Directly Grown Multiwall Carbon Nanotube and Hydrothermal MnO2 Composite for High-Performance Supercapacitor Electrodes
Abstract
:1. Introduction
2. Materials and Methods
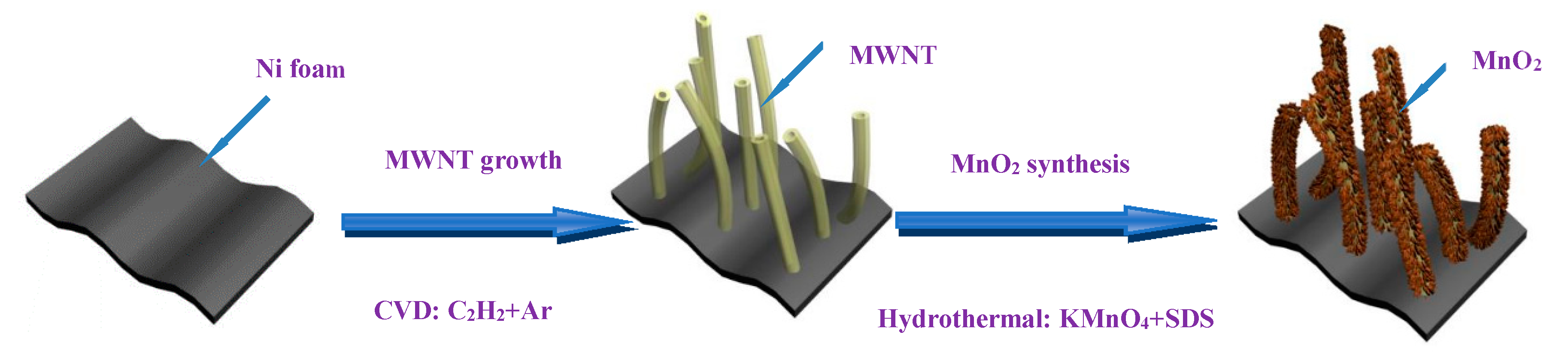
2.1. Synthesis of the MnO2–MWNT–Ni Foam Composite Electrode
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
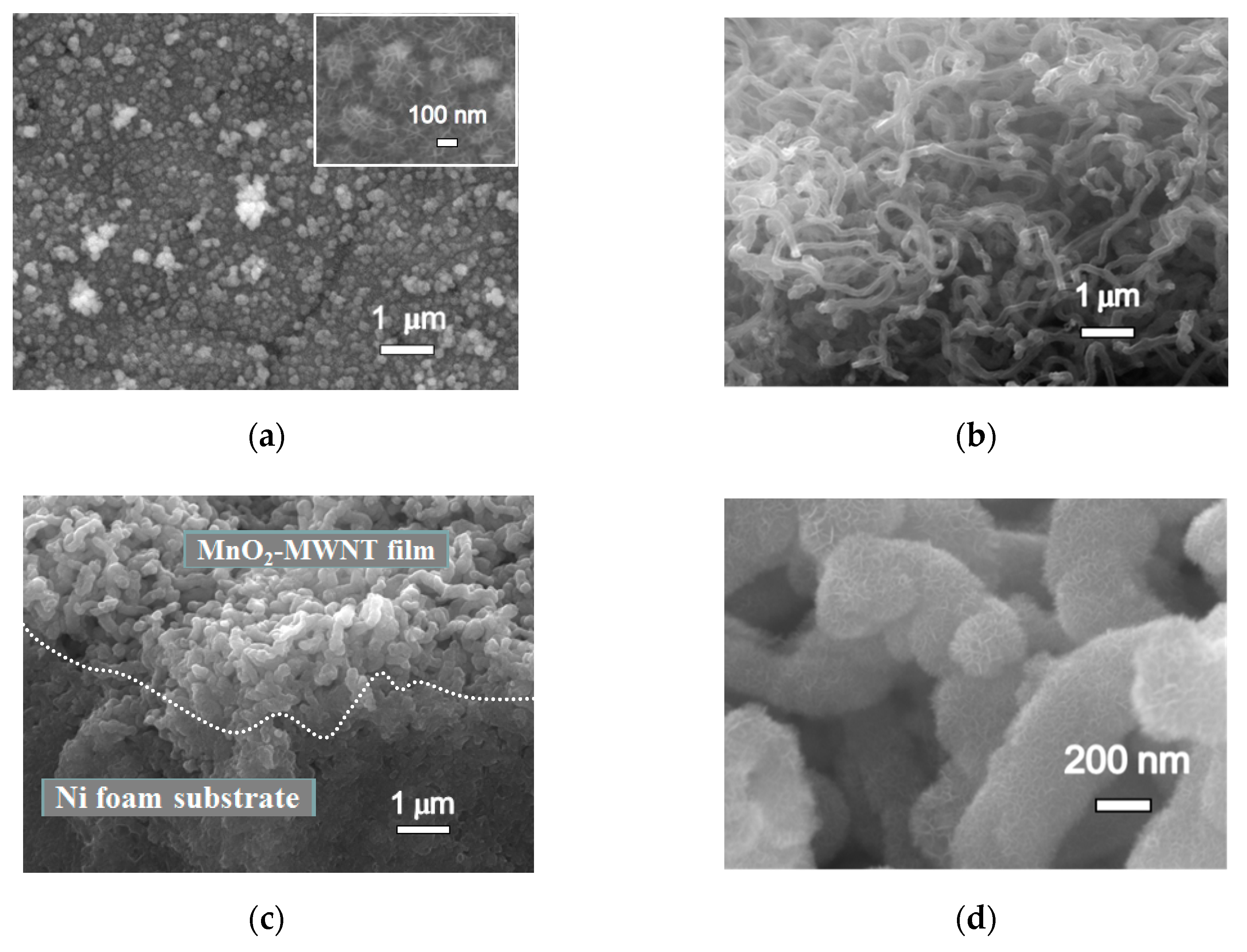
3.1. Structure Characterization
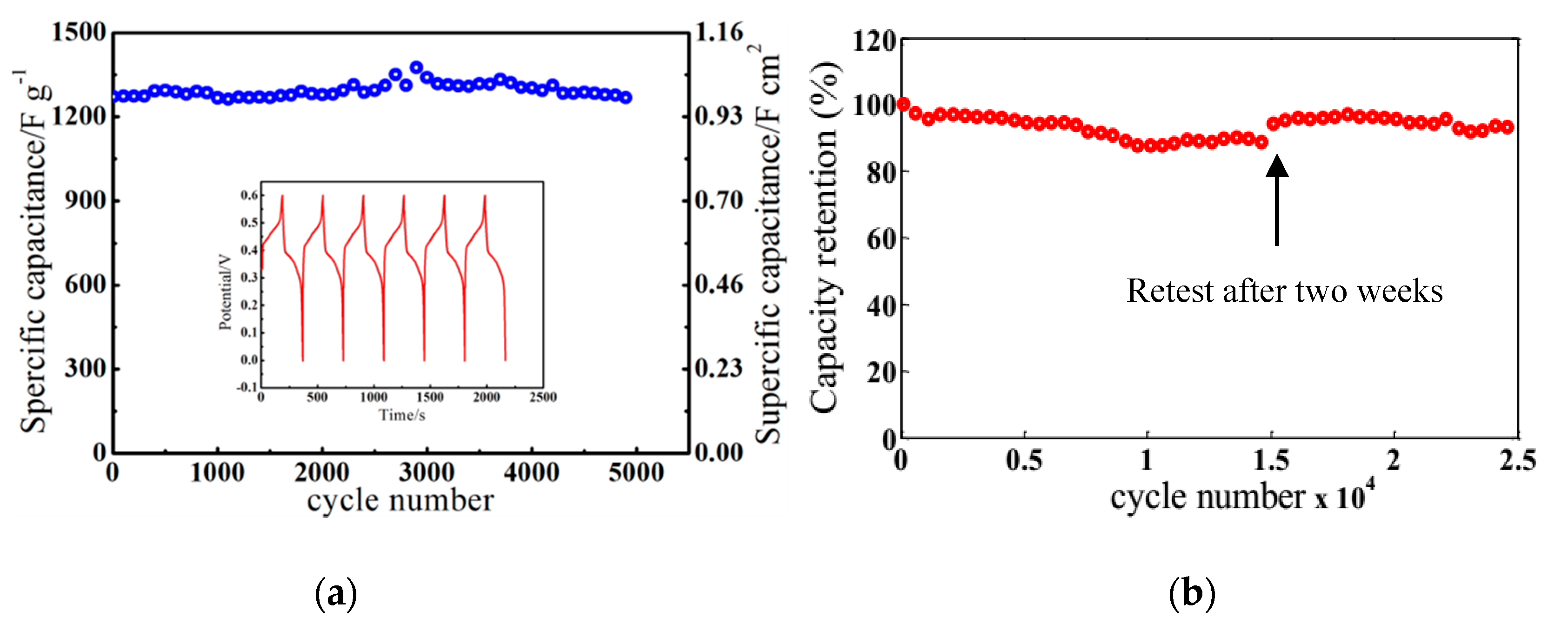
3.2. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Deng, T.; Zhang, W.; Arcelus, O.; Kim, J.G.; Carrasco, J.; Yoo, S.J.; Zheng, W.T.; Wang, J.F.; Tian, H.W.; Zhang, H.B.; et al. Atomic-level energy storage mechanism of cobalt hydroxide electrode for pseudocapacitors. Nat. Commun. 2017, 8, 15194. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhou, E.; He, W.; Deng, X.; Huang, J.; Ding, M.; Wei, X.; Liu, X.; Xu, X. NiCo2O4-Based Supercapacitor Nanomaterials. Nanomaterials 2017, 7, 41. [Google Scholar] [CrossRef]
- Ramkumar, R.; Sundaram, M.M. Electrochemical synthesis of polyaniline cross-linked NiMoO4 nanofibre dendrites for energy storage devices. New J. Chem. 2016, 40, 456–7464. [Google Scholar] [CrossRef]
- Ramkumar, R.; Sundaram, M.M. A biopolymer gel-decorated cobalt molybdate nanowafer: Effective graft polymer cross-linked with an organic acid for better energy storage. New J. Chem. 2016, 40, 2863–2877. [Google Scholar] [CrossRef]
- Lei, W.; Liu, H.P.; Xiao, J.L.; Wang, Y.; Lin, L.X. Moss-Derived Mesoporous Carbon as Bi-Functional Electrode Materials for Lithium–Sulfur Batteries and Supercapacitors. Nanomaterials 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, L.; Li, Y.; Wang, Y.; Zhang, J.; Guan, G.; Pan, Z.; Zheng, G.; Peng, H. Nitrogen-Doped Core-Sheath Carbon Nanotube Array for Highly Stretchable Supercapacitor. Adv. Energy Mater. 2017, 7, 1601814. [Google Scholar] [CrossRef]
- He, N.; Yildiz, O.; Pan, Q.; Zhu, J.D.; Zhang, X.W.; Bradford, P.D.; Gao, W. Pyrolytic-carbon coating in carbon nanotube foams for better performance in supercapacitors. J. Power Sources 2017, 343, 492–501. [Google Scholar] [CrossRef]
- Hu, M.; Liu, Y.; Zhang, M.; Wei, H.L.; Gao, Y.H. Wire-type MnO2/Multilayer graphene/Ni electrode for high-performance supercapacitors. J. Power Sources 2016, 335, 113–120. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, L.; Long, C.; Fan, Z. Graphene and nanostructured MnO2 composite electrodes for supercapacitors. Carbon 2011, 49, 2917–2925. [Google Scholar]
- Zhang, Y.Z.; Wang, Y.; Xie, Y.L.; Cheng, T.; Lai, W.Y.; Pang, H.; Huang, W. Porous hollow Co3O4 with rhombic dodecahedral structures for high-performance supercapacitors. Nanoscale 2014, 6, 14354–14359. [Google Scholar] [CrossRef]
- Wang, D.C.; Ni, W.B.; Pang, H.; Lu, Q.Y.; Huang, Z.J.; Zhao, J.W. Preparation of mesoporous NiO with a bimodal pore size distribution and application in electrochemical capacitors. Electrochim. Acta 2010, 55, 6830–6835. [Google Scholar] [CrossRef]
- Xiao, W.; Xia, H.; Fuh, J.Y.H.; Lu, L. Growth of single-crystal α-MnO2 nanotubes prepared by a hydrothermal route and their electrochemical properties. J. Power Sources 2009, 193, 935–938. [Google Scholar] [CrossRef]
- Zhai, T.; Wang, F.X.; Yu, M.H.; Xie, S.L.; Liang, C.L.; Li, C.; Xiao, F.M.; Tang, R.H.; Wu, Q.X.; Lu, X.H.; et al. 3D MnO2–graphene composites with large areal capacitance for high-performance asymmetric supercapacitors. Nanoscale 2013, 5, 6790–6796. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Sumboja, A.; Wang, X.; Fu, C.P.; Kumar, V.; Lee, P.S. Insights on the fundamental capacitive behavior: A case study of MnO2. Small 2014, 11, 3568–3578. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.J.; Slade, R.C.T. Performance loss of aqueous MnO2/carbon supercapacitors at elevated temperature: Cycling vs. storage. J. Mater. Chem. A 2013, 1, 14140–14146. [Google Scholar] [CrossRef]
- Hassan, S.; Suzuki, M.; Mori, S.; Moneim, A.A. MnO2/carbon nanowalls composite electrode for supercapacitor application. J. Power Sources 2014, 249, 21–27. [Google Scholar] [CrossRef]
- Zhu, G.; He, Z.; Chen, J.; Zhao, J.; Feng, X.M.; Ma, Y.W.; Fan, Q.L.; Wang, L.H.; Huang, W. Highly conductive three-dimensional MnO2–carbon nanotube–graphene–Ni hybrid foam as a binder-free supercapacitor electrode. Nanoscale 2014, 6, 1079–1085. [Google Scholar] [CrossRef]
- Hu, L.B.; Chen, W.; Xie, X.; Liu, N.; Yang, Y.; Wu, H.; Yao, Y.; Pasta, M.; Alshareef, H.N.; Cui, Y. Symmetrical MnO2–carbon nanotube–textile nanostructures for wearable pseudocapacitors with high mass loading. ACS Nano 2011, 5, 8904–8913. [Google Scholar] [CrossRef]
- Wang, K.; Gao, S.; Du, Z.L.; Yuan, A.B.; Lu, W.; Chen, L.W. MnO2-Carbon nanotube composite for high-areal-density supercapacitors with high rate performance. J. Power Sources 2016, 305, 30–36. [Google Scholar] [CrossRef]
- Zhao, D.D.; Yang, Z.; Zhang, L.Y.; Feng, X.L.; Zhang, Y.F. Electrodeposited manganese oxide on nickel foam–supported carbon nanotubes for electrode of supercapacitors. Electrochem. Solid-State Lett. 2011, 14, A93–A96. [Google Scholar] [CrossRef]
- Rangom, Y.; Tang, X.; Nazar, L.F. Carbon nanotube-based supercapacitors with excellent ac line filtering and rate capability via improved interfacial impedance. ACS Nano 2015, 9, 7248–7255. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Dong, C.K.; Qian, W.J.; Zhai, Y.; Li, L.; Li, D.T. Electrochemical properties of nickel-metal hydride battery based on directly grown multiwalled carbon nanotubes. Int. J. Hydrogen Energy 2015, 40, 8935–8940. [Google Scholar] [CrossRef]
- Chen, L.L.; Li, L.; Qian, W.J.; Dong, C.K. MnO2/multiwall carbon nanotube/Ni-foam hybrid electrode for electrochemical capacitor. IOP Conf. Ser. Mater. Sci. Eng. 2018, 292, 012018. [Google Scholar] [CrossRef]
- Wang, J.G.; Yang, Y.; Huang, Z.H.; Kang, F.Y. A high-performance asymmetric supercapacitor based on carbon and carbon–MnO2 nanofiber electrodes. Carbon 2013, 61, 190–199. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wang, Y.H.; Zang, J.B.; Xin, G.X.; Yuan, Y.G.; Qu, X.H. Electrophoretic deposition of MnO2-coated carbon nanotubes on a graphite sheet as a flexible electrode for supercapacitors. Carbon 2012, 50, 5196–5202. [Google Scholar] [CrossRef]
- Yang, C.Z.; Zhou, M.; Xu, Q. Three-dimensional ordered macroporous MnO2/carbon nanocomposites as high-performance electrodes for asymmetric supercapacitors. Phys. Chem. Chem. Phys. 2013, 15, 19730–19740. [Google Scholar] [CrossRef]
- Chen, J.J.; Huang, Y.; Zhang, X.; Chen, X.F.; Li, C. MnO2 grown in situ on graphene@ CNTs as electrode materials for supercapacitors. Ceram. Int. 2015, 41, 12680–12685. [Google Scholar] [CrossRef]
- Sun, P.; Yi, H.; Peng, T.Q.; Jing, Y.T.; Wang, R.J.; Wang, H.W.; Wang, X.F. Ultrathin MnO2 nanoflakes deposited on carbon nanotube networks for symmetrical supercapacitors with enhanced performance. J. Power Sources 2017, 341, 27–35. [Google Scholar] [CrossRef]
- Xiang, C.; Xiao, L.X.; Ying, J.; Chen, W.S.; Xue, L.L. Rational synthesis of alpha-MnO2 and gamma-Mn2O3 nanowires with the electrochemical characterization of alpha-MnO2 nanowires for supercapacitor. Solid State Commun. 2005, 136, 94–96. [Google Scholar]
- Shu, L.C.; Fan, L.; Quan, J.X.; Xiong, H.F.; Guo, H.Q. Synthesis of Mn2O3 microstructures and their energy storage ability studies. Electrochim. Acta 2013, 106, 360–371. [Google Scholar]
- Wen, Y.L.; Jia, J.S.; Qian, L.; Xi, J.L.; Xi, Y.Z.; Jun, Q.H. Facile synthesis of porous Mn2O3 nanocubics for high-rate supercapacitors. Electrochim. Acta 2015, 157, 108–114. [Google Scholar]
- Liu, Y.; Yan, D.; Zhuo, R.F.; Li, S.K.; Wu, Z.G.; Wang, J.; Ren, P.Y.; Yan, P.X.; Geng, Z.R. Design, hydrothermal synthesis and electrochemical properties of porous birnessite-type manganese dioxide nanosheets on graphene as a hybrid material for supercapacitors. J. Power Sources 2013, 242, 78–85. [Google Scholar] [CrossRef]
- Xia, H.; Huo, C. Electrochemical properties of MnO2/CNT nanocomposite in neutral aqueous electrolyte as cathode material for asymmetric supercapacitors. Int. J. Smart Nano Mater. 2011, 2, 283–291. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Minakshi, M. Lithium intercalation into amorphous FePO4 cathode in aqueous solutions. Electrochim. Acta 2010, 55, 9174–9178. [Google Scholar] [CrossRef]
- Ranjusha, R.; Nair, A.S.; Ramakrishna, S.; Anjali, P.; Sujith, K.; Subramanian, K.R.V.; Sivakumar, N.; Kim, T.N.; Nair, S.V.; Balakrishnan, A. Ultrafine MnO2 nanowire based high performance thin film rechargeable electrodes: Effect of surface morphology, electrolytes and concentrations. J. Mater. Chem. 2012, 22, 20465–20471. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, S.; Jiang, H.; Qu, F.Y.; Wu, X. Phase-controlled synthesis of polymorphic MnO2 structures for electrochemical energy storage. J. Mater. Chem. A 2015, 3, 5722–5729. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, X.; Su, L.H.; Gao, B.; Shen, L.F. Facile synthesis and self-assembly of hierarchical porous NiO nano/micro spherical superstructures for high performance supercapacitors. J. Mater. Chem. 2009, 19, 5772–5777. [Google Scholar] [CrossRef]
- Chen, G.Z. Understanding supercapacitors based on nano-hybrid materials with interfacial conjugation. Prog. Nat. Sci. 2013, 23, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Zhao, X.L.; Li, F.; Li, W.; Zhang, B.; Zhang, Y.X. Synthesis of Co3O4/SnO2@ MnO2 core–shell nanostructures for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 12852–12857. [Google Scholar] [CrossRef]
- Wang, J.G.; Yang, Y.; Huang, Z.H.; Kong, F.Y. Interfacial synthesis of mesoporous MnO2/polyaniline hollow spheres and their application in electrochemical capacitors. J. Power Sources 2012, 204, 236–243. [Google Scholar] [CrossRef]
- Barmi, M.J.; Minakshi, M. Tuning the Redox Properties of the Nanostructured CoMoO4Electrode: Effects of Surfactant Content and Synthesis Temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Y.; Liu, L.; Wu, Y. Graphene–hollow PPy sphere 3D-nanoarchitecture with enhanced electrochemical performance. Nanoscale 2013, 5, 3052–3057. [Google Scholar] [CrossRef]
- Mai, L.Q.; Minhas-Khan, A.; Tian, X. Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance. Nat. Commun. 2013, 4, 2923. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Chen, L.; Qian, W.; Xie, F.; Dong, C. Directly Grown Multiwall Carbon Nanotube and Hydrothermal MnO2 Composite for High-Performance Supercapacitor Electrodes. Nanomaterials 2019, 9, 703. https://doi.org/10.3390/nano9050703
Li L, Chen L, Qian W, Xie F, Dong C. Directly Grown Multiwall Carbon Nanotube and Hydrothermal MnO2 Composite for High-Performance Supercapacitor Electrodes. Nanomaterials. 2019; 9(5):703. https://doi.org/10.3390/nano9050703
Chicago/Turabian StyleLi, Li, Lihui Chen, Weijin Qian, Fei Xie, and Changkun Dong. 2019. "Directly Grown Multiwall Carbon Nanotube and Hydrothermal MnO2 Composite for High-Performance Supercapacitor Electrodes" Nanomaterials 9, no. 5: 703. https://doi.org/10.3390/nano9050703



