Growth and Functionalization of Particle-Based Mesoporous Silica Films and Their Usage in Catalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Syntheses
2.2.1. Functionalization of Substrates
2.2.2. Film Synthesis
2.2.3. Direct Sulfonation (SBA-15-DS)
2.2.4. Carbon Infiltration and Sulfonation (SBA-15-CMK-5)
2.3. Characterization
2.4. Esterification Reaction
3. Results
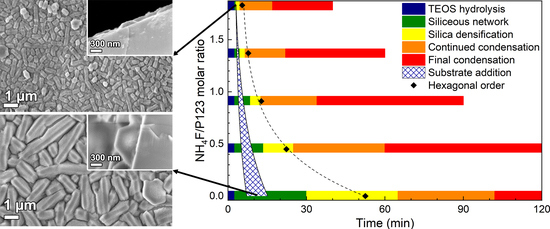
3.1. Film Growth
3.2. Surfactant Removal
3.3. Substrate Effects
3.4. Functionalization
3.5. Catalytic Performance
4. Discussion
4.1. Film Formation
4.2. Functionalization
4.3. Catalytic Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walcarius, A. Silica-Based Electrochemical Sensors and Biosensors: Recent Trends. Curr. Opin. Electrochem. 2018, 10, 88–97. [Google Scholar] [CrossRef]
- Wagner, T.; Haffer, S.; Weinberger, C.; Klaus, D.; Tiemann, M. Mesoporous Materials as Gas Sensors. Chem. Soc. Rev. 2013, 42, 4036–4053. [Google Scholar] [CrossRef]
- Innocenzi, P.; Malfatti, L. Mesoporous Thin Films: Properties and Applications. Chem. Soc. Rev. 2013, 42, 4198–4216. [Google Scholar] [CrossRef] [PubMed]
- Mutschler, A.; Stock, V.; Ebert, L.; Björk, E.M.; Leopold, K.; Lindén, M. Mesoporous silica-gold films for straightforward, highly reproducible trace-level monitoring of mercury traces in water. Nanomaterials 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Wiltschka, O.; Scheitenberger, P.; Lindén, M. Control of Particle Uptake Kinetics from Particulate Mesoporous Silica Films by Cells through Covalent Linking of Particles to the Substrate – Towards Sequential Drug Delivery for Tissue Engineering Applications. J. Mater. Chem. B 2016, 4, 7669–7675. [Google Scholar] [CrossRef]
- Ehlert, N.; Mueller, P.P.; Stieve, M.; Lenarz, T.; Behrens, P. Mesoporous Silica Films as a Novel Biomaterial: Applications in the Middle Ear. Chem. Soc. Rev. 2013, 42, 3847–3861. [Google Scholar] [CrossRef] [PubMed]
- Cauda, V.; Mühlstein, L.; Onida, B.; Bein, T. Tuning Drug Uptake and Release Rates through Different Morphologies and Pore Diameters of Confined Mesoporous Silica. Microporous Mesoporous Mater. 2009, 118, 435–442. [Google Scholar] [CrossRef]
- Song, S.; Liang, Y.; Li, Z.; Wang, Y.; Fu, R.; Wu, D.; Tsiakaras, P. Effect of Pore Morphology of Mesoporous Carbons on the Electrocatalytic Activity of Pt Nanoparticles for Fuel Cell Reactions. Appl. Catal. B Environ. 2010, 98, 132–137. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Kruk, M. Facile Method to Synthesize Platelet Sba-15 Silica with Highly Ordered Large Mesopores. J. Colloid Interface Sci. 2011, 361, 472–476. [Google Scholar] [CrossRef]
- Johansson, E.M.; Cordoba, J.M.; Oden, M. The Effects on Pore Size and Particle Morphology of Heptane Additions to the Synthesis of Mesoporous Silica Sba-15. Microporous Mesoporous Mater. 2010, 133, 66–74. [Google Scholar] [CrossRef]
- Björk, E.M.; Söderlind, F.; Odén, M. Tuning the Shape of Mesoporous Silica Particles by Alterations in Parameter Space: From Rods to Platelets. Langmuir 2013, 29, 13551–13561. [Google Scholar] [CrossRef] [PubMed]
- Björk, E.M.; Militello, M.P.; Tamborini, L.H.; Coneo Rodriguez, R.; Planes, G.A.; Acevedo, D.F.; Moreno, M.S.; Odén, M.; Barbero, C.A. Mesoporous Silica and Carbon Based Catalysts for Esterification and Biodiesel Fabrication-the Effect of Matrix Surface Composition and Porosity. Appl. Catal. A Gen. 2017, 533, 49–58. [Google Scholar] [CrossRef]
- Pirez, C.; Caderon, J.-M.; Dacquin, J.-P.; Lee, A.F.; Wilson, K. Tunable Kit-6 Mesoporous Sulfonic Acid Catalysts for Fatty Acid Esterification. ACS Catal. 2012, 2, 1607–1614. [Google Scholar] [CrossRef]
- Gustafsson, H.; Johansson, E.M.; Barrabino, A.; Odén, M.; Holmberg, K. Immobilization of Lipase from Mucor Miehei and Rhizopus Oryzae into Mesoporous Silica—The Effect of Varied Particle Size and Morphology. Colloids Surf. B Biointerfaces 2012, 100, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, H.; Zheng, Q.; Xu, J.; Li, X. Amine-Functionalized Sba-15 with Uniform Morphology and Well-Defined Mesostructure for Highly Sensitive Chemosensors to Detect Formaldehyde Vapor. Langmuir 2012, 28, 7843–7850. [Google Scholar] [CrossRef]
- Grosso, D.; Cagnol, F.; Soler-Illia, G.J.A.A.; Crepaldi, E.L.; Amenitsch, H.; Brunet-Bruneau, A.; Bourgeois, A.; Sanchez, C. Fundamentals of Mesostructuring through Evaporation-Induced Self-Assembly. Adv. Funct. Mater. 2004, 14, 309–322. [Google Scholar] [CrossRef]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-Induced Self-Assembly: Nanostructures Made Easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Bottein, T.; Loizillon, J.; Grosso, D. Full Investigation of Angle Dependence in Dip-Coating Sol–Gel Films. J. Phys. Chem. B 2017, 121, 6220–6225. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, D.R.; Sheth, P.H.; Garcia, F.L.; Brinker, C.J. Enlarged Pore Size in Mesoporous Silica Films Templated by Pluronic F127: Use of Poloxamer Mixtures and Increased Template/Sio2 Ratios in Materials Synthesized by Evaporation-Induced Self-Assembly. Chem. Mater. 2015, 27, 75–84. [Google Scholar] [CrossRef]
- Wiltschka, O.; Böcking, D.; Miller, L.; Brenner, R.E.; Sahlgren, C.; Lindén, M. Preparation, Characterization, and Preliminary Biocompatibility Evaluation of Particulate Spin-Coated Mesoporous Silica Films. Microporous Mesoporous Mater. 2014, 188, 203–209. [Google Scholar] [CrossRef]
- Znamenskaya Falk, Y.; Schmitt, J.; Alfredsson, V. Langmuir—Blodgett Monolayers of SBA-15 Particles with Different Morphologies. Microporous Mesoporous Mater. 2018, 256, 32–38. [Google Scholar] [CrossRef]
- Björk, E.M.; Söderlind, F.; Odén, M. Single-Pot Synthesis of Ordered Mesoporous Silica Films with Unique Controllable Morphology. J. Colloid Interface Sci. 2014, 413, 1–7. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Kim, T.W.; Ryoo, R. Synthesis and Characterization of Hexagonally Ordered Carbon Nanopipes. Chem. Mater. 2003, 15, 2815–2823. [Google Scholar] [CrossRef]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D. Direct Syntheses of Ordered SBA-15 Mesoporous Silica Containing Sulfonic Acid Groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Aldana-Pérez, A.; Lartundo-Rojas, L.; Gómez, R.; Niño-Gómez, M.E. Sulfonic Groups Anchored on Mesoporous Carbon Starbons-300 and Its Use for the Esterification of Oleic Acid. Fuel 2012, 100, 128–138. [Google Scholar] [CrossRef]
- Tamborini, L.H.; Militello, M.P.; Balach, J.; Moyano, J.M.; Barbero, C.A.; Acevedo, D.F. Application of Sulfonated Nanoporous Carbons as Acid Catalysts for Fischer Esterification Reactions. Arabian J. Chem. 2015. [Google Scholar] [CrossRef]
- Björk, E.M.; Mäkie, P.; Rogström, L.; Atakan, A.; Schell, N.; Odén, M. Formation of Block-Copolymer-Templated Mesoporous Silica. J. Colloid Interface Sci. 2018, 521, 183–189. [Google Scholar] [CrossRef]
- Pirez, C.; Wilson, K.; Lee, A.F. An Energy-Efficient Route to the Rapid Synthesis of Organically-Modified SBA-15 Via Ultrasonic Template Removal. Green Chem. 2014, 16, 197–202. [Google Scholar] [CrossRef]
- Song, X.; Zhao, S.; Fang, S.; Ma, Y.; Duan, M. Mesoscopic Simulations of Adsorption and Association of PEO-PPO-PEO Triblock Copolymers on a Hydrophobic Surface: From Mushroom Hemisphere to Rectangle Brush. Langmuir 2016, 32, 11375–11385. [Google Scholar] [CrossRef]
- Liu, X.; Wu, D.; Turgman-Cohen, S.; Genzer, J.; Theyson, T.W.; Rojas, O.J. Adsorption of a Nonionic Symmetric Triblock Copolymer on Surfaces with Different Hydrophobicity. Langmuir 2010, 26, 9565–9574. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.K.; Hee Han, O. Removal of Copolymer Template from SBA-15 Studied by 1h MAS NMR. Microporous Mesoporous Mater. 2007, 106, 304–307. [Google Scholar]
- Kecht, J.; Bein, T. Oxidative Removal of Template Molecules and Organic Functionalities in Mesoporous Silica Nanoparticles by H2O2 Treatment. Microporous Mesoporous Mater. 2008, 116, 123–130. [Google Scholar] [CrossRef]
- Wu, J.; Ling, L.; Xie, J.; Ma, G.; Wang, B. Surface Modification of Nanosilica with 3-Mercaptopropyl Trimethoxysilane: Experimental and Theoretical Study on the Surface Interaction. Chem. Phys. Lett. 2014, 591, 227–232. [Google Scholar] [CrossRef]
- Nassor, E.C.O.; Ávila, L.R.; Pereira, P.F.S.; Ciuffi, K.J.; Calefi, P.S.; Nassar, E.J. Influence of the Hydrolysis and Condensation Time on the Preparation of Hybrid Materials. Mater. Res. 2011, 14, 1–6. [Google Scholar] [CrossRef]
- Tamborini, L.H.; Casco, M.E.; Militello, M.P.; Silvestre-Albero, J.; Barbero, C.A.; Acevedo, D.F. Sulfonated Porous Carbon Catalysts for Biodiesel Production: Clear Effect of the Carbon Particle Size on the Catalyst Synthesis and Properties. Fuel Process. Technol. 2016, 149, 209–217. [Google Scholar] [CrossRef]
- Rocha, R.P.; Pereira, M.F.R.; Figueiredo, J.L. Carbon as a Catalyst: Esterification of Acetic Acid with Ethanol. Catal. Today 2013, 218–219, 51–56. [Google Scholar] [CrossRef]
- Fraile, J.M.; García-Bordejé, E.; Roldán, L. Deactivation of Sulfonated Hydrothermal Carbons in the Presence of Alcohols: Evidences for Sulfonic Esters Formation. J. Catal. 2012, 289, 73–79. [Google Scholar] [CrossRef]










| Material | Specific Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | Unit Cell Parameter (nm) | Acidic Sites (mmol/g) |
|---|---|---|---|---|---|
| SBA-15_0.0_no DS a | 920 | 11.2 | 1.19 | 13.6 | 0.003 |
| SBA-15_0.0_1DS | 948 | 8.9 | 1.10 | 11.2 | 0.024 |
| SBA-15_0.0_2DS | 898 | 10.2 | 1.11 | 12.6 | 0.009 |
| SBA-15_0.0_4DS | 870 | 10.4 | 1.07 | 13.6 | 0.020 |
| SBA-15_0.0_20DS | 951 | 11.3 | 1.22 | 14.1 | 0.008 |
| SBA-15_0.0_carbon temp b | 697 | 9.8 | 0.86 | 12.9 | - |
| SBA-15_0.0_CMK-5 | 521 | 9.4 | 0.68 | 12.4 | - |
| SBA-15_0.0_CMK-5_SO3H | 462 | 9.3 | 0.63 | 12.5 | 0.191 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.-H.; Mäkie, P.; Odén, M.; Björk, E.M. Growth and Functionalization of Particle-Based Mesoporous Silica Films and Their Usage in Catalysis. Nanomaterials 2019, 9, 562. https://doi.org/10.3390/nano9040562
Wu P-H, Mäkie P, Odén M, Björk EM. Growth and Functionalization of Particle-Based Mesoporous Silica Films and Their Usage in Catalysis. Nanomaterials. 2019; 9(4):562. https://doi.org/10.3390/nano9040562
Chicago/Turabian StyleWu, Pei-Hsuan, Peter Mäkie, Magnus Odén, and Emma M. Björk. 2019. "Growth and Functionalization of Particle-Based Mesoporous Silica Films and Their Usage in Catalysis" Nanomaterials 9, no. 4: 562. https://doi.org/10.3390/nano9040562