanoparticles (AgNPs) Formation Conditions onto Titanium Dioxide (TiO2) Nanotubes Based Electrodes on Their Impedimetric Response. Nanomaterials, 9(8), 1072. https://doi.org/10.3390/nano9081072