Modification of Layered Graphitic Carbon Nitride by Nitrogen Plasma for Improved Electrocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Catalytic Activity Evaluation
3. Results and Discussion
3.1. Morphology and Crystalline Phases
3.2. Surface Chemical Composition
3.3. Surface Wettability
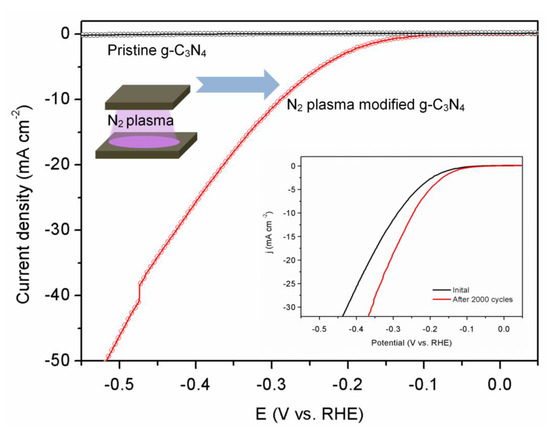
3.4. Catalytic Activity and Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 463, 1256–1260. [Google Scholar] [CrossRef]
- Esposito, D.V.; Hunt, S.T.; Stottlemyer, A.L.; Dobson, K.D.; McCandless, B.E.; Birkmire, R.W.; Chen, J.G. Low-cost hydrogen-evolution catalysts based on monolayer platinum on tungsten monocarbide substrates. Angew. Chem. Int. Ed. 2010, 49, 9859–9862. [Google Scholar] [CrossRef]
- Ledendecker, M.; Mondschein, J.S.; Kasian, O.; Geiger, S.; Gohl, D.; Schalenbach, M.; Zeradjanin, A.; Cherevko, S.; Schaak, R.E.; Mayrhofer, K. Stability and activity of non-nobl e-metal-based catalysts toward the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2017, 56, 9767–9771. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Emerging two-dimensional nanomaterials for electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jia, J.; Lu, J.; Yang, L.; Hou, D.; Li, G.; Chen, S. Recent developments of carbon-based electrocatalysts for hydrogen evolution reaction. Nano Energy 2016, 28, 29–43. [Google Scholar]
- Zheng, Y.; Lin, L.; Wang, B.; Wang, X. Graphitic carbon nitride polymers toward sustainable photoredox catalysis. Angew. Chem. Int. Ed. 2015, 54, 12868–12884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, H.; Guo, X.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S. Metal-free hybrids of graphitic carbon nitride and nanodiamonds for photoelectrochemical and photocatalytic applications. J. Colloid Interf. Sci. 2017, 493, 275–280. [Google Scholar] [CrossRef]
- Nikokavoura, A.; Trapalis, C. Graphene and g-C3N4 based photocatalysts for NOx removal: A review. Appl. Surf. Sci. 2018, 430, 18–52. [Google Scholar]
- Zheng, Y.; Yan, J.; Zhu, Y.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Tahini, H.A.; Smith, S.C. p-Doped graphene/graphitic carbon nitride hybrid electrocatalysts: Unraveling charge transfer mechanisms for enhanced hydrogen evolution reaction performance. ACS Catal. 2016, 6, 7071–7077. [Google Scholar] [CrossRef]
- Pei, Z.; Zhao, J.; Huang, Y.; Huang, Y.; Zhu, M.; Wang, Z.; Chen, Z.; Zhi, C. Toward enhanced activity of a graphitic carbon nitride-based electrocatalyst in oxygen reduction and hydrogen evolution reactions via atomic sulfur doping. J. Mater. Chem. A 2016, 4, 12205–12211. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.L.; Liu, C.J. Catalyst preparation with plasmas: How does it work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Dou, S.; Tao, L.; Wang, R.; Hankari, S.E.; Chen, R.; Wang, S. Plasma-assisted synthesis and surface modification of electrode materials for renewable energy. Adv. Mater. 2018, 30, 1705850. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Cheng, H.; Lv, H.; Wang, J.; Liu, L.; Liu, S.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. Controllable surface reorganization engineering on cobalt phosphide nanowire arrays for efficient alkaline hydrogen evolution reaction. Adv. Mater. 2018, 30, 1703322. [Google Scholar] [CrossRef]
- Zhang, L.; Sadanandam, G.; Liu, X.; Scurrell, M.S. Carbon surface modifications by plasma for catalyst support and electrode materials applications. Top. Catal. 2017, 60, 823–830. [Google Scholar]
- Muramatsu, H.; Takahashi, M.; Kang, C.S.; Kim, J.H.; Kim, Y.A.; Hayashi, T. Synthesis of outer tube-selectively nitrogen-doped double-walled carbon nanotubes by nitrogen plasma treatment. Nanoscale 2018, 10, 15938–15942. [Google Scholar] [CrossRef]
- Gao, M.; Sun, L.; Guo, Y.; Shi, J.; Zhang, J. Modification of polyethylene terephthalate (PET) films surface with gradient roughness and homogenous surface chemistry by dielectric barrier discharge plasma. Chem. Phys. Lett. 2017, 689, 179–184. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, J.; Yang, Y.; Bie, L.; Fahlman, B.D.; Wang, D. Modification of surface properties and enhancement of photocatalytic performance for g-C3N4 via plasma treatment. Carbon 2017, 123, 651–659. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Ge, C.; Xing, Z.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Ultrathin graphitic carbon nitride nanosheet: A low-cost, green, and highly efficient electrocatalyst toward the reduction of hydrogen peroxide and its glucose biosensing application. Nanoscale 2013, 5, 8921–8924. [Google Scholar] [CrossRef]
- Tian, Y.; Ye, Y.; Wang, X.; Peng, S.; Wei, Z.; Zhang, X.; Liu, W. Three-dimensional N-doped, plasma-etched graphene: Highly active metal-free catalyst for hydrogen evolution reaction. Appl. Catal. A Gen. 2017, 529, 127–133. [Google Scholar] [CrossRef]
- Gao, M.; Tong, R.; Huang, H.; Kang, Y.; Luo, Q.; Huang, Y.; Chu, P.K. Activation of graphitic carbon nitride by surface discharge plasma treatment for enhanced photocatalysis. Vacuum 2019, 159, 235–238. [Google Scholar] [CrossRef]
- Dey, A.; Chroneos, A.; Braithwaite, N.S.; Gandhiraman, R.P.; Krishnamurthy, S. Plasma engineering of graphene. Appl. Phys. Rev. 2016, 3, 021301. [Google Scholar] [Green Version]
- Ji, X.; Yuan, X.; Wu, J.; Yu, L.; Guo, H.; Wang, H.; Zhang, H.; Yu, D.; Zhao, Y. Tuning the photocatalytic activity of graphitic carbon nitride by plasma-based surface modification. ACS Appl. Mater. Interfaces 2017, 9, 24616–24624. [Google Scholar]
- Fang, J.; Fan, H.; Li, M.; Long, C. Nitrogen self-doped graphitic carbon nitride as efficient visible light photocatalyst for hydrogen evolution. J. Mater. Chem. A 2015, 3, 13819–13826. [Google Scholar]
- Wang, R.; Jiang, W.; Xia, D.; Liu, T.; Gan, L. Improving the wettability of thin-film rotating disk elctrodes for reliable activity evaluation of oxygen electrocatalysts by triggering oxygen reduction at the catalyst-electrolyte-bubble triple phase boundaries. J. Electrochem. Soc. 2018, 165, 436–440. [Google Scholar] [CrossRef]
- Chien, H.H.; Cheng, Y.C.; Hao, Y.C.; Hsu, C.C.; Cheng, I.C.; Yu, I.S.; Chen, J.Z. Nitrogen DC-pulse atmospheric-pressure-plasma jet (APPJ)-processed reduced graphene oxide (rGO)-carbon black (CB) nanocomposite electrodes for supercapacitor applications. Diam. Relat. Mater. 2018, 88, 23–31. [Google Scholar]
- Li, W.; Liu, D.; Yang, N.; Wang, J.; Huang, M.; Liu, L.; Peng, X.; Wang, G.; Yu, X.F.; Chu, P.K. Molybdenum diselenide—Black phosphorus heterostructures for electrocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 467, 328–334. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Liu, D.; Yang, H.; Huang, H.; Luo, Q.; Huang, Y.; Yu, X.-F.; Chu, P.K. Modification of Layered Graphitic Carbon Nitride by Nitrogen Plasma for Improved Electrocatalytic Hydrogen Evolution. Nanomaterials 2019, 9, 568. https://doi.org/10.3390/nano9040568
Gao M, Liu D, Yang H, Huang H, Luo Q, Huang Y, Yu X-F, Chu PK. Modification of Layered Graphitic Carbon Nitride by Nitrogen Plasma for Improved Electrocatalytic Hydrogen Evolution. Nanomaterials. 2019; 9(4):568. https://doi.org/10.3390/nano9040568
Chicago/Turabian StyleGao, Ming, Danni Liu, Huanhuan Yang, Hao Huang, Qian Luo, Yifan Huang, Xue-Feng Yu, and Paul K. Chu. 2019. "Modification of Layered Graphitic Carbon Nitride by Nitrogen Plasma for Improved Electrocatalytic Hydrogen Evolution" Nanomaterials 9, no. 4: 568. https://doi.org/10.3390/nano9040568