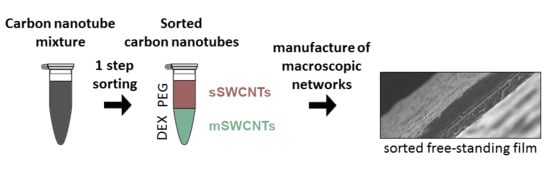
Manufacture of Networks from Large Diameter Single-Walled Carbon Nanotubes of Particular Electrical Character
Abstract
:1. Introduction
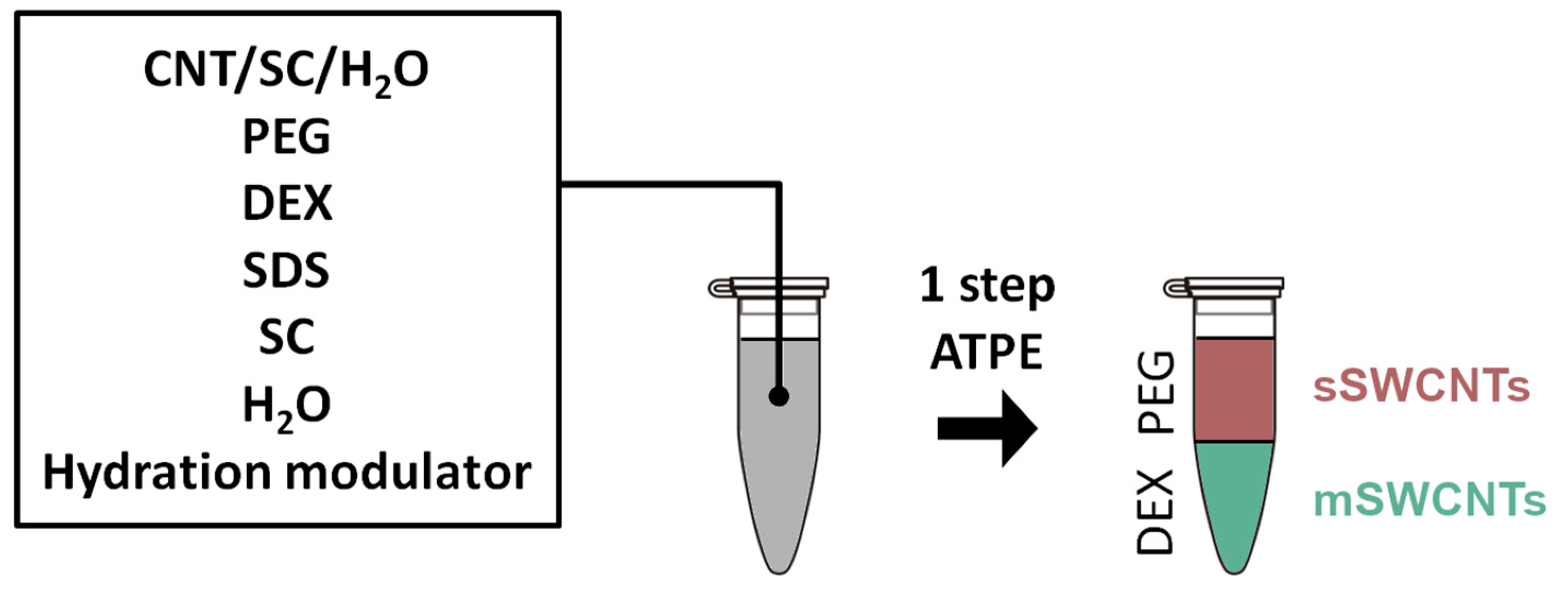
2. Materials and Methods
3. Results and Discussion
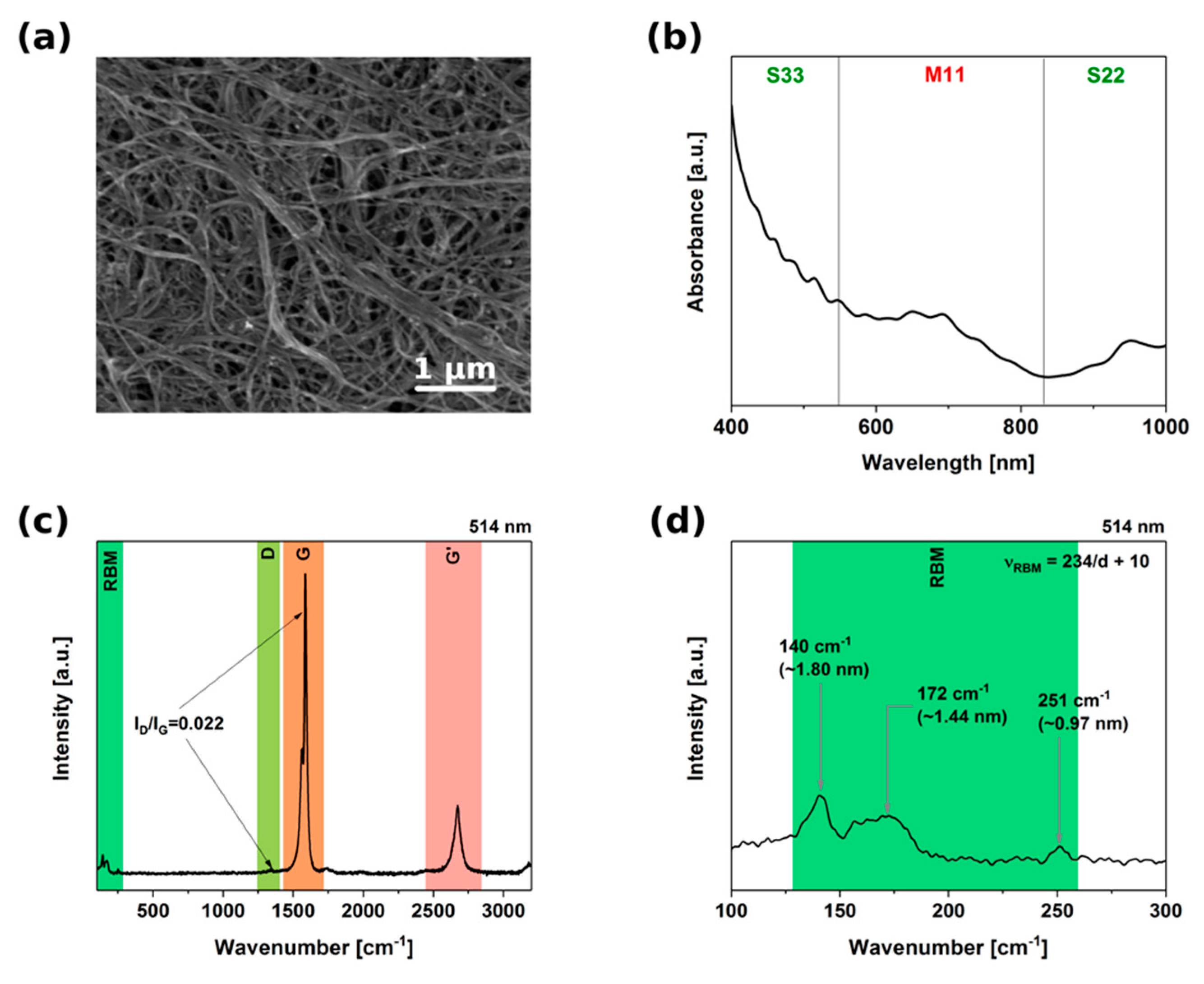
3.1. Characterization of the Material
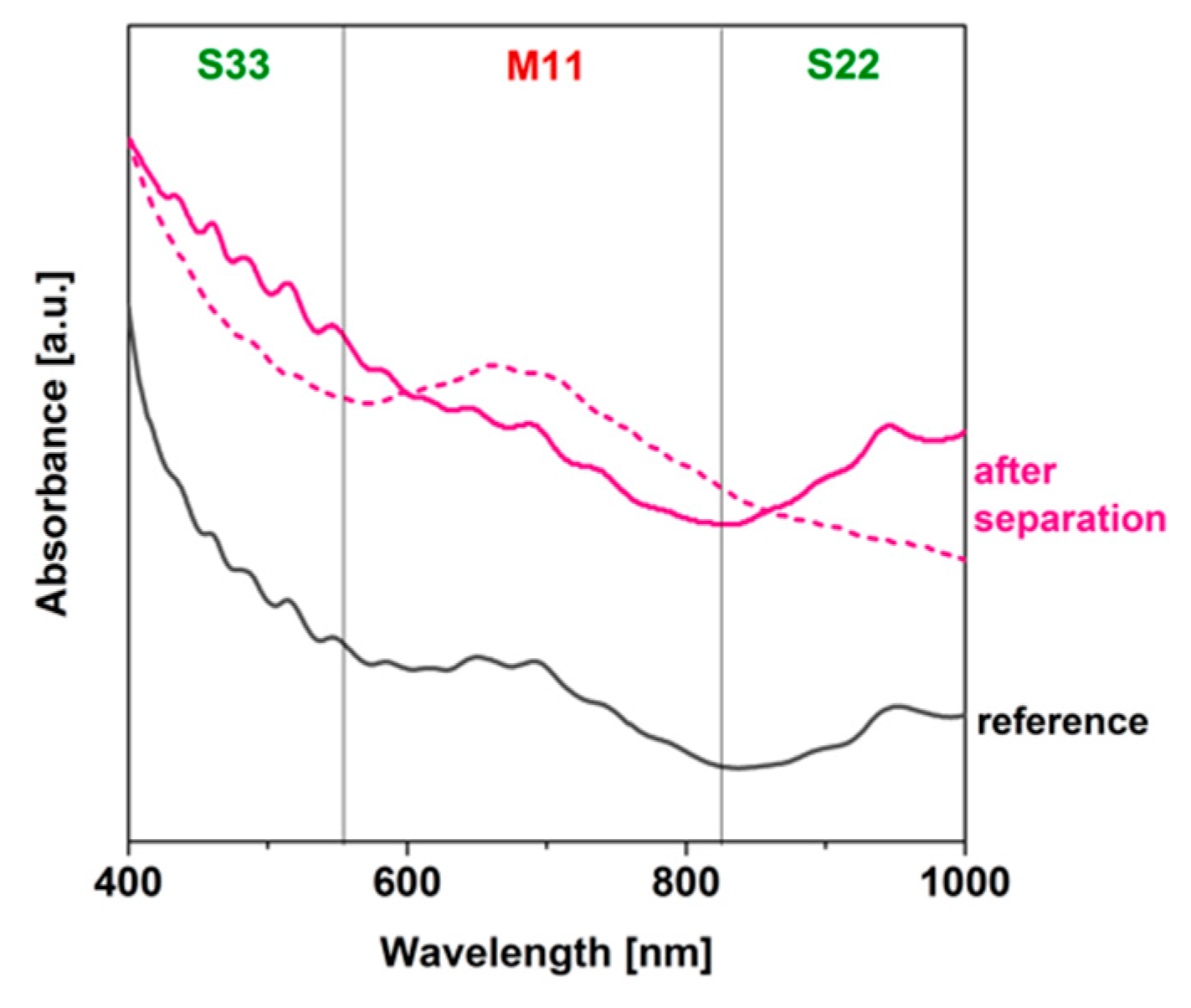
3.2. Regular ATPE Separation
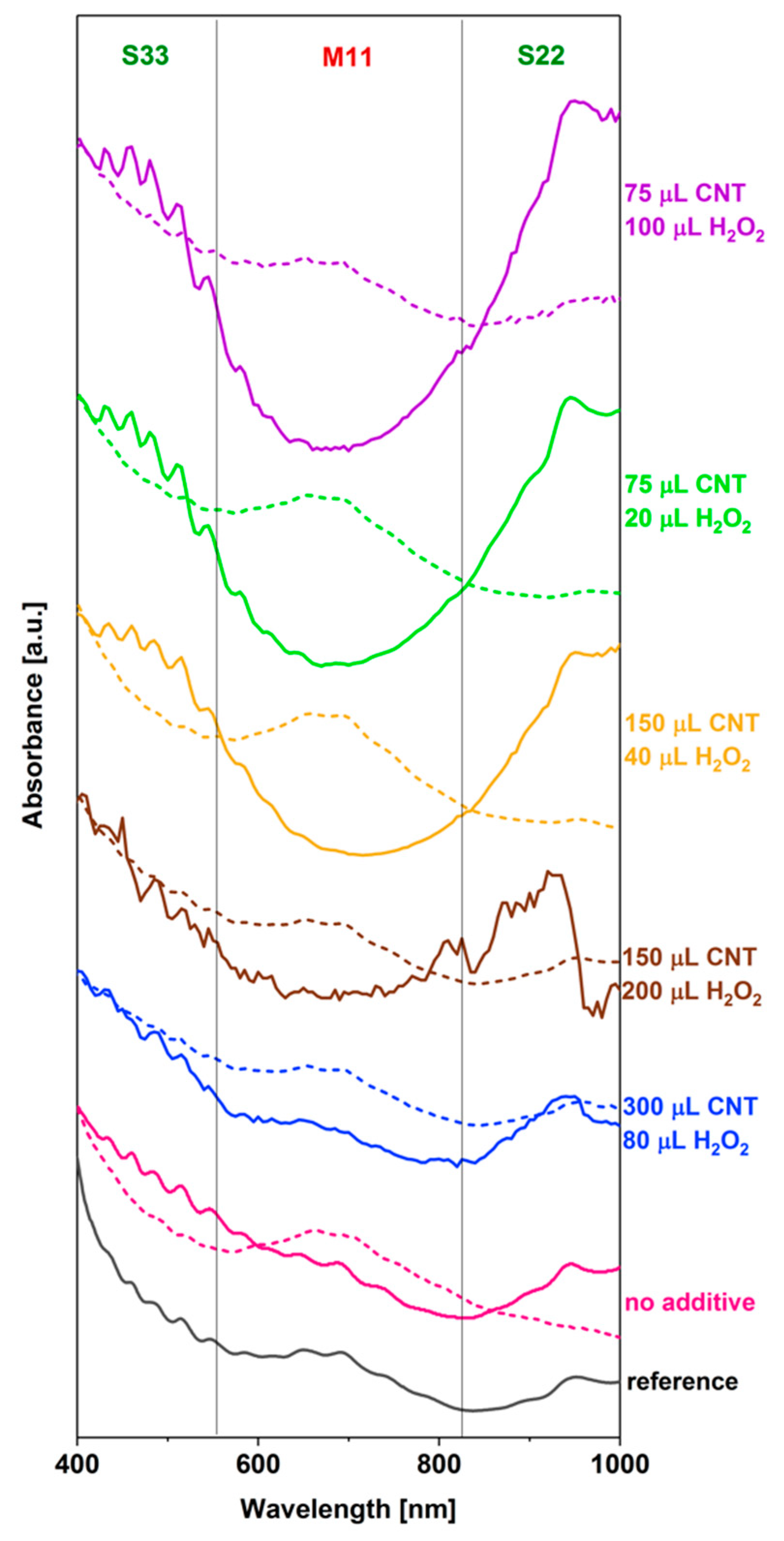
3.3. Effect of H2O2 Addition
3.4. Effect of Poly(Ethylene Glycol) Methyl Ether Addition
3.5. Effect of Addition of Other Hydration Modulators
3.6. Removal of ATPE Remnants
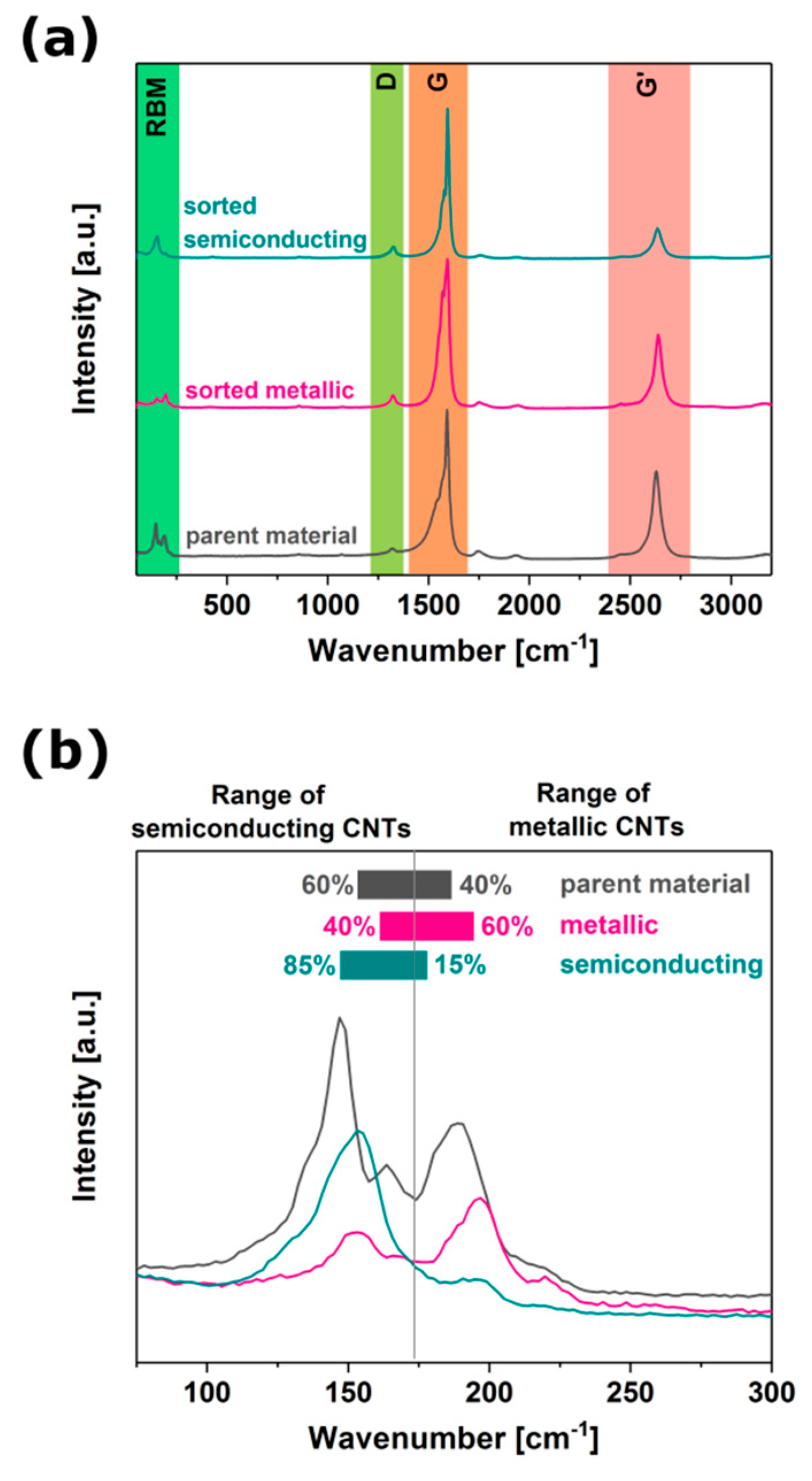
3.7. Sorting Outcome Characterization by Raman Spectroscopy
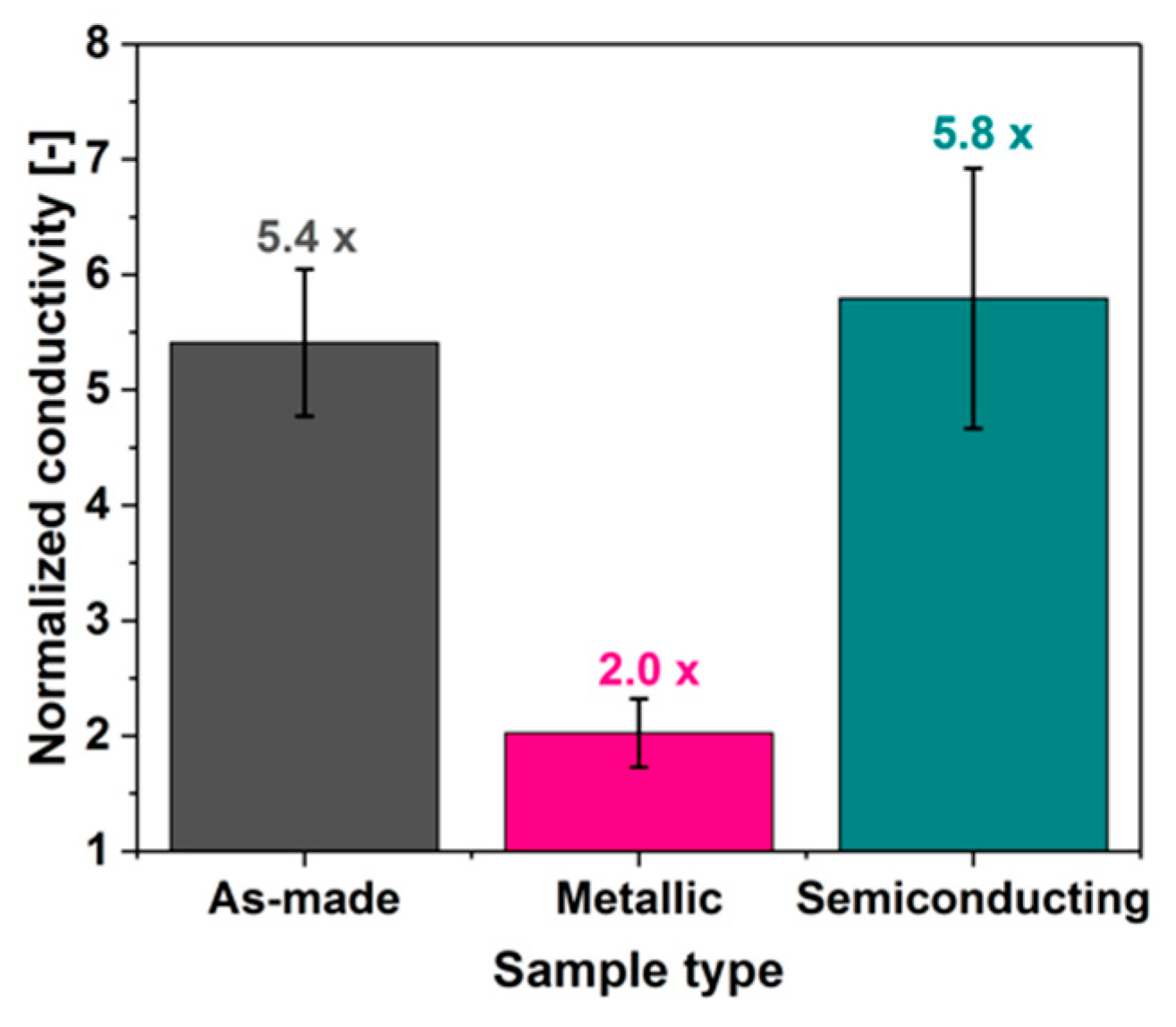
3.8. Electrical Conductivity of Sorted CNT Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Janas, D.; Milowska, K.Z.; Bristowe, P.D.; Koziol, K.K. Improving the electrical properties of carbon nanotubes with interhalogen compounds. Nanoscale 2017, 9, 3212–3221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, J. Carbon nanotube in different shapes. Mater. Today 2009, 12, 12–18. [Google Scholar] [CrossRef]
- Kumanek, B.; Janas, D. Thermal conductivity of carbon nanotube networks—Review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef]
- Mir, M.; Ebrahimnia-Bajestan, E.; Niazmand, H.; Mir, M. A novel approach for determining thermal properties of single-walled carbon nanotubes. Comput. Mater. Sci. 2012, 63, 52–57. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical properties of single-wall carbon nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Sfeir, M.Y.; Beetz, T.; Wang, F.; Huang, L.M.; Huang, X.M.H.; Huang, M.Y.; Hone, J.; O'Brien, S.; Misewich, J.A.; Heinz, T.F.; et al. Optical spectroscopy of individual single-walled carbon nanotubes of defined chiral structure. Science 2006, 312, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Janas, D.; Czechowski, N.; Krajnik, B.; Mackowski, S.; Koziol, K.K. Electroluminescence from carbon nanotube films resistively heated in air. Appl. Phys. Lett. 2013, 102, 181104. [Google Scholar] [CrossRef]
- Janas, D. Towards monochiral carbon nanotubes: A review of progress in the sorting of single-walled carbon nanotubes. Mater. Chem. Front. 2018, 2, 36–63. [Google Scholar] [CrossRef]
- Yang, D.H.; Hu, J.W.; Liu, H.P.; Li, S.L.; Su, W.; Li, Q.; Zhou, N.G.; Wang, Y.C.; Zhou, W.Y.; Xie, S.S.; et al. Structure Sorting of Large-Diameter Carbon Nanotubes by NaOH Tuning the Interactions between Nanotubes and Gel. Adv. Funct. Mater. 2017, 27, 1700278. [Google Scholar] [CrossRef]
- He, X.W.; Htoon, H.; Doorn, S.K. Tunable Room-Temperature Single-Photon Emission at Telecom Wavelengths from sp3 Defects in Carbon Nanotubes. Nat. Photonics 2017, 11, 577. [Google Scholar] [CrossRef]
- Graf, A.; Zakharko, Y.; Schiessl, S.P.; Backes, C.; Pfohl, M.; Flavel, B.S.; Zaumseil, J. Large scale, selective dispersion of long single-walled carbon nanotubes with high photoluminescence quantum yield by shear force mixing. Carbon 2016, 105, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Tulevski, G.S.; Franklin, A.D.; Frank, D.; Lobez, J.M.; Cao, Q.; Park, H.; Afzali, A.; Han, S.J.; Hannon, J.B.; Haensch, W. Toward High-Performance Digital Logic Technology with Carbon Nanotubes. ACS Nano 2014, 8, 8730–8745. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Appenzeller, J.; Knoch, J.; Lin, Y.M.; Avouris, P. The role of metal-nanotube contact in the performance of carbon nanotube field-effect transistors. Nano Lett. 2005, 5, 1497–1502. [Google Scholar] [CrossRef]
- He, X.; Gao, W.; Xie, L.; Li, B.; Zhang, Q.; Lei, S.; Robinson, J.M.; Haroz, E.H.; Doorn, S.K.; Wang, W.; et al. Wafer-scale monodomain films of spontaneously aligned single-walled carbon nanotubes. Nat. Nanotechnol. 2016, 11, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Turek, E.; Shiraki, T.; Shiraishi, T.; Shiga, T.; Fujigaya, T.; Janas, D. Single-step isolation of carbon nanotubes with narrow-band light emission characteristics. Sci. Rep. 2019, 9, 535. [Google Scholar] [CrossRef]
- Gui, H.; Streit, J.K.; Fagan, J.A.; Walker, A.R.H.; Zhou, C.W.; Zheng, M. Redox Sorting of Carbon Nanotubes. Nano Lett. 2015, 15, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Janas, D.; Rdest, M.; Koziol, K.K.K. Free-standing films from chirality-controlled carbon nanotubes. Mater. Des. 2017, 121, 119–125. [Google Scholar] [CrossRef]
- Clancy, A.J.; White, E.R.; Tay, H.H.; Yau, H.C.; Shaffer, M.S.P. Systematic comparison of conventional and reductive single-walled carbon nanotube purifications. Carbon 2016, 108, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Fagan, J.A.; Haroz, E.H.; Ihly, R.; Gui, H.; Blackburn, J.L.; Simpson, J.R.; Lam, S.; Walker, A.R.H.; Doorn, S.K.; Zheng, M. Isolation of > 1 nm Diameter Single-Wall Carbon Nanotube Species Using Aqueous Two-Phase Extraction. ACS Nano 2015, 9, 5377–5390. [Google Scholar] [CrossRef]
- Souza, A.G.; Chou, S.G.; Samsonidze, G.G.; Dresselhaus, G.; Dresselhaus, M.S.; An, L.; Liu, J.; Swan, A.K.; Unlu, M.S.; Goldberg, B.B.; et al. Stokes and anti-Stokes Raman spectra of small-diameter isolated carbon nanotubes. Phys. Rev. B 2004, 69, 115428. [Google Scholar] [CrossRef]
- Fantini, C.; Jorio, A.; Souza, M.; Strano, M.S.; Dresselhaus, M.S.; Pimenta, M.A. Optical transition energies for carbon nanotubes from resonant Raman spectroscopy: Environment and temperature effects. Phys. Rev. Lett. 2004, 93, 147406. [Google Scholar] [CrossRef]
- Goran, J.M.; Phan, E.N.H.; Favela, C.A.; Stevenson, K.J. H2O2 Detection at Carbon Nanotubes and Nitrogen-Doped Carbon Nanotubes: Oxidation, Reduction, or Disproportionation? Anal. Chem. 2015, 87, 5989–5996. [Google Scholar] [CrossRef]
- Miao, Z.Y.; Zhang, D.; Chen, Q. Non-enzymatic Hydrogen Peroxide Sensors Based on Multi-wall Carbon Nanotube/Pt Nanoparticle Nanohybrids. Materials 2014, 7, 2945–2955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, A.J.; Liu, Q.X.; Ge, H.L.; Kong, Y. Novel H2O2 electrochemical sensor based on graphene-polyacrylamide composites. Mater. Technol. 2015, 30, 50–53. [Google Scholar] [CrossRef]
- Janas, D. Powerful doping of chirality-sorted carbon nanotube films. Vacuum 2018, 149, 48–52. [Google Scholar] [CrossRef]
- Bulmer, J.S.; Gspann, T.S.; Orozco, F.; Sparkes, M.; Koerner, H.; Di Bernardo, A.; Niemiec, A.; Robinson, J.W.A.; Koziol, K.K.; Elliott, J.A.; et al. Photonic Sorting of Aligned, Crystalline Carbon Nanotube Textiles. Sci. Rep. 2017, 7, 12977. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turek, E.; Kumanek, B.; Boncel, S.; Janas, D. Manufacture of Networks from Large Diameter Single-Walled Carbon Nanotubes of Particular Electrical Character. Nanomaterials 2019, 9, 614. https://doi.org/10.3390/nano9040614
Turek E, Kumanek B, Boncel S, Janas D. Manufacture of Networks from Large Diameter Single-Walled Carbon Nanotubes of Particular Electrical Character. Nanomaterials. 2019; 9(4):614. https://doi.org/10.3390/nano9040614
Chicago/Turabian StyleTurek, Edyta, Bogumila Kumanek, Slawomir Boncel, and Dawid Janas. 2019. "Manufacture of Networks from Large Diameter Single-Walled Carbon Nanotubes of Particular Electrical Character" Nanomaterials 9, no. 4: 614. https://doi.org/10.3390/nano9040614