A Review on Sulfonated Polymer Composite/Organic-Inorganic Hybrid Membranes to Address Methanol Barrier Issue for Methanol Fuel Cells
Abstract
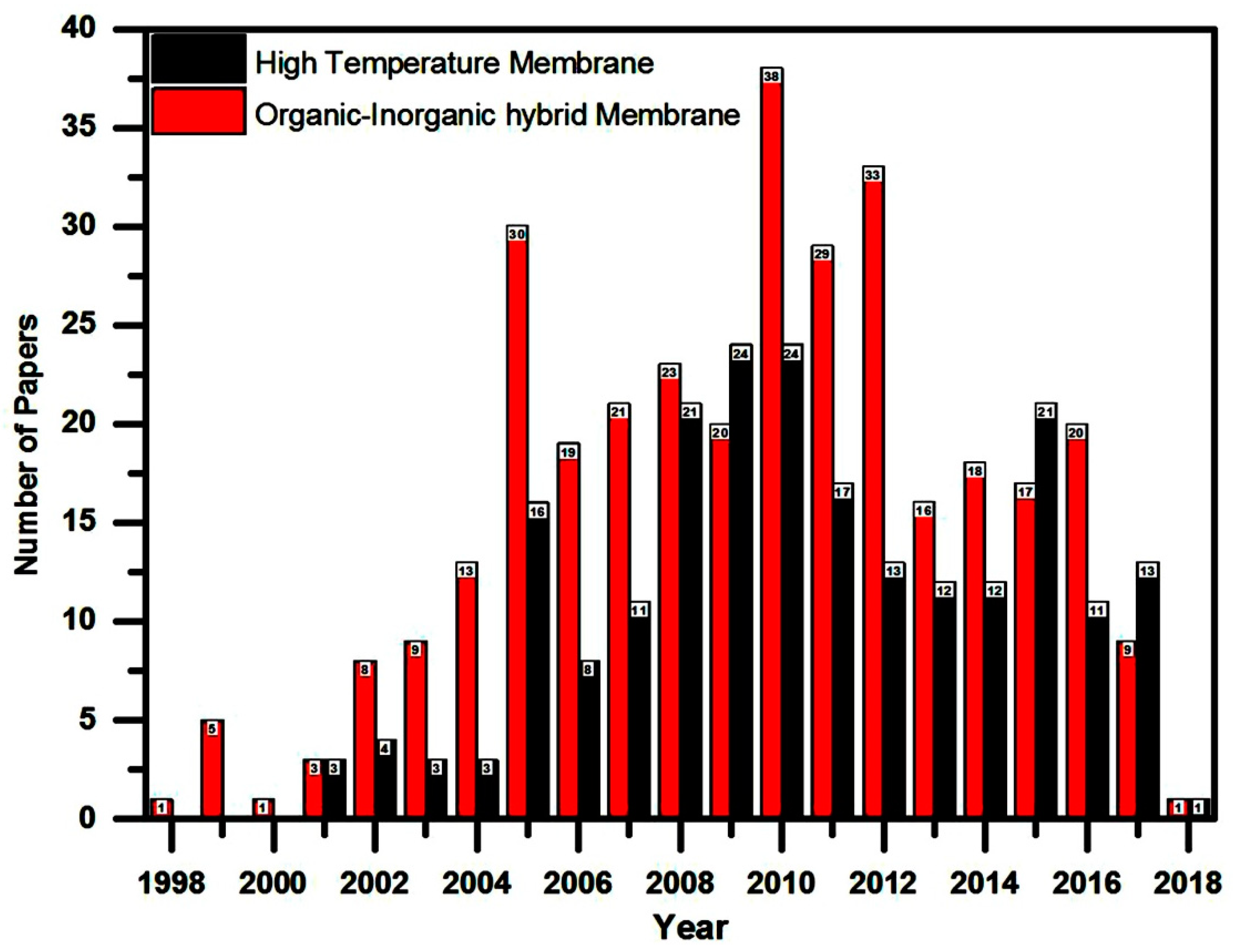
:1. Introduction
2. Composite Membranes
2.1. Sulfonated Composite Membranes
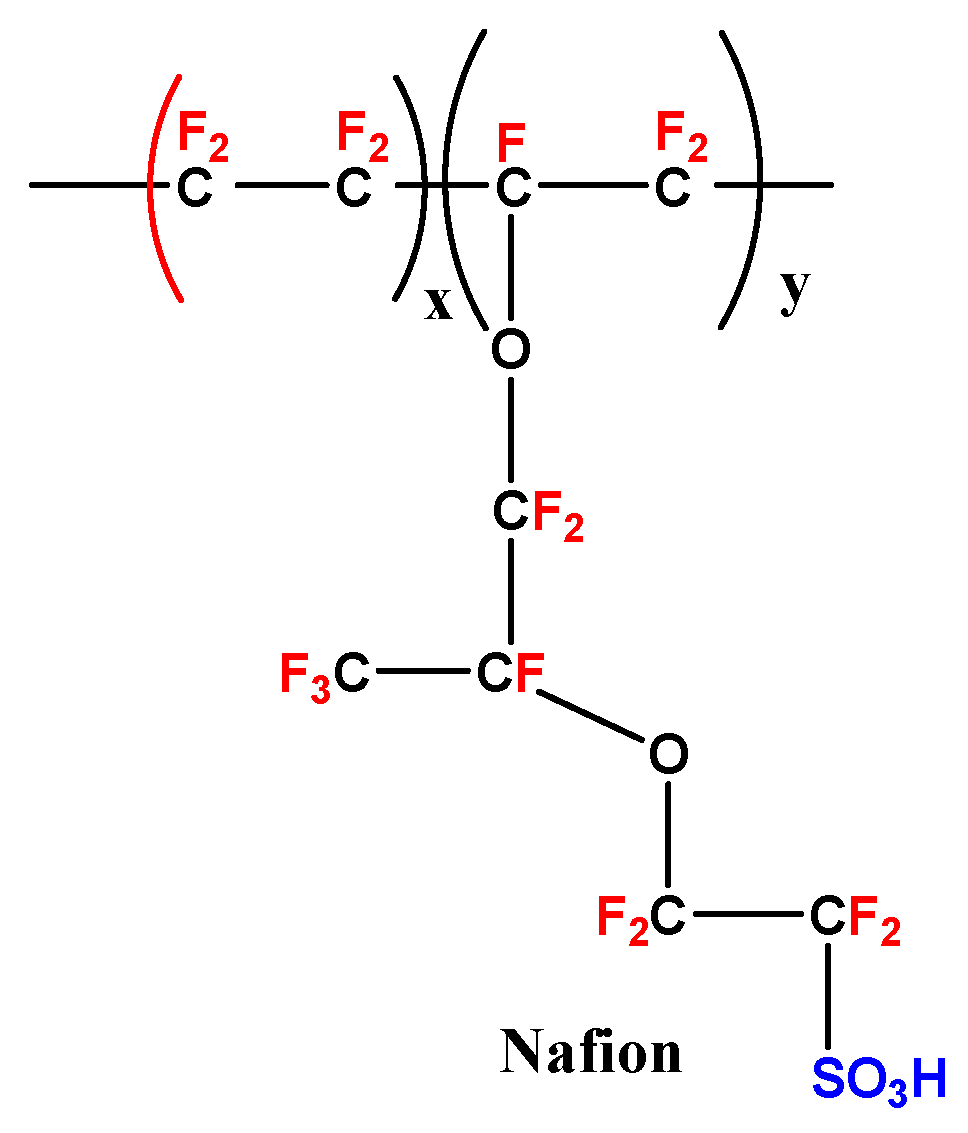
2.2. Sulfonated Fluoride Composite Membranes
2.3. Hydroxyl Sulfonated Composite Membranes
2.4. Sulfonated Chitosan Containing Composite Membranes
3. Sulfonated-Based Modified Membranes
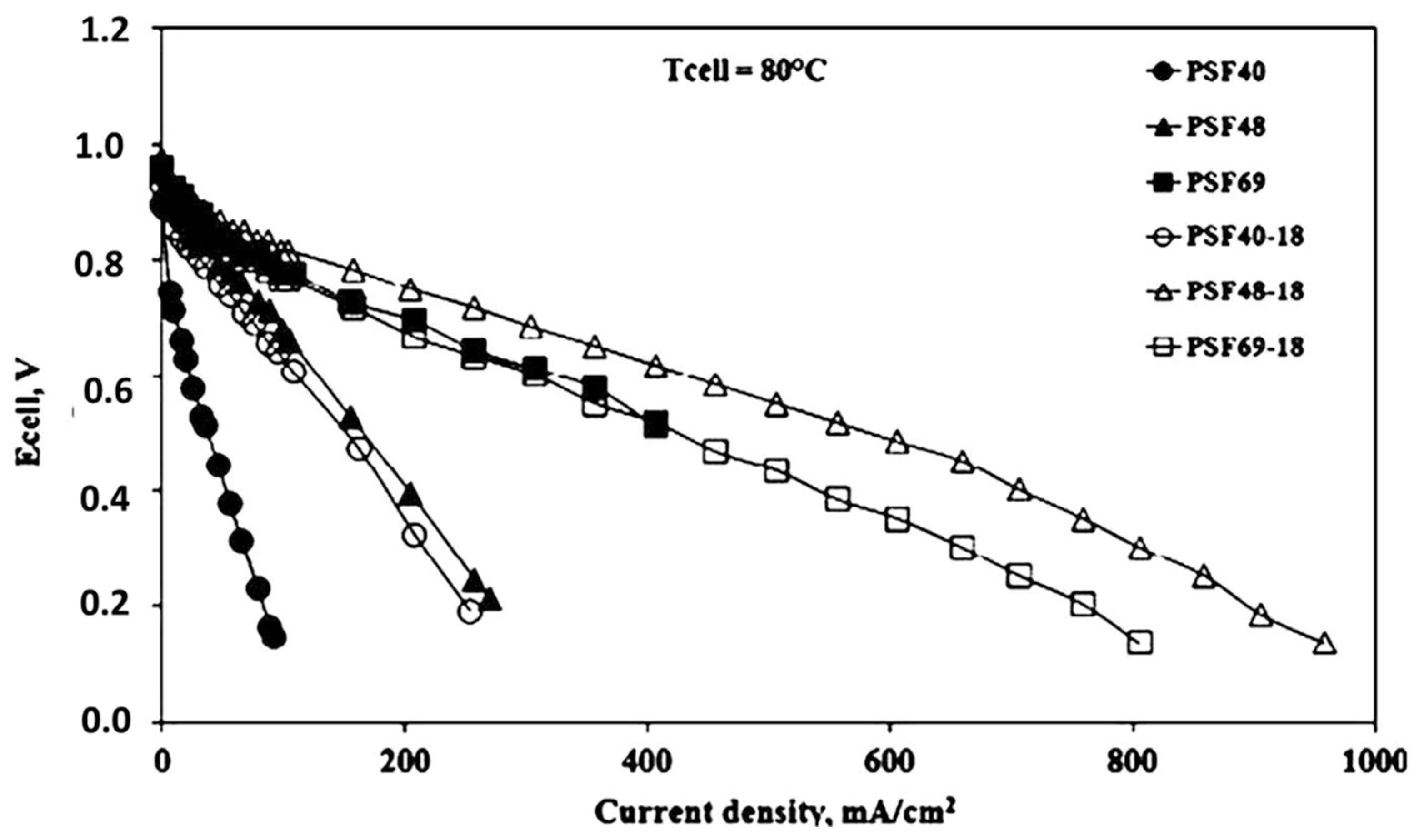
3.1. Sulfonated Organic Membranes
3.2. Sulfonated Inorganic Membranes
3.3. Sulfonated Organic–Inorganic Hybrid Membranes
4. Future Prospective
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEM | Alkaline anion exchange membrane |
| APTES | Aminopropyl Triethoxysilane |
| ATRA | Atom transfer radical addition |
| ADMFCs | Alkaline direct methanol fuel cells |
| Ba | Benzoxazine |
| BisPFPPO-OH | Bis ((p-hydroxy- tetrafluoro) phenyl) phenyl phosphine oxide |
| Bisphenol-AF | Bis (4-hydroxyphenyl) hexafluoropropane |
| BM | Blend membrane |
| BPPO | Bromomethylated poly phenylene oxide |
| BrPEEK | Bromomethylated poly ether ether ketone |
| cSMMs | Charged surface modified macromolecules |
| CS | Chitosan |
| CCSM 110 | Chitosan Sulfate Composite Membranes |
| C30B | Cloisite 30B |
| cSPAES | Cross-linked sulfonated poly arylene ether sulfone |
| DCDPS | Dichlorodiphenyl sulfone |
| DS | Degree of sulfonation |
| DMFCs | Direct methanol fuel cells |
| FSiO2 | Functionalization of silica |
| GA | Glutaraldehyde |
| GPTMS | Glycidoxy propyl trimethoxysilane |
| G-POSS | Glycidyl ether of polyhedral oligomeric silsesquioxanes |
| SGO | Sulfonated Graphene oxide |
| g-C3N4 | Graphitic carbon nitride |
| HTPEM | High temperature proton exchange membranes |
| HGMs | Hollow glass microspheres |
| IEC | Ion-exchange capacity |
| ICPTES | Isocyanatopropyl triethoxysilane |
| MEAs | Membrane electrode assemblies |
| MPTES | Mercaptopropyl triethoxysilane |
| MPTMS | Mercaptopropyl- trimethoxysilane |
| MSiSQ | Mesoporous organosilicate |
| MOR | Mordenite |
| NM | Nanocomposites membranes |
| NMP | N-methyl-2-pyrrolidone |
| NPHCs | N-phthaloyl chitosan |
| OCV | Open circuit voltage |
| PEM | Polymer electrolyte membrane |
| PEMFCs | Proton-exchange membrane fuel cells |
| PA | Phosphoric acid |
| PBa | Polybenzoxazine |
| PWA-IL | Phosphotungstate |
| PWA | Phosphotungstic Acid |
| AMPS | Poly 2-Acrylamido-2-Methyl Propanesulfonic Acid |
| ABPBI | Poly 2,5-Benzimidazole |
| AM-POSS | Octa Ammonium Polyhedral Oligosilsesquioxane |
| PES 70 | Poly Ether Sulfone 70 |
| PFPPO-OH | Bis (P-Hydroxy- Tetrafluoro) Phenyl) Phenyl Phosphine Oxide |
| PPEES | Poly Phenylene Ether Ether Sulfone |
| PSSA | Poly Styrene Sulfonic Acid |
| PVA | Poly Vinyl Alcohol |
| PVDF | Poly Vinylidene Fluoride |
| PVDF-HFP | Poly Vinylidene Fluoride-Co-Hexafluoro Propylene |
| PBI | Polybenzimidazole |
| PES | Polyethersulfone |
| PEFC | Polymer Electrolyte Fuel Cell |
| PSSA | Polystyrene Sulfonic Acid |
| PU | Polyurethane |
| PCS | Polyvinyl Alcohol/Chitosan |
| PVB | Polyvinyl Butyral |
| PEMFCs | Proton Exchange Membrane Fuel Cells |
| PEMs | Proton Exchange Membranes |
| PMAV | Poly Methacrylic Acid-2-Acrylamido- 2-Methyl-1-Propanesulfimponic Acid-Vinyltriethoxysilicone |
| QCS | Quaternized Chitosan |
| RH | Relative Humidity |
| Semi-IPN | Semi-Interpenetrating Network |
| Si-PWA | Silica Immobilized Phosphotungstic Acid |
| SisPS/A | Silicon-Containing Sulfonated Polystyrene/Acrylate |
| SFBC | Sulfonated Fluorinated Block Copolymer |
| SGO | Sulfonated Graphene oxide |
| SPVDF-co-HFP | Sulfonated Poly Vinylidene Fluoride-Co-Hexafluoro Propylene |
| SPEES | Sulfonated Poly1,4-Phenylene Ether-Ether-Sulfone |
| SPAES | Sulfonated Polyarylene Ether Ketones |
| SPAEKS | Sulfonated Poly Arylene Ether Ketone Sulfone |
| SPEEK | Sulfonated Poly Ether Ether Ketone |
| SPEKES | Sulfonated Poly Ether Ketone Ether Sulfone |
| SPES | Sulfonated Poly Ether Sulfone |
| CBrSPIBIs | Sulfonated Poly Imide- Benzimidazole |
| SPPO | Sulfonated Poly Phenylene Oxide |
| sPAEK | Sulfonated Poly Arylene Ether Ketone |
| SPAni | Sulfonated Polyaniline |
| SPI | Sulfonated Polyimide |
| SPIBI | Sulfonated Polyimides Containing Benzimidazole |
| s-Poly | Sulfonated Polymer |
| SPSU | Sulfonated Polysulfone |
| SSA | Sulfosuccinic Acid |
| SPSF | Sulphonated Polysulphone |
| TBT | Tetrabutyl Titanate |
| TEOS | Tetraethoxysilane |
| VBIm-Br | Vinyl-3-Butylimdazolium- Bromide |
| VPSIm-Cl | Vinyl-Propyl-Triethoxy Silane Imidazolium Chloride |
References
- Kamarudin, S.K.; Achmad, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrogen Energy 2009, 34, 6902–6916. [Google Scholar] [CrossRef]
- Ilbeygi, H.; Ghasemi, M.; Emadzadeh, D.; Ismail, A.F.; Zaidi, S.M.J.; Aljlil, S.A.; Jaafer, J.; Martin, D.; Keshani, S. Power generation and wastewater treatment using a novel SPEEK nanocomposite membrane in a dual chamber microbial fuel cell. Int. J. Hydrogen Energy 2015, 40, 477–487. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Hickner, M.A.; Einsla, B.; Harrison, W.L.; McGrath, J.E. Synthesis and characterization of partially disulfonated hydroquinone-based poly(arylene ether sulfone)s random copolymers for application as proton exchange membranes. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 384–391. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hickner, M.A.; Dong, L.; Pivovar, B.S.; McGrath, J.E. Sulfonated poly(arylene ether sulfone) copolymer proton exchange membranes: Composition and morphology effects on the methanol permeability. J. Membr. Sci. 2004, 243, 317–326. [Google Scholar] [CrossRef]
- Felix, C.; Jao, T.C.; Pasupathi, S.; Pollet, B.G. Optimisation of electrophoretic deposition parameters for gas diffusion electrodes in high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2013, 243, 40–47. [Google Scholar] [CrossRef]
- Musse Branco, C.; Sharma, S.; de Camargo Forte, M.M.; Steinberger-Wilckens, R. New approaches towards novel composite and multilayer membranes for intermediate temperature polymer electrolyte fuel cells and direct methanol fuel cells. J. Power Sources 2016, 316, 139–159. [Google Scholar] [CrossRef]
- Yang, C.; Srinivasan, S.; Arico, A.S.; Cretı, P.; Baglio, V.; Antonucci, V. Composite Nafion/Zirconium Phosphate Membranes for Direct Methanol Fuel Cell Operation at High Temperature. Electrochem. Solid State Lett. 2001, 4, A31–A34. [Google Scholar] [CrossRef]
- Gamburzev, S.; Appleby, A.J. Recent progress in performance improvement of the proton exchange membrane fuel cell (PEMFC). J. Power Sources 2007, 107, 5–12. [Google Scholar] [CrossRef]
- Rikukawa, M.; Sanui, K. Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog. Polym. Sci. 2000, 25, 1463–1502. [Google Scholar] [CrossRef]
- Shoesmith, J.P.; Collins, R.D.; Oakley, M.J.; Stevenson, D.K. Status of solid polymer fuel cell system development. J. Power Sources 1994, 49, 129–142. [Google Scholar] [CrossRef]
- Kim, D.S.; Robertson, G.P.; Guiver, M.D.; Lee, Y.M. Synthesis of highly fluorinated poly(arylene ether)s copolymers for proton exchange membrane materials. J. Membr. Sci. 2006, 281, 111–120. [Google Scholar] [CrossRef]
- Neelakandan, S.; Kanagaraj, P.; Sabarathinam, R.M.; Nagendran, A. Polypyrrole layered SPEES/TPA proton exchange membrane for direct methanol fuel cells. Appl. Surf. Sci. 2015, 359, 272–279. [Google Scholar] [CrossRef]
- Schonert, M.; Jakoby, K.; Schlumbohm, C.; Glusen, A.; Mergel, J.; Stolten, D. Manufacture of robust catalyst layers for the DMFC. Fuel Cells 2004, 4, 175–179. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Colpan, C.O.; Cruickshank, C.A.; Matida, E.; Hamdullahpur, F. 1D modeling of a flowing electrolyte-direct methanol fuel cell. J. Power Sources 2011, 196, 3572–3582. [Google Scholar] [CrossRef]
- Liang, W.; Tongwen, X.; Dan, W.; Xin, Z. Preparation and characterization of CPPO/BPPO blend membranes for potential application in alkaline direct methanol fuel cell. J. Membr. Sci. 2008, 310, 577–585. [Google Scholar]
- Prabhuram, J.; Manoharan, R. Investigation of methanol oxidation on unsupported platinum electrodes in strong alkali and strong acid. J. Power Sources 1998, 74, 54–61. [Google Scholar] [CrossRef]
- Tripkovic, A.V.; Popovic, K.D.; Grgur, B.N.; Blizanac, B.; Ross, P.N.; Markovic, N.M. Methanol electro- oxidation on supported Pt and PtRu catalysts in acid and alkaline solutions. Electrochim. Acta 2002, 47, 3707–3714. [Google Scholar] [CrossRef]
- Yang, C.; Srinivasan, S.; Bocarsly, A.B. A comparison of physical properties and fuel cell performance of Nafion and zirconium phosphate/Nafion composite membranes. J. Membr. Sci. 2004, 237, 145–161. [Google Scholar] [CrossRef]
- Scott, K.; Taama, W.M.; Argyropoulos, P. Performance of the direct methanol fuel cell with radiation-grafted polymer membranes. J. Membr. Sci. 2000, 171, 119–130. [Google Scholar] [CrossRef]
- Dimitrova, P.; Friedrich, K.A.; Vogt, B.; Stimming, U. Transport properties of ionomer composite membranes for direct methanol fuel cells. J. Electroanal. Chem. 2002, 532, 75–83. [Google Scholar] [CrossRef]
- Barragan, V.M.; Heinzel, A. Estimation of the membrane methanol diffusion coefficient from open circuit voltage measurements in a direct methanol fuel cell. J. Power Sources 2002, 104, 66–72. [Google Scholar] [CrossRef]
- Qi, Z.; Kaufman, A. Open circuit voltage and methanol crossover in DMFCs. J. Power Sources 2002, 110, 177–185. [Google Scholar] [CrossRef]
- Scott, K.; Taama, W.; Cruickshank, J. Performance and modelling of a direct methanol solid polymer electrolyte fuel cell. J. Power Sources 1997, 65, 159–171. [Google Scholar] [CrossRef]
- Gurauand, B.; Smotkin, E.S. Methanol crossover in direct methanol fuel cells: A link between power and energy density. J. Power Sources 2002, 112, 339–352. [Google Scholar]
- Basri, S.; Kamarudin, S.K.; Daud, W.R.W.; Yaakub, Z. Nanocatalyst for direct methanol fuel cell (DMFC). Int. J. Hydrogen Energy 2012, 35, 7957–7970. [Google Scholar] [CrossRef]
- Neburchilov, V.; Martin, J.; Wang, H.J.; Zhang, J.J. A review of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 2007, 169, 221–238. [Google Scholar] [CrossRef]
- Kerres, J.A. Development of ionomer membranes for fuel cells. J. Membr. Sci. 2001, 185, 3–27. [Google Scholar] [CrossRef]
- Tsai, J.-C.; Lin, C.-K.; Kuo, J.-F.; Chen, C.-Y. Preparation and properties of cross- linked sulphonated poly(arylene ether sulphone) blends for direct methanol fuel cell applications. J. Power Sources 2010, 195, 4072–4079. [Google Scholar] [CrossRef]
- Liu, B.; Robertson, G.; Kim, D.; Guiver, M.; Hu, W.; Jiang, Z. Aromatic poly(ether ketone)s with pendant sulfonic acid phenyl groups prepared by a mild sulfonation method for proton exchange membranes. Macromolecules 2007, 40, 1934–1944. [Google Scholar] [CrossRef]
- Jiang, R.; Kunz, H.R.; Fenton, J.M. Composite silica/Nafion® membranes prepared by tetraethylorthosilicate sol–gel reaction and solution casting for direct methanol fuel cells. J. Membr. Sci. 2006, 272, 116–124. [Google Scholar] [CrossRef]
- Song, M.K.; Kim, Y.T.; Fenton, J.M.; Russell Kunz, H.; Rhee, H.W. Chemically-modified Nafion®/poly (vinylidene fluoride) blend ionomers for proton exchange membrane fuel cells. J. Power Sources 2003, 117, 14–21. [Google Scholar] [CrossRef]
- Shao, Z.G.; Wang, X.; Hsing, I.M. Composite Nafion/polyvinyl alcohol membranes for the direct methanol fuel cell. J. Membr. Sci. 2002, 210, 147–153. [Google Scholar] [CrossRef]
- Shao, Z.G.; Hsing, I.M. Nafion Membrane Coated with Sulfonated Poly(vinyl alcohol) Nafion Film for Direct Methanol Fuel Cells. Electrochem. Solid State 2002, 5, A185–A187. [Google Scholar] [CrossRef]
- Shim, J.; Ha, H.Y.; Hong, S.A.; Oh, I.H. Characteristics of the Nafion ionomer-impregnated composite membrane for polymer electrolyte fuel cells. J. Power Sources 2002, 109, 412–417. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Wang, S. Study on the preparation and properties of pvdf-composite nafion membranes. Acta Polym. Sin. 2002, 4, 540–543. [Google Scholar]
- Lin, J.C.; Ouyang, M.; Fenton, J.M.; Kunz, H.R.; Koberstein, J.T. Study of blend membranes consisting of NafionR and vinylidene fluoride–hexafluoropropylene copolymer. J. Appl. Polym. Sci. 1998, 70, 121–127. [Google Scholar] [CrossRef]
- Jagur-Grodzinki, J. Polymeric materials for fuel cells: Concise review of recent studies. Polym. Adv. Technol. 2007, 18, 785–799. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Wang, Y. Sulfonated poly(ether ether ketone) membranes for direct methanol fuel cell. J. Membr. Sci. 2003, 226, 159–167. [Google Scholar] [CrossRef]
- Woo, Y.; Oh, S.Y.; Kang, Y.S.; Jung, B. Synthesis and characterization of sulfonated polyimide membranes for direct methanol fuel cell. J. Membr. Sci. 2003, 220, 31–45. [Google Scholar] [CrossRef]
- Deluca, N.W.; Elabd, Y.A. Polymer electrolyte membranes for the direct methanol fuel cell: A review. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2201–2225. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef]
- Liu, K.L.; Lee, H.C.; Wang, B.Y.; Lue, S.J.; Lu, C.Y.; Tsai, L.D.; Fang, J.; Chao, C.Y. Sulfonated poly(styrene-block-(ethylene-ran-butylene)-block-styrene (SSEBS)-zirconium phosphate (ZrP) composite membranes for direct methanol fuel cells. J. Membr. Sci. 2015, 495, 110–120. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Krishnan, P.; Park, J.S.; Kim, C.S. Preparation and characterization of protonconducting sulfonated poly(ether ether ketone)/phosphatoantimonic acid composite membranes. Eur. Polym. J. 2007, 43, 4019–4027. [Google Scholar] [CrossRef]
- Heinzel, A.; Barragan, V.M. A review of the state-of-the-art of the methanol crossover in direct methanol fuel cells. J. Power Sources 1999, 84, 70–74. [Google Scholar] [CrossRef]
- Chun, J.H.; Kim, S.G.; Lee, J.Y.; Hyeon, D.H.; Chun, B.-H.; Kim, S.H.; Park, K.T. Cross-linked sulfonated poly(arylene ether sulfone)/silica hybrid membranes for high temperature proton exchange membrane fuel cells. Renew. Energy 2013, 51, 22–28. [Google Scholar] [CrossRef]
- Lee, H.S.; Roy, A.; Lane, O.; Dunn, S.; McGrath, J.E. Hydrophilicehydrophobic multiblock copolymers based on poly(arylene ether sulfone) via low temperature coupling reactions for proton exchange membrane fuel cells. Polymer 2008, 49, 715–723. [Google Scholar] [CrossRef]
- Lee, J.K.; Li, W.; Manthiram, A. Poly(arylene ether sulfone)s containing pendant sulfonic acid groups as membrane materials for direct methanol fuel cells. J. Membr. Sci. 2009, 330, 73–79. [Google Scholar] [CrossRef]
- Badami, A.S.; Lane, O.; Lee, H.S.; Roy, A.; McGrath, J.E. Fundamental investigations of the effect of the linkage group on the behavior of hydrophilic-hydrophobic poly(arylene ether sulfone) multiblock copolymers for proton exchange membrane fuel cells. J. Membr. Sci. 2009, 333, 1–11. [Google Scholar] [CrossRef]
- Goh, Y.T.; Patel, R.; Im, S.J.; Kim, J.H.; Min, B.R. Synthesis and characterization of poly(ether sulfone) grafted poly(styrene sulfonic acid) for proton conducting membranes. Korean J. Chem. Eng. 2009, 26, 518–522. [Google Scholar] [CrossRef]
- Neelakandan, S.; Kanagaraj, P.; Nagendran, A.; Rana, D.; Matsuura, T.; Muthumeenal, A. Enhancing proton conduction of sulfonated poly (phenylene etherether sulfone) membrane by charged surface modifying macromolecules for H2/O2 fuel cells. Renew. Energy 2015, 78, 306–313. [Google Scholar] [CrossRef]
- Jin, L.; Li, Z.; Wang, S.; Wang, Z.; Dong, F.; Yin, X. Highly conductive proton exchange membranes based on sulfonated poly (phthalazinone ether sulfone) an cerium sulfophenyl phosphate. React. Funct. Polym. 2012, 72, 549–555. [Google Scholar] [CrossRef]
- Jisu, C.; Dong, H.K.; Hyung, K.K.; Chongkyu, S.; Sung, C.K. Polymer blend membranes of sulfonated poly(arylene ether ketone) for direct methanol fuel cell. J. Membr. Sci. 2008, 310, 384–392. [Google Scholar]
- Bi, H.; Wang, J.; Chen, S.; Hua, Z.; Gao, Z.; Wang, L.; Okamoto, K. Preparation and properties of cross-linked sulfonated poly(arylene ether sulfone)/sulfonated polyimide blend membranes for fuel cell application. J. Membr. Sci. 2010, 350, 109–116. [Google Scholar] [CrossRef]
- Pedicini, R.; Saccà, A.; Carbone, A.; Gatto, I.; Patti, A.; Passalacqua, E. Study on sulphonated polysulphone/polyurethane blend membranes for fuel cell applications. Chem. Phys. Lett. 2013, 579, 100–104. [Google Scholar] [CrossRef]
- Zhao, C.; He, D.; Li, Y.; Xiang, J.; Li, P.; Sue, H.J. High performance proton exchange membranes for direct methanol fuel cells based on a SPEEK/polybenzoxazine cross-linked structure. RSC Adv. 2015, 5, 47284–47293. [Google Scholar] [CrossRef]
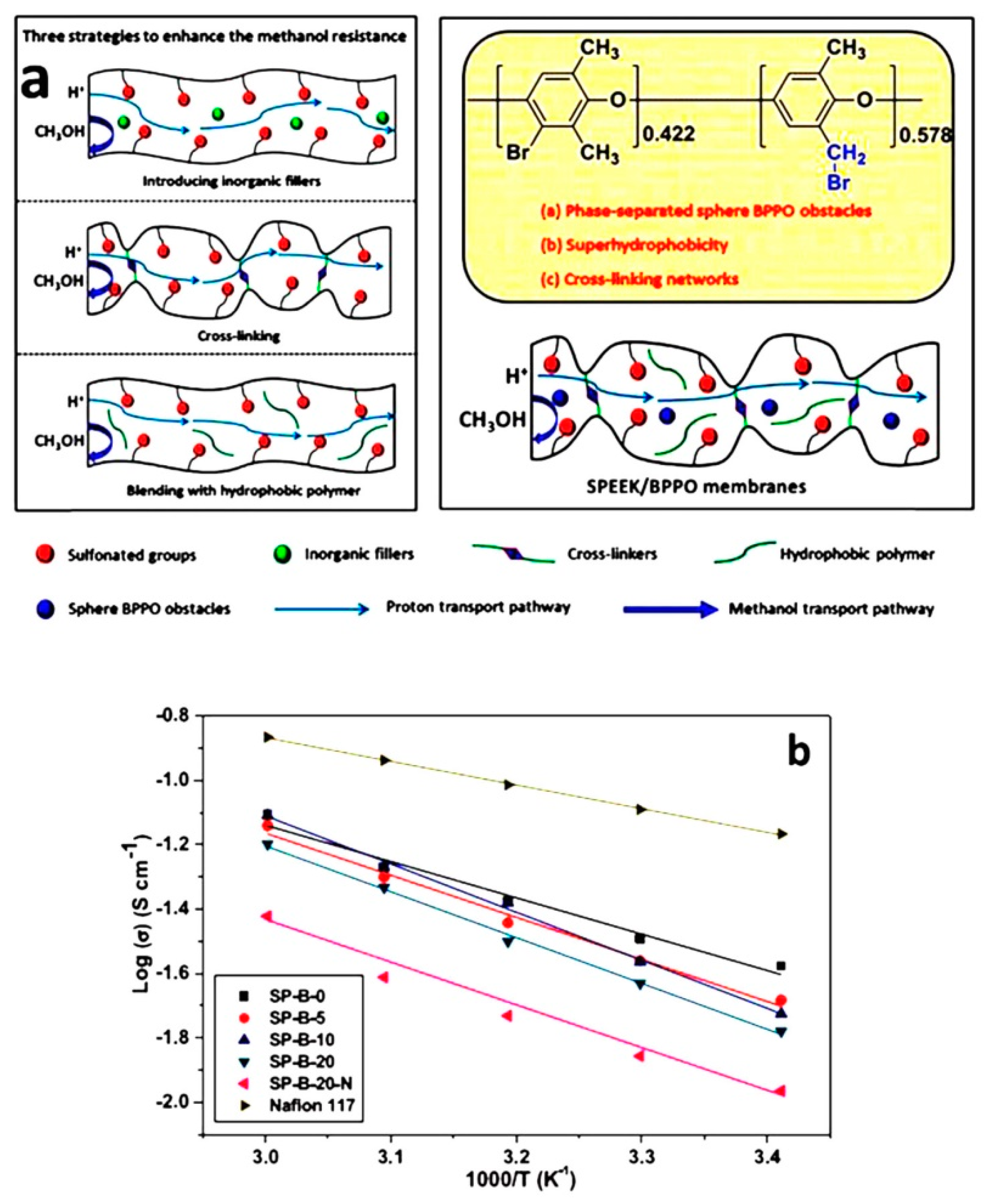
- Liu, X.; Zhang, Y.; Chen, Y.; Li, C.; Dong, J.; Zhang, Q.; Wang, J.; Yang, Z.; Cheng, H. A superhydrophobic bromomethylated poly(phenyleneoxide) as a multi- functional polymer filler in SPEEK membrane towards neat methanol operation of direct methanol fuel cells. J. Membr. Sci. 2017, 544, 58–67. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, C.H.; Guiver, M.D.; Lee, Y.M. Sulfonated hydrocarbon membranes for medium-temperature and low-humidity proton exchange membrane fuel cells (PEMFCs). Prog. Polym. Sci. 2011, 36, 1443–1498. [Google Scholar] [CrossRef]
- Ruichun, J.; Timothy, F.; Shelly, B.; Craig, G. Perfluorocyclobutane and poly(vinylidene fluoride) blend membranes for fuel cells. Electrochim. Acta 2013, 110, 306–315. [Google Scholar]
- Gnana kumar, G.; Kim, P.; Kim, A.; Nahm, K.S.; Nimma Elizabeth, R. Structural, thermal and ion transport studies of different particle size nano composite fillers incorporated PVdF-co-HFP hybrid membranes. Mater. Chem. Phys. 2009, 115, 40–46. [Google Scholar] [CrossRef]
- Tulay, Y.; Inan, H.D.; Elif, E.; Unveren, E.E. Sulfonated PEEK and fluorinated polymer based blends for fuel cell applications: Investigation of the effect of type and molecular weight of the fluorinated polymers on the membrane’s properties. Int. J. Hydrogen Energy 2010, 35, 12038–12053. [Google Scholar]
- Deuk, J.K.; Hye, J.L.; Sang, Y.N. Sulfonated poly(arylene ether sulfone) membranes blended with hydrophobic polymers for direct methanol fuel cell applications. Int. J. Hydrogen Energy 2013, 39, 17524–17532. [Google Scholar]
- Unveren, E.E.; Inan, T.Y.; Çelebi, S.S. Partially sulfonated poly(1,4-phenylene ether-ether-sulfone) and poly(vinylidene fluoride) blend membranes for fuel cells. Fuel Cells 2013, 13, 862–872. [Google Scholar] [CrossRef]
- Kingshuk, D.; Suparna, D.; Patit, P.K. Low methanol permeable and highly selective membranes composed of pure and/or partially sulfonated PVdF-co-HFP and polyaniline. J. Membr. Sci. 2014, 468, 42–51. [Google Scholar]
- Merve, G.S.; Emre, B.; Tülay, Y.I.; Nilhan, K.A.; Atilla, G. Synthesis and fuel cell characterization of blend membranes from phenyl phosphine oxide containing flou- rinated novel polymers. J. Power Sources 2014, 271, 465–479. [Google Scholar]
- Prasad, M.; Smita, M.; Sanjay, N.K. Polymer electrolyte membranes from Cloisite30Bbased solid proton conductor and sulfonated polyether ether ketone/polyvinylidene fluoride-cohexafluoro propylene blend s for direct methanol fuel cells. RSC Adv. 2014, 4, 61178–61186. [Google Scholar] [CrossRef]
- Kumar, P.; Dutta, K.; Das, S.; Kundu, P.P. Membrane prepared by incorporation of cross-linked sulfonated polystyrene in the blend of PVdF-co-HFP/Nafion: A preliminary evaluation for application in DMFC. Appl. Energy 2014, 123, 66–74. [Google Scholar] [CrossRef]
- Liu, X.; Meng, X.; Wu, J.; Huo, J.; Cui, L.; Zhou, Q. Microstructure and Properties of Novel SPEEK/PVDF-g-PSSA blends for Proton Exchange Membrane with Improved Compatibility. RSC Adv. 2015, 5, 69621–69628. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Hazra, S.; Kundu, P.P. Partially Sulfonated Poly(vinylidene fluoride) Induced Enhancements of Properties and DMFC Performance of Nafion Electrolyte Membrane. Fuel Cells 2015, 15, 505–515. [Google Scholar] [CrossRef]
- Mondal, S.; Soam, S.; Kundu, P.P. Reduction of methanol crossover and improved electrical efficiency in direct methanol fuel cell by the formation of a thin layer on Nafion 117 membrane: Effect of dip-coating of a blend of sulphonated PVdF-co-HFP and PBI. J. Membr. Sci. 2015, 474, 140–147. [Google Scholar] [CrossRef]
- Bagheri, A.; Javanbakht, M.; Beydaghi, H.; Salarizadeh, P.; Shabanikia, A.; Amoli, H.S. Sulfonated poly(etheretherketone) and sulfonated polyvinylidene fluoride-co- hexafluoro- propylene based blend proton exchange membranes for direct methanol fuel cell applications. RSC Adv. 2016, 6, 39500–39510. [Google Scholar] [CrossRef]
- Uma, D.; Muthumeenal, A.; Sabarathinam, R.M.; Nagendran, A. Fabrication and electrochemical properties of SPVdF-co-HFP/SPES blend proton exchange membranes for direct methanol fuel cells. Renew. Energy 2017, 102, 258–265. [Google Scholar]
- Kim, A.R.; Vinothkannan, M.; Kim, J.S.; Yoo, D.J. Proton-conducting phosphotungstic acid/sulfonated fluorinated block copolymer composite membrane for polymer electrolyte fuel cells with reduced hydrogen permeability. Polym. Bull. 2018, 75, 2779–2804. [Google Scholar] [CrossRef]
- Liang, Y.; Gong, C.; Qi, Z.; Li, H.; Wu, Z.; Zhang, Y.; Zhang, S.; Li, Y. Intermolecular ionic cross-linked sulfonated poly(ether ether ketone) membranes containing diazafluorene for direct methanol fuel cell applications. J. Power Sources 2015, 284, 86–94. [Google Scholar] [CrossRef]
- Attaran, A.M.; Javanbakht, M.; Hooshyari, K.; Enhessari, M. New proton conducting nanocomposite membranes based on poly vinylalcohol/poly vinyl pyrrolidone/BaZrO3 for proton exchange membrane fuel cells. Solid State Ionics 2015, 269, 98–105. [Google Scholar] [CrossRef]
- Liu, C.; Dai, C.; Chao, C.; Chang, S. Novel proton exchange membrane based on cross-linked PVA for direct methanol fuel cells. J. Power Sources 2014, 249, 285–298. [Google Scholar] [CrossRef]
- Molla, S.; Compan, V. Polymer blends of SPEEK for DMFC application at intermediate temperatures. Int. J. Hydrogen Energy 2014, 39, 5121–5136. [Google Scholar] [CrossRef]
- Lin, H.; Wang, S. Nafion/poly(vinyl alcohol) nano-fiber composite and Nafion/poly(vinyl alcohol) blend membranes for direct methanol fuel cells. J. Membr. Sci. 2014, 452, 253–262. [Google Scholar] [CrossRef]
- Yang, T. Preliminary study of SPEEK/PVA blend membranes for DMFC applications. Int. J. Hydrogen Energy 2008, 33, 6772–6779. [Google Scholar] [CrossRef]
- Bhat, S.D.; Sahu, A.K.; George, C.; Pitchumani, S.; Sridhar, P.; Chandrakumar, N.; Singh, K.K.; Krishna, N.; Shukla, A.K. Mordenite-incorporated PVA–PSSA membranes as electrolytes for DMFCs. J. Membr. Sci. 2009, 340, 73–83. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Amirinejad, S.; Amirinejad, M. Phosphotungstic acid doped poly(vinyl alcohol)/poly(ether sulfone) blend composite membranes for direct methanol fuel cells. J. Membr. Sci. 2011, 380, 132–137. [Google Scholar] [CrossRef]
- Yang, J.; Wang, N.; Chiu, H. Preparation and characterization of poly(vinyl alcohol)/sodium alginate blended membrane for alkaline solid polymer electrolytes membrane. J. Membr. Sci. 2014, 457, 139–148. [Google Scholar] [CrossRef]
- Mollá, S.; Compañ, V. Nanocomposite SPEEK-based membranes for direct methanol fuel cells at intermediate temperatures. J. Membr. Sci. 2015, 492, 123–136. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M.; Bagheri, A.; Salarizadeh, P.; Zahmatkesh, H.G.; Kashefi, S.; Kowsari, E. Novel nanocomposite membranes based on blended sulfonated poly(ether ether ketone)/poly(vinyl alcohol) containing sulfonated graphene oxide/Fe3O4 nanosheets for DMFC applications. RSC Adv. 2015, 5, 74054–74064. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Tam Bui, V. Chitosan-based electrolyte composite membranes: II mechanical properties and ionic conductivity. J. Membr. Sci. 2006, 284, 331–338. [Google Scholar] [CrossRef]
- Muthumeenal, A.; Neelakandan, S.; Kanagaraj, P.; Nagendran, A. Synthesis and properties of novel proton exchange membranes based on sulfonated polyethersulfone and N-phthaloyl chitosan blends for DMFC applications. Renew. Energy 2016, 86, 922–929. [Google Scholar] [CrossRef]
- Meenakshi, S.; Bhat, S.D.; Sahu, A.K.; Sridhar, P.; Pitchumani, S.; Shukla, A.K. Chitosan-polyvinyl alcohol-sulfonated polyethersulfone mixed-matrix membranes as methanol-barrier electrolytes for DMFCs. J. Appl. Polym. Sci. 2012, 124, e73–e82. [Google Scholar] [CrossRef]
- Yang, J.M.; Chiu, H.C. Preparation and characterization of polyvinyl alcohol/chitosan blended membrane for alkaline direct methanol fuel cells. J. Membr. Sci. 2012, 419–420, 65–71. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, M.; Guo, Z.; Cui, Z. Alternatively chitosan sulfate blending membrane as methanol-blocking polymer electrolyte membrane for direct methanol fuel cell. J. Membr. Sci. 2009, 337, 318–323. [Google Scholar] [CrossRef]
- Lee, S.; Lim, Y.; Hossain, M.A.; Jang, H.; Jeon, Y.; Lee, S.; Jin, L.; Kim, W. Synthesis and properties of grafting sulfonated polymer containing isatin by super acid-catalyzed polyhydroxyalkylation reaction for PEMFC. Renew. Energy 2015, 79, 72–77. [Google Scholar] [CrossRef]
- Allcock, H.R.; Hofmann, M.A.; Ambler, C.M.; Lvov, S.N.; Zhou, X.Y.; Chalkova, E. Phenylphosphonic acid functionalized poly[aryloxyphosphazenes] as proton-conducting membranes for direct methanol fuel cells. J. Membr. Sci. 2002, 201, 47–54. [Google Scholar] [CrossRef]
- Jones, D.J.; Roziere, J. Recent advances in the functionalization of polybenzimidazole and polyetherketone for fuel cell applications. J. Membr. Sci. 2001, 185, 41–58. [Google Scholar] [CrossRef]
- Mohd Norddin, M.N.A.; Ismail, A.F.; Rana, D.; Matsuura, T.; Mustafa, A.; Tabe Mohammadi, A. Characterization and performance of proton exchange membranes for direct methanol fuel cell: Blending of sulfonated poly(ether ether ketone) with charged surface modifying macromolecule. J. Membr. Sci. 2008, 323, 404–413. [Google Scholar] [CrossRef]
- Mohd Norddin, M.N.A.; Ismail, A.F.; Rana, D.; Matsuura, T.; Tabe, S. The effect blending sulfonated poly(ether ether ketone) with various charged surface modifying macromolecules on proton exchange membrane performance. J. Membr. Sci. 2009, 328, 148–155. [Google Scholar] [CrossRef]
- Krishnan, N.N.; Lee, H.-J.; Kim, H.-J.; Kim, J.-Y.; Hwang, I.; Jang, J.H.; Cho, E.A.; Kim, S.-K.; Henkensmeier, D.; Hong, S.-A.; et al. Sulfonated poly(ether sulfone)/sulfonated polybenzimidazole blend membrane for fuel cell applications. Eur. Polym. J. 2010, 46, 1633–1641. [Google Scholar] [CrossRef]
- Yang, T.; Liu, C. SPEEK/sulfonated cyclodextrin blend membranes for direct methanol fuel cell. Int. J. Hydrogen Energy 2011, 36, 5666–5674. [Google Scholar] [CrossRef]
- Jithunsaa, M.; Tashiro, K.; Nunes, S.P.; Chirachanchai, S. Poly(acrylic acid-co-4-vinylimidazole)/Sulfonated poly(ether ether ketone) composite membranes: A role of polymer chain with proton acceptor and donor for enhancing proton transfer in anhydrous system. Int. J. Hydrogen Energy 2011, 36, 10384–10391. [Google Scholar] [CrossRef]
- Yue, Z.; Cai, Y.B.; Xu, S. Phosphoric acid-doped cross-linked sulfonated poly(imide-benzimidazole) for proton exchange membrane fuel cell applications. J. Membr. Sci. 2016, 501, 220–227. [Google Scholar] [CrossRef]
- Alvarez, A.; Guzmán, C.; Carbone, A.; Saccà, A.; Gatto, I.; Pedicini, R.; Passalacqua, E.; Nava, R.; Ornelas, R.; Ledesma-Garcia, J.; et al. Composite membranes based on micro and mesostructured silica: A comparison of physicochemical and transport properties. J. Power Sources 2011, 196, 5394–5401. [Google Scholar] [CrossRef]
- Sacca, A.; Carbone, A.; Gatto, I.; Pedicini, R.; Freni, A.; Patti, A.; Passalacqua, E. Composites Nafion-titania membranes for Polymer Electrolyte Fuel Cell (PEFC) applications at low relative humidity levels: Chemical physical properties and electrochemical performance. Polym. Test. 2016, 56, 10–18. [Google Scholar] [CrossRef]
- Rico-Zavala, A.; Gurrola, M.P.; Arriaga, L.G.; Jennifer, B.A.; Alvarez-Contreras, L.; Carbone, A.; Sacca, A.; Fabio Matera, V.; Pedicini, R.; Alvarez, A.; et al. Synthesis and characterization of composite membranes modified with Halloysite nanotubes and phosphotungstic acid for electrochemical hydrogen pumps. Renew. Energy 2018, 122, 163–172. [Google Scholar] [CrossRef]
- Lu, C.; Chang, C.; Guo, Y.; Yeh, T.; Su, Y.; Wang, P.; Hsueh, K.; Tseng, F. High-performance and low-leakage phosphoric acid fuel cell with synergic composite membrane stacking of micro glass microfiber and nano PTFE. Renew. Energy 2019, 134, 982–988. [Google Scholar] [CrossRef]
- Changkhamchom, S.; Sirivat, A. Polymer Electrolyte Composite Membrane Based on Molecular Sieve 13X Mixed with Sulfonated Poly(ether ketone ether sulfone)/ Poly (phenylene ether ether sulfone) blended Membrane for Use in Direct Methanol Fuel Cell. Adv. Polym. Technol. 2017, 36, 385–391. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Xu, J.; Xu, L.; Liu, F.; Tian, X. Organic-inorganic composite membrane based on sulfonated poly (arylene ether ketone sulfone) with excellent long-term stability for proton exchange membrane fuel cells. J. Membr. Sci. 2017, 529, 243–251. [Google Scholar] [CrossRef]
- Lu-Lu, W.; Wang, J.; Zhang, Y.; Feng, R. Alkaline hybrid composite membrane for direct methanol fuel cells application. J. Electroanal. Chem. 2015, 759, 174–183. [Google Scholar]
- Shabanikia, A.; Javanbakht, M.; Amoli, H.S.; Hooshyari, K.; Enhessari, M. Polybenzimidazole/strontium cerate nanocomposites with enhanced proton conductivity for proton exchange membrane fuel cells operating at high temperature. Electrochim. Acta 2015, 154, 370–378. [Google Scholar] [CrossRef]
- de Bonis, C.; Cozzi, D.; Mecheri, B.; D’Epifanio, A.; Rainer, A.; de Porcellinis, D.; Licoccia, S. Effect of filler surface functionalization on the performance of Nafion/Titanium oxide composite membranes. Electrochim. Acta 2014, 147, 418–425. [Google Scholar] [CrossRef]
- Pandey, J.; Mir, F.Q.; Shukla, A. Performance of PVDF supported silica immobilized phosphotungstic acid membrane (Si-PWA/PVDF) in direct methanol fuel cell. Int. J. Hydrogen Energy 2014, 39, 17306–17313. [Google Scholar] [CrossRef]
- Pandey, J.; Shukla, A. PVDF supported silica immobilized phosphotungstic acid membrane for DMFC application. Solid State Ionics 2014, 262, 811–814. [Google Scholar] [CrossRef]
- Zhong, S.; Cui, X.; Sun, C.; Dou, S.; Liu, W. Cross-linked organic/inorganic proton exchange membranes with multilayer structure. Solid State Ionics 2012, 227, 91–95. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Hasran, U.A.; Daud, W.R.W. A novel hybrid Nafion-PBI-ZP membrane for direct methanol fuel cells. Int. J. Hydrogen Energy 2011, 36, 14668–14677. [Google Scholar] [CrossRef]
- Khan, M.A.; Kumar, M.; Alothman, Z.A. Preparation and characterization of organic–inorganic hybrid anion-exchange membranes for electrodialysis. J. Ind. Eng. Chem. 2015, 21, 723–730. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Li, Y.; Xu, T.; Fu, Y. PVA–silica anion-exchange hybrid membranes prepared through a copolymer crosslinking agent. J. Membr. Sci. 2010, 350, 322–332. [Google Scholar] [CrossRef]
- Kumari, M.; Sodaye, H.S.; Bindal, R.C. Cross-linked sulfonated poly(ether ether ketone)-poly ethylene glycol/silica organic–inorganic nanocomposite membrane for fuel cell application. J. Power Sources 2018, 398, 137–148. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; JinYoo, D. Artificially designed, low humidifying organic–inorganic (SFBC-50/FSiO2) composite membrane for electrolyte applications of fuel cells. Compos. Part B Eng. 2017, 130, 103–118. [Google Scholar] [CrossRef]
- Han, H.; Li, H.Q.; Liu, M.; Xu, L.; Xu, J.; Wang, S.; Ni, H.; Wang, Z. Effect of “bridge” on the performance of organic-inorganic cross-linked hybrid proton exchange membranes via KH550. J. Power Sources 2017, 340, 126–138. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Q.; Ni, N.; Lu, F.; Zhang, R.; Hu, S.; Bao, X.; Zhang, F.; Zhao, F.; Li, X. Novel octopus shaped organic-inorganic composite membranes for PEMFCs. Int. J. Hydrogen Energy 2016, 41, 16160–16166. [Google Scholar] [CrossRef]
- Peng, K.-J.; Lai, J.-Y.; Liu, Y.-L. Nanohybrids of graphene oxide chemically-bonded with Nafion: Preparation and application for proton exchange membrane fuel cells. J. Membr. Sci. 2016, 514, 86–94. [Google Scholar] [CrossRef]
- Feng, T.; Lin, B.; Zhang, S.; Yuan, N.; Chu, F.; Hickner, M.A.; Wang, C.; Zhu, L.; Ding, J. Imidazolium-based organic–inorganic hybrid anion exchange membranes for fuel cell applications. J. Membr. Sci. 2016, 508, 7–14. [Google Scholar] [CrossRef]
- Gang, M.; He, G.; Li, Z.; Cao, K.; Li, Z.; Yin, Y.; Wu, H.; Jiang, Z. Graphitic carbon nitride nanosheets/sulfonated poly(ether ether ketone) nanocomposite membrane for direct methanol fuel cell application. J. Membr. Sci. 2016, 507, 1–11. [Google Scholar] [CrossRef]
- He, Y.; Fu, Y.; Geng, L.; Zhao, Y.; Lü, C. A facile route to enhance the properties of polymer electrolyte-based organic–inorganic hybrid proton exchange membranes. Solid State Ionics 2015, 283, 1–9. [Google Scholar] [CrossRef]
- Mosa, J.; Duran, A.; Aparicio, M. Sulfonic acid-functionalized hybrid organic-inorganic proton exchange membranes synthesized by sol-gel using 3-mercaptopropyltrimethoxysilane (MPTMS). J. Power Sources 2015, 297, 208–216. [Google Scholar] [CrossRef]
- Hattori, M.; Yamaura, S.; Zhang, W.; Sakamoto, W.; Yogo, T. Proton-conductive inorganic-organic hybrid membranes synthesized from a trimethoxysilylmethylstyrene–fluorophenylvinyl acid copolymer. J. Membr. Sci. 2015, 488, 166–172. [Google Scholar] [CrossRef]
- Prapainainar, P.; Theampetch, A.; Kongkachuichay, P.; Laosiripojana, N.; Holmes, S.M.; Prapainainar, C. Effect of solution casting temperature on properties of nafion composite membrane with surface modified mordenite for direct methanol fuel cell. Surf. Coat. Technol. 2015, 271, 63–73. [Google Scholar] [CrossRef]
- Ahn, K.; Kim, M.; Kim, K.; Oh, I.; Ju, H.; Kim, J. Low methanol permeable cross-linked sulfonated poly(phenylene oxide) membranes with hollow glass microspheres for direct methanol fuel cells. Polymer 2015, 56, 178–188. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, B.; Zhao, C.; Zhang, Y.; Bu, F.; Cui, Y.; Li, X.; Na, H. Dual cross-linked organic-inorganic hybrid polymer electrolyte membranes based on quaternized poly(ether ether ketone) and (3-aminopropyl) triethoxysilane. J. Power Sources 2015, 275, 815–822. [Google Scholar] [CrossRef]
- Zhong, S.; Cui, X.; Gao, Y.; Liu, W.; Dou, S. Fabrication and properties of poly(vinyl alcohol)-based polymer electrolyte membranes for direct methanol fuel cell applications. Int. J. Hydrogen Energy 2014, 39, 17857–17864. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, Y.; Pu, H.; Chang, Z. Organic-inorganic hybrid proton exchange membrane based on polyhedral oligomeric silsesquioxanes and sulfonated polyimides containing benzimidazole. J. Power Sources 2014, 263, 195–202. [Google Scholar] [CrossRef]
- Wu, H.; Cao, Y.; Shen, X.; Li, Z.; Xu, T.; Jiang, Z. Preparation and performance of different amino acids functionalized titania-embedded sulfonated poly (ether ether ketone) hybrid membranes for direct methanol fuel cells. J. Membr. Sci. 2014, 463, 134–144. [Google Scholar] [CrossRef]
- Chen, B.; Li, G.; Wang, L.; Chen, R.; Yin, F. Proton conductivity and fuel cell performance of organic-inorganic hybrid membrane based on poly(methyl methacrylate)/silica. Int. J. Hydrogen Energy 2013, 38, 7913–7923. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, S.; Liu, Y.; Wang, Y.; Pang, J.; Wang, Q.; Wang, G. A novel cross-linking organic–inorganic hybrid proton exchange membrane based on sulfonated poly(arylene ether sulfone) with 4-amino-phenyl pendant group for fuel cell application. J. Membr. Sci. 2013, 434, 161–170. [Google Scholar] [CrossRef]
- Kima, A.R.; Park, C.J.; Vinothkannan, M.; Yoo, D.J. Sulfonated poly ether sulfone/heteropoly acid composite membranes as electrolytes for the improved power generation of proton exchange membrane fuel cells. Compos. Part B 2018, 155, 272–281. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Nahm, K.S.; Yoo, D.J. Ternary hybrid (SPEEK/SPVdF-HFP/GO) based membrane electrolyte for the applications of fuel cells: Profile of improved mechanical strength, thermal stability and proton conductivity. RSC Adv. 2016, 6, 108851–108863. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Kumar, G.G.; Yoond, J.; Yoo, D.J. Toward improved mechanical strength, oxidative stability and proton conductivity of an aligned quadratic hybrid (SPEEK/FPAPB/Fe3O4-FGO) membrane for application in high temperature and low humidity fuel cells. RSC Adv. 2017, 7, 39034–39048. [Google Scholar] [CrossRef]


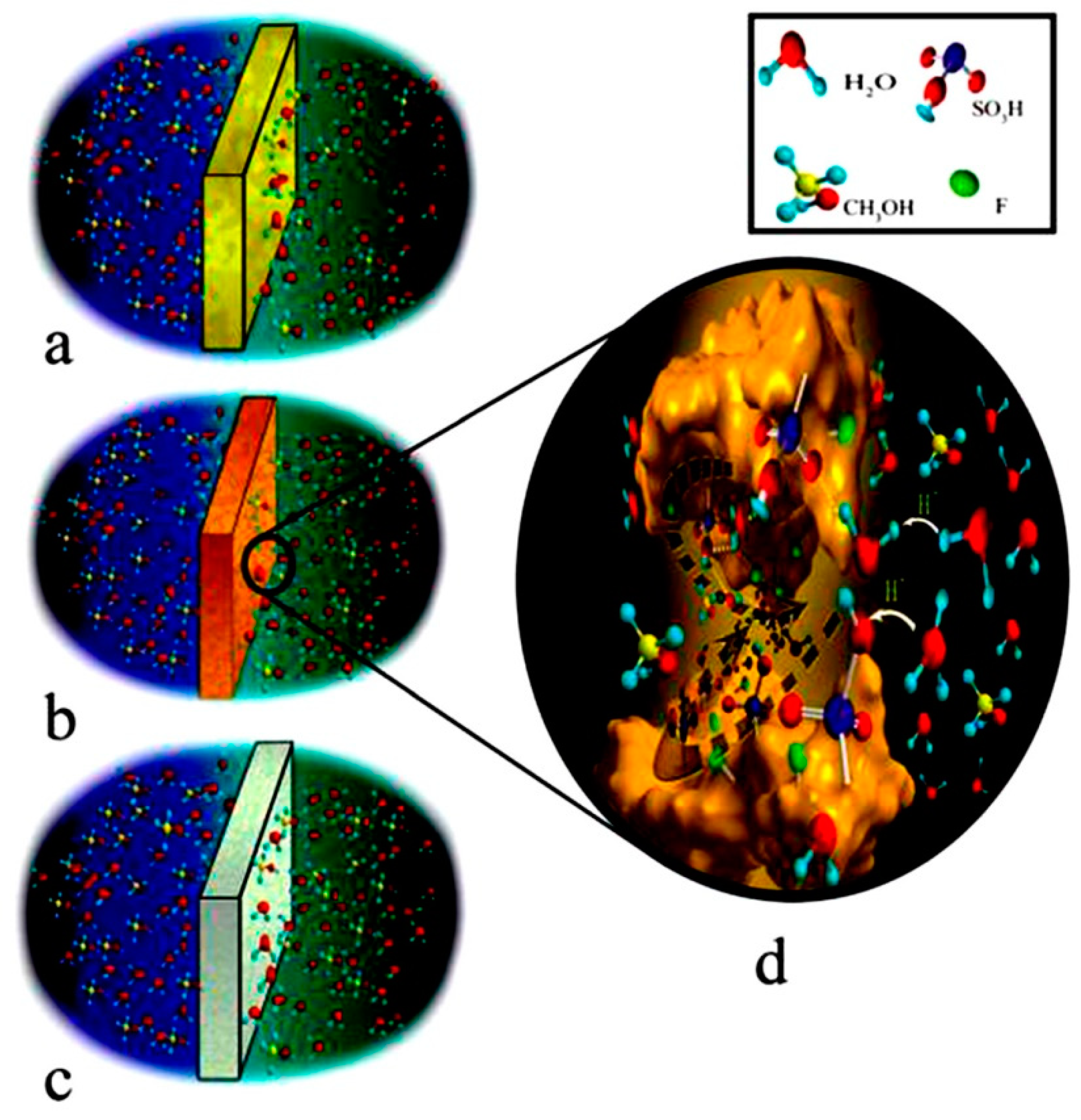
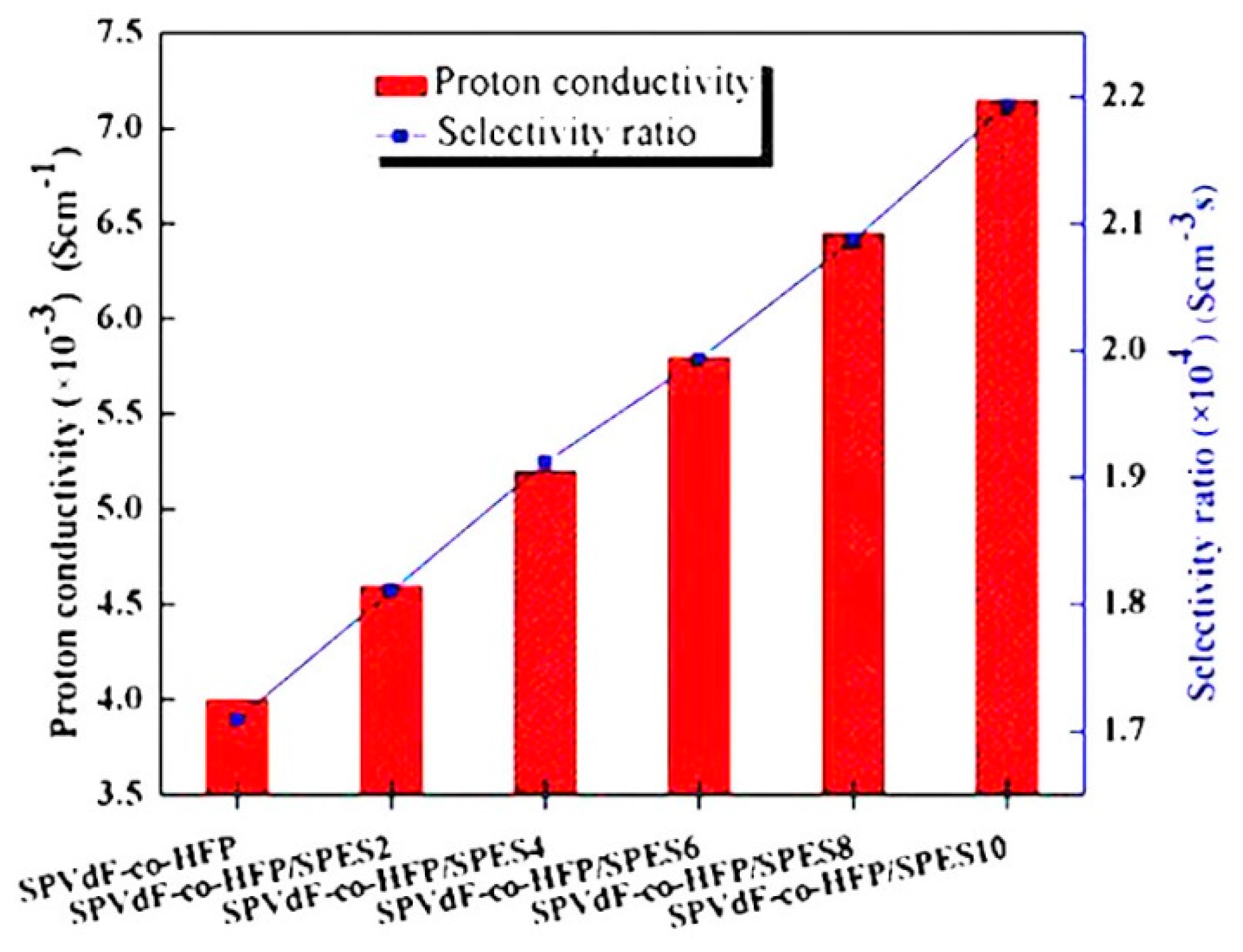


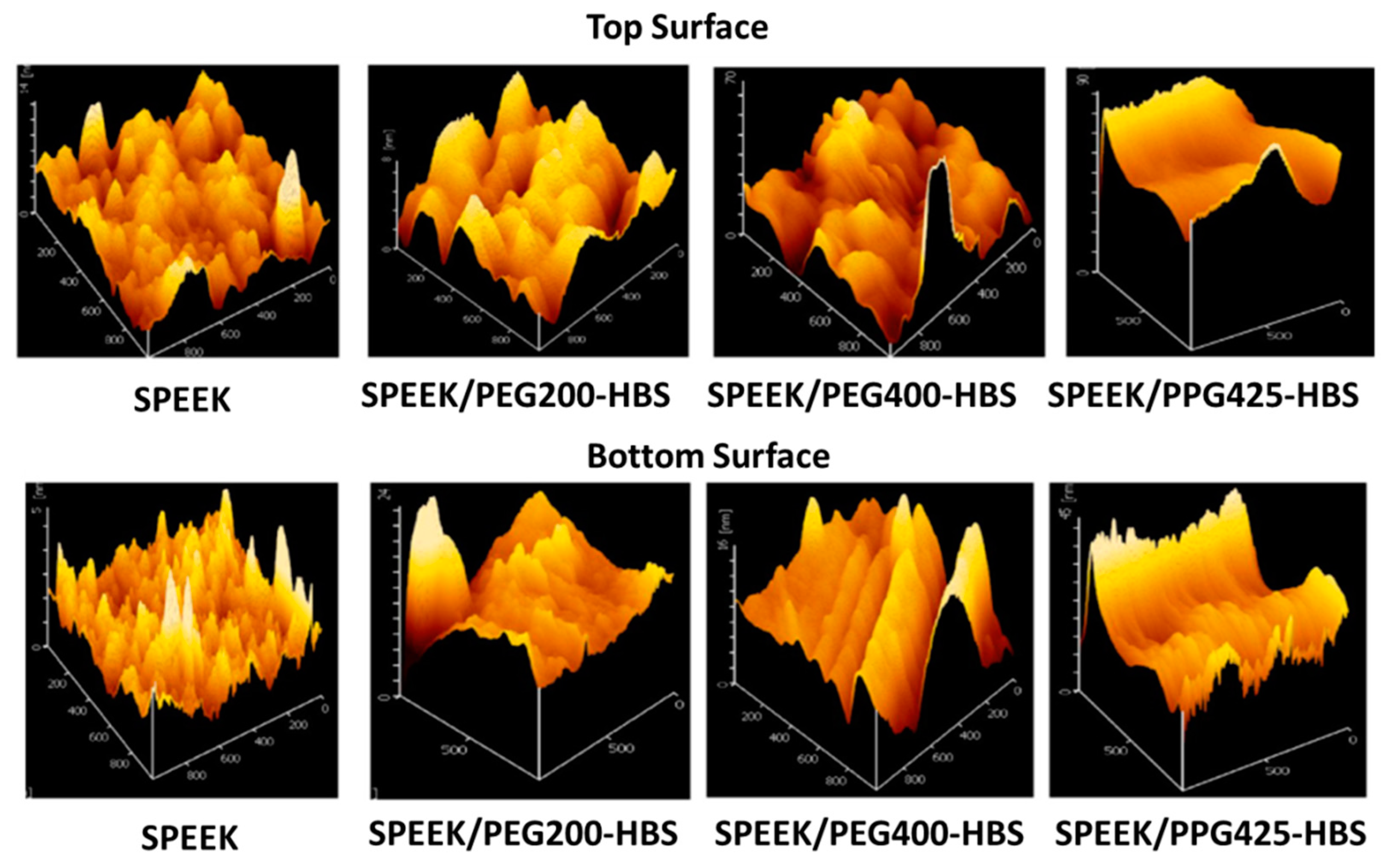

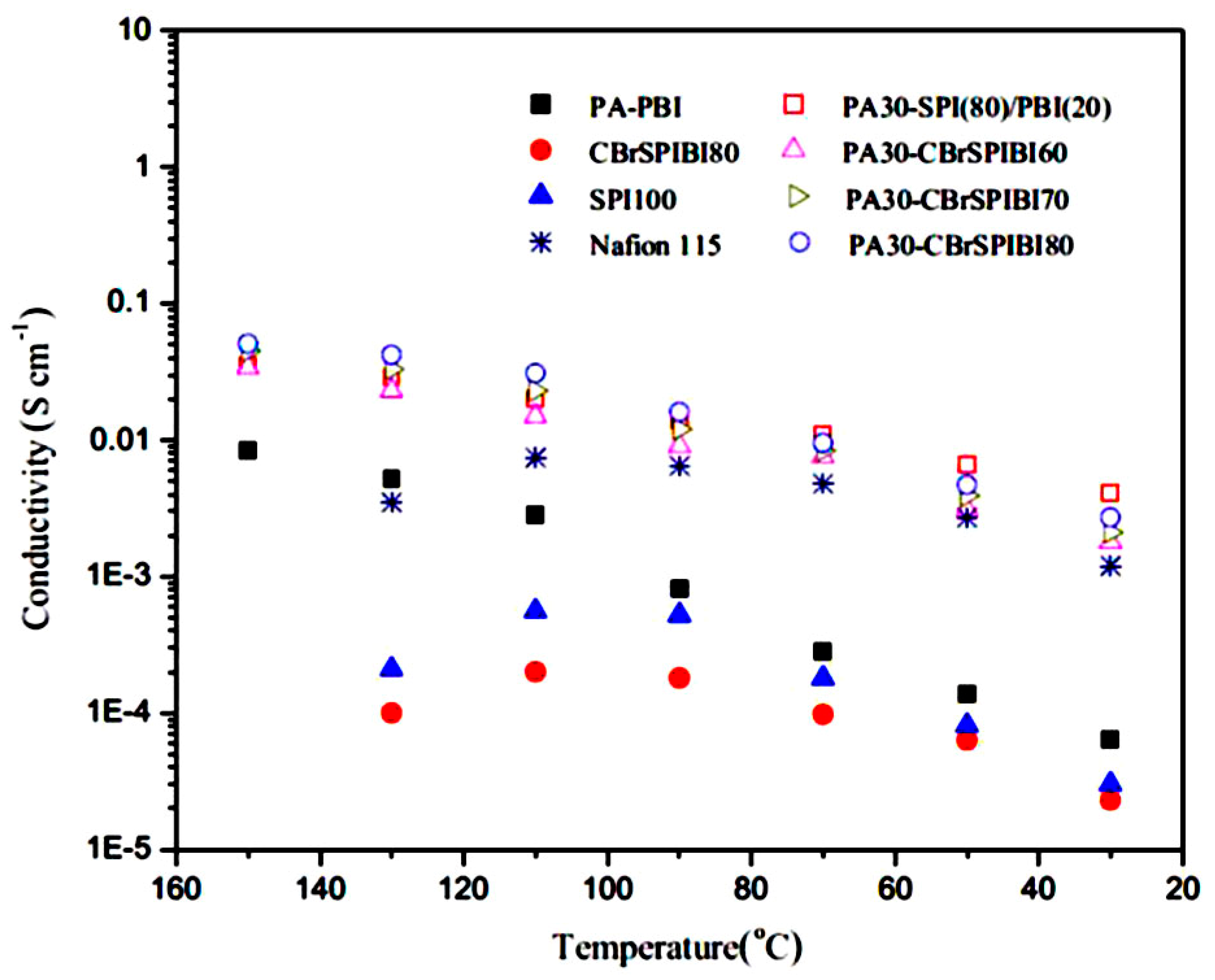

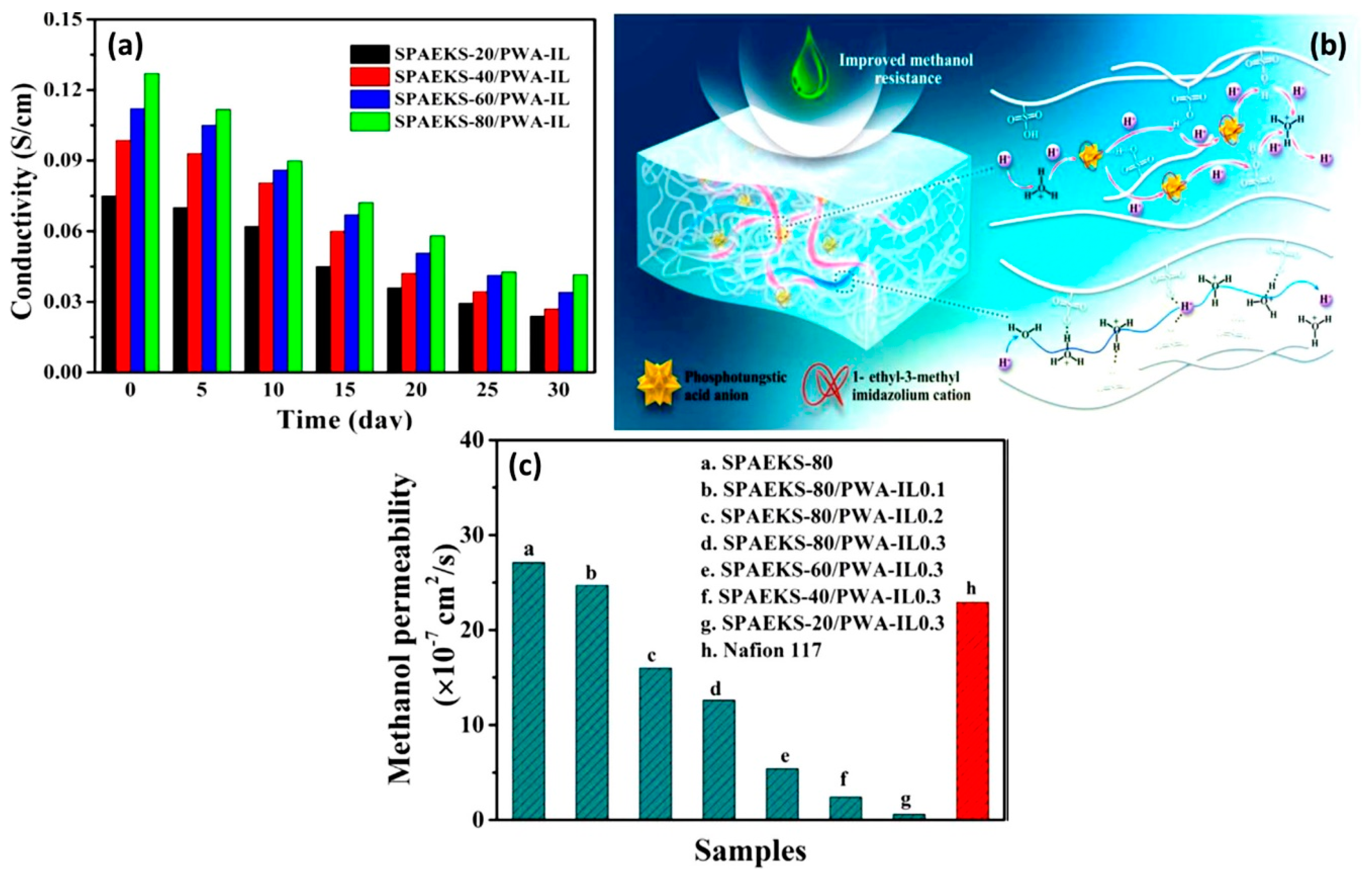
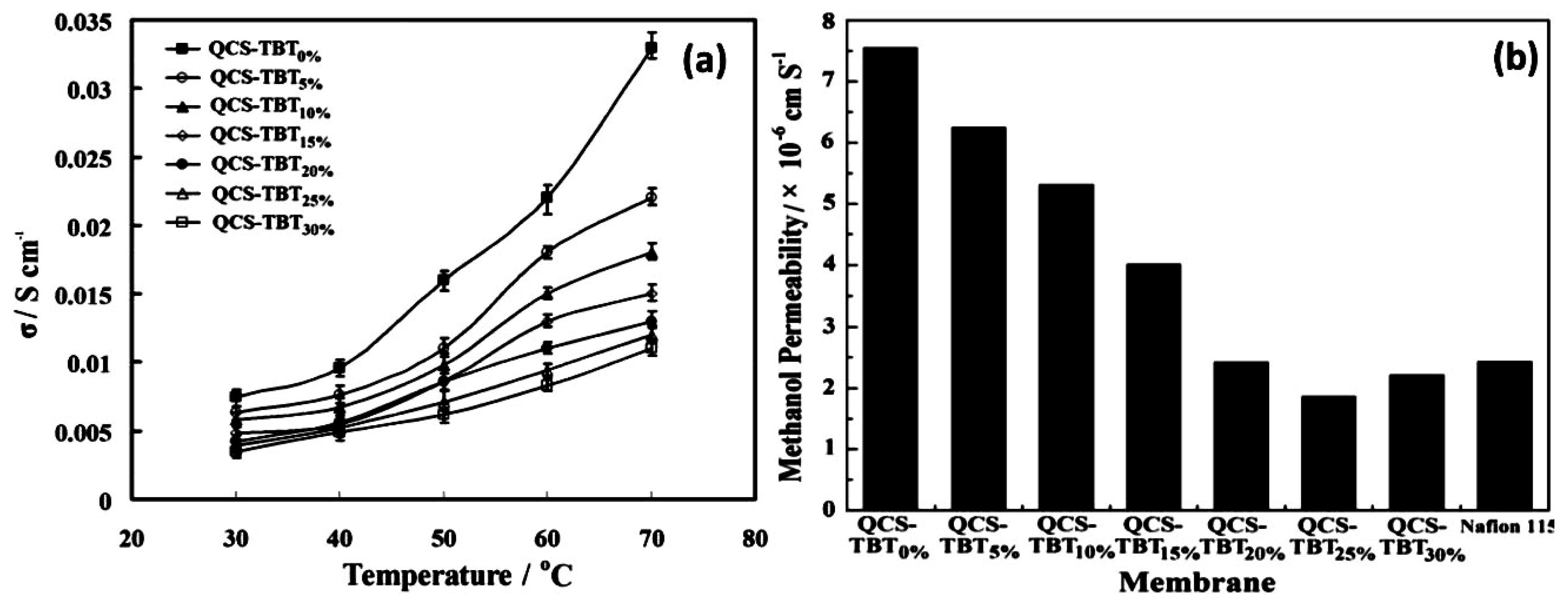
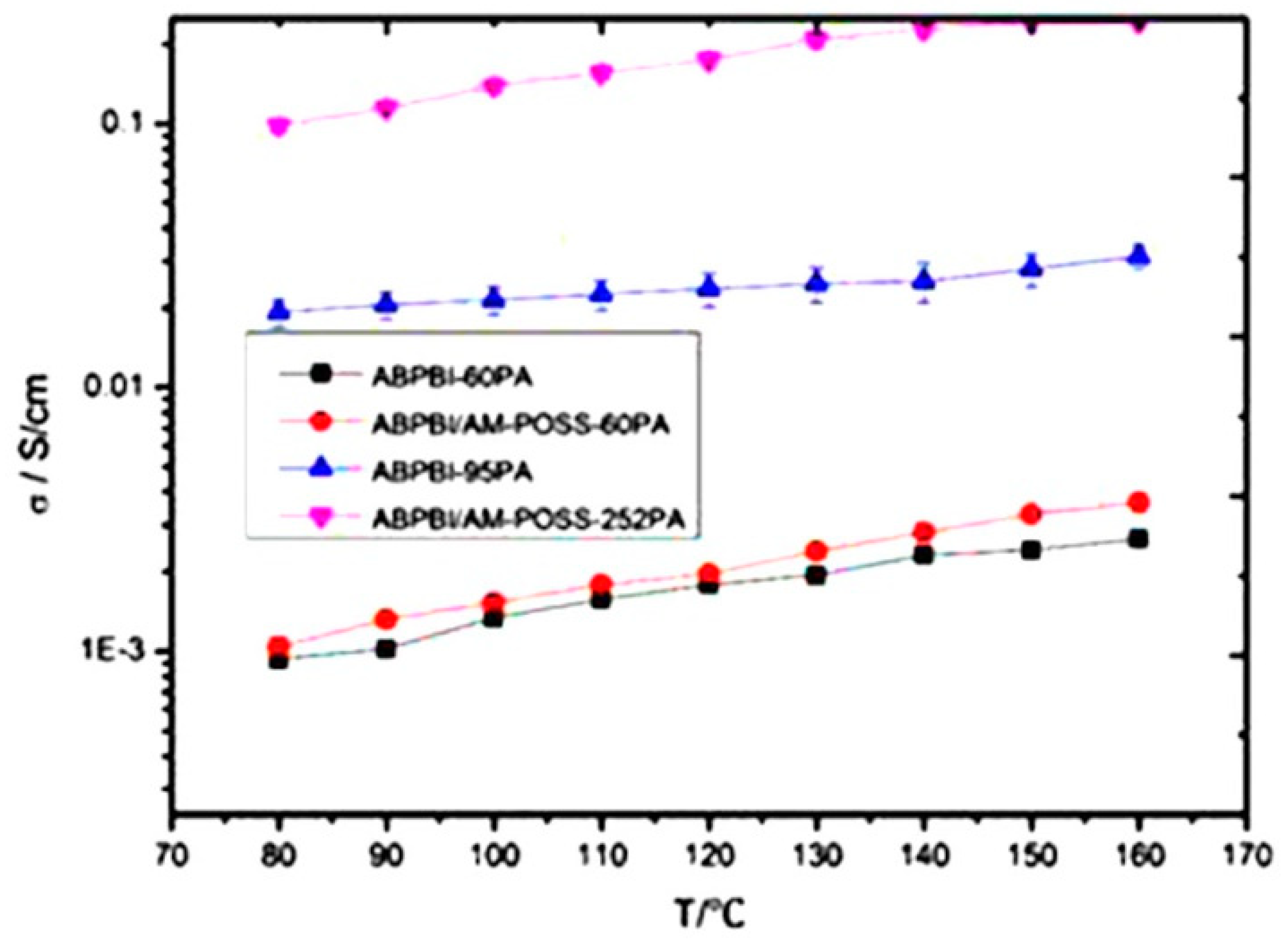

| Author | Blend Membrane Composition | Methanol Permeability (cm2 s−1) 10−6 | Proton Conductivity (S cm−1) | Membrane Selectivity (Ss cm−3) |
|---|---|---|---|---|
| Bi et al. | cSPAES70-5/SPI(5/5) | - | 11.1 ** | - |
| Zhao et al. | SPEEK/PBa-15% | 0.126 (at 30 °C) & 5.53 (at 80 °C) | 4.25 × 10−3 (at 30 °C) & 2.46 × 10−2 (at 80 °C) | 3.12 × 104 (at 30 °C) & 4.45 × 103 (at 80 °C) |
| Inan et al. | SPEEK70/PVDF(Mw¼275.000) | 0.313 ** | - | - |
| Unveren et al. | SPEES72/PVDF180 (10 wt %) | 2.4 ** | 144.0 * | 6.0 × 107 * |
| Dutta et al. | PAni/PVdF-co-HFP/Pani | 0.0000981 ** | - | 2.38 × 10−6 |
| PAni/SPVdF-co-HFP/PAni | 0.0172 ** | - | 2.45 × 105 | |
| SPAni/SPVdF-co-HFP/SPAni | 0.0529 ** | - | 9.39 × 104 | |
| Seden et al. | SPEEK70/Copolymer 1a | 0.082 ** | 84.4 | - |
| SPEEK70/Copolymer 1b | 0.0013 ** | 30.5 | - | |
| Prasad et al. | SPEEK BNCM D-2 | 0.135 ** | 1.31 & 2.14 × 10−3 | 9.63 × 104 |
| Kumar et al. | S-20 | 1.76 | 3.16 × 10−2 * | 1.80 × 104 |
| Mondal et al. | M2 (70/30 S-PVdF-co-HFP/PBI coated) | 0.492 * | 1.51 × 10−2 ** | 3.0695.3 * |
| Bagheri et al. | MSSP20 | 0.211 | 32.71 | 15.51 × 104 |
| Devi et al. | SPVdF-co-HFP/SPES blends | 3.26 * | 7.2 ** | 2.193 * |
| Molla and Compan | SPEEK-35%PVA | 4.70 ** | 1.1 × 10−2 (at 120 °C) ** | - |
| Muthumeenal et al. | SPES/NPHCs (1) | 0.0171 * | 9.2 × 10−3 (at 30 °C) * & 12.1 × 10−3 (at 80 °C) | 5.3 × 104 * |
| Meenakshi et al. | CS-PVA-SPES (25 wt %) | - | - | 2.41 × 10−4 * |
| Norddin et al. | SPEEK/cSMM | 0.275 ** | 6.4 × 10−3 * | - |
| Changkhamchom et al. | (15% v/v) Molecular sieve13X/S-PEKES/PPEES (5:1) | 0.0487 | 1.44 × 10−2 | 2.95 × 105 |
| Ahmad et al. | Nafion-PBI 1%-ZP 1% | 0.233927 ** | 0.02022 ** | 86,437.06 * |
| Kim et al. | FSiO2-12 | - | 100 ** | - |
| Han et al. | C-SPAEKS/K-SiO2-8 | 0.667 (at 60 °C) | 0.110 (at 120 °C) * | - |
| Peng et al. | NM/GO-0.10 | - | 40.8 (at 20 °C) 82.3 (at 95 °C) | - |
| Feng et al. | Membrane-5 | - | 4.10 × 10−2 (at 30 °C) 9.05 × 10−2 (at 90 °C) | - |
| He et al. | SPI-40-MsiSQ | 0.018 ** | 0.566 (at 80 °C) * | 12.8 × 106 * |
| Ahn et al. | SPPO-HGM (9 wt %)/C-SPPO | 0.267 | 0.0278 | 110,317.46 * |
| Zhang et al. | PA-QPEEK-10%APTES | - | 61.7 (at 200 °C) | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhanapal, D.; Xiao, M.; Wang, S.; Meng, Y. A Review on Sulfonated Polymer Composite/Organic-Inorganic Hybrid Membranes to Address Methanol Barrier Issue for Methanol Fuel Cells. Nanomaterials 2019, 9, 668. https://doi.org/10.3390/nano9050668
Dhanapal D, Xiao M, Wang S, Meng Y. A Review on Sulfonated Polymer Composite/Organic-Inorganic Hybrid Membranes to Address Methanol Barrier Issue for Methanol Fuel Cells. Nanomaterials. 2019; 9(5):668. https://doi.org/10.3390/nano9050668
Chicago/Turabian StyleDhanapal, Duraibabu, Min Xiao, Shuanjin Wang, and Yuezhong Meng. 2019. "A Review on Sulfonated Polymer Composite/Organic-Inorganic Hybrid Membranes to Address Methanol Barrier Issue for Methanol Fuel Cells" Nanomaterials 9, no. 5: 668. https://doi.org/10.3390/nano9050668





