Influence of Boron, Tungsten and Molybdenum Modifiers on Zirconia Based Pt Catalyst for Glycerol Valorization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Catalytic Systems
2.2. Characterization of the Catalysts
2.3. Reactivity Tests
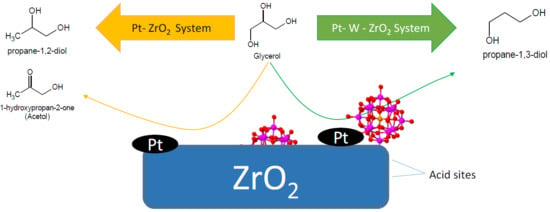
3. Results and Discussion
3.1. Textural and Structural Characterization of the Solids
3.2. Surface Acid Properties of the Catalysts
3.3. Glycerol Conversion under Hydrogenolysis Conditions
3.3.1. Influence of the Reaction Temperature
3.3.2. Acetol Conversion under Reaction Conditions
3.3.3. Reusability Study for Pt//W/ZrO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Russbueldt, B.M.E.; Hoelderich, W.F. New rare earth oxide catalyst for the transesterification of triglycerides with methanol resulting in biodediesel and pure glycerol. J. Catal. 2010, 271, 290–304. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Hezaveh, H. Glycerol for renewable acrolein production by catalytic dehydration. Renew. Sustain. Energy Rev. 2014, 40, 28–59. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; US Department of Energy: Oak Ridge, TN, USA, 2004; pp. 1–76.
- Pagliaro, M.; Rossi, M. The Future of Glycerol: New Uses of a Versatile Raw Material; RSC: Cambridge, UK, 2008. [Google Scholar]
- Johnson, D.T.; Taconi, K.A. The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. 2007, 26, 338–348. [Google Scholar] [CrossRef]
- Gholami, Z.; Abdullah, A.Z.; Lee, K.T. Catalytic upgrading to polyglycerols and other value-added products. Renew. Sustain. Energy Rev. 2014, 39, 327–341. [Google Scholar] [CrossRef]
- Chaminand, J.; Djakovitch, L.A.; Gallezot, P.; Marion, P.; Pinel, C.; Rosier, C. Glycerol hydrogenolysis on heterogeneous catalysts. Green Chem. 2004, 6, 359–361. [Google Scholar] [CrossRef]
- Vila, F.; Granados, M.L.; Ojeda, M.; Fierro, J.L.G.; Mariscal, R. Glycerol hydrogenolysis to 1,2-propanediol with Cu/g-Al2O3: Effect of the activation process. Catal. Today 2012, 187, 122–128. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Pina, C.D. Recent advances in the conversion of bioglycerol into value-added products. Eur. J. Lipid Sci. Technol. 2009, 111, 788–799. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Shinmi, Y.; Koso, S.; Tomishige, K. Direct Hydrogenolysis of Glycerol into 1,3-Propanediol over Rhenium-modified Iridium Catalyst. J. Catal. 2010, 272, 191–194. [Google Scholar] [CrossRef]
- Feng, J.; Xu, B. Reaction mechanism for the hetrogeneous hydrogenolysis of biomass-derived glicerol to propanediols. Prog. React. Kinet. Mech. 2014, 39, 1–15. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Lemonidou, A.A. Glycerol transformation to value added C3 diols: Reaction mechanism, kinetic, and engineering aspects. WIREs Energy Environ. 2014, 4, 486–520. [Google Scholar] [CrossRef]
- Yuan, Z.; Wu, P.; Gao, J.; Lu, X.; Hou, Z.; Zheng, X. Pt7solid-base: A predominant catalyst for glycerol hydrogenolysis in a base-free aqueous solution. Catal. Lett. 2009, 130, 261–265. [Google Scholar] [CrossRef]
- Marinas, A.; Bruijnincx, P.; Ftouni, J.; Urbano, F.J.; Pinel, C. Sustainability metrics for a fossil- and renewable-based route for 1,2-propanediol production: A comparison. Catal. Today 2015, 239, 31–37. [Google Scholar] [CrossRef]
- Montes, V.; Checa, M.; Marinas, A.; Boutonnet, M.; Marinas, J.M.; Urbano, F.J.; Järas, S.; Pinel, C. Synthesis of different ZnO-supported metal systems through microemulsion technique and application to catalytic transformation of glycerol to acetol and 1,2-propanedio. Catal. Today 2014, 223, 129–137. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, Y.; Hao, S.; Chen, L.; Zhang, B.; Li, Y. Aqueous-phase hydrogenolysis of glycerol to 1,3-propanediol over Pt-H4SiW12O40/SiO2. Catal. Lett. 2012, 142, 267–274. [Google Scholar] [CrossRef]
- Priya, S.; Kumar, V.; Kantam, M.; Bhargava, S.; Chary, K. Vapour-phase hydrogenolysis of glycerol to 1,3-propanediol over supported pt catalysts: The effect of supports on the catalytic functionalities. Catal. Lett. 2014, 144, 2129–2143. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Catalyst development for the hydrogenolysis of biomass derived chemicals to value-added ones. Catal. Surv. Asia 2011, 15, 111–116. [Google Scholar] [CrossRef]
- Montes, V.; Boutonnet, M.; Järås, S.; Lualdi, M.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Mora, M. Preparation and characterization of Pt-modified Co-based catalysts through the microemulsion technique: Preliminary results on the Fischer-Tropsch synthesis. Catal. Today 2014, 223, 66–75. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic materials for the hydrogenolysis of glycerol to 1,3-propanediol. J. Mater. Chem. A 2014, 2, 6688–6702. [Google Scholar] [CrossRef]
- Checa, M.; Auneau, F.; Hidalgo-Carrillo, J.; Marinas, A.; Marinas, J.M.; Pinel, C.; Urbano, F.J. Catalytic transformation of glycerol on several metal systems supported on ZnO. Catal. Today 2012, 196, 91–100. [Google Scholar] [CrossRef]
- Checa, M.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Deactivation study of supported Pt catalyst on glycerol Hydrogenolysis. Appl. Catal. A Gen. 2015, 507, 34–43. [Google Scholar] [CrossRef]
- Dam, J.; Djanashvili, K.; Kapteijn, F.; Hanefeld, U. Pt/Al2O3 Catalyzed 1,3-Propanediol Formation from Glycerol using Tungsten Additives. ChemCatChem 2013, 5, 497–505. [Google Scholar]
- Hu, J.; Liu, X.; Wang, B.; Pei, Y.; Qiao, M.; Fan, K. Reforming and Hydrogenolysis of Glycerol over Ni/ZnO Catalysts Prepared by Different Methods. Chin. J. Catal. 2012, 33, 1266–1275. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy, B.V.S.; Reddy, B.M. Development of cerium promoted copper–magnesium catalysts for biomass valorization: Selective hydrogenolysis of bioglycerol. Appl. Catal. B Environ. 2016, 181, 47–57. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Ma, L.; He, D. Glycerol hydrogenolysis to propanediols over supported Pd–Re catalysts. RSC Adv. 2014, 4, 5503–5512. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Li, Y. Promoting effect of WOx on selective hydrogenolysis of glycerol to 1,3-propanediol over bifunctional Pt–WOx/Al2O3 catalysts. J. Mol. Catal. A Chem. 2015, 398, 391–398. [Google Scholar] [CrossRef]
- Kittisakmontree, P.; Yoshida, H.; Fujita, S.; Arai, M.; Panpranot, J. The effect of TiO2 particle size on the characteristics of Au-Pd/TiO 2 catalysts. Catal. Commun. 2015, 58, 70–75. [Google Scholar] [CrossRef]
- Zanin, C.I.C.B.; Jordao, E.; Mandelli, D.; Figueiredo, F.C.A.; Carvalho, W.A.; Oliveira, E.V. Hydrogenolysis of glycerol to alcohols catalyzed by transition metals supported on pillared clay. React. Kinet. Mech. Catal. 2015, 115, 293–311. [Google Scholar] [CrossRef]
- García-Fernández, S.; Gandarias, I.; Requies, J.; Güemez, M.B.; Bennici, S.; Auroux, A.; Arias, P.L. New approaches to the Pt/WOx/Al2O3 catalytic system behavior for the selective glycerol hydrogenolysis to 1,3-propanediol. J. Catal. 2015, 323, 65–75. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.; Wang, Y.; Wang, S.; Ma, X. Glycerol Hydrogenolysis to 1,3-Propanediol on Tungstate/Zirconia-Supported Platinum: Hydrogen Spillover Facilitated by Pt(1 1 1) Formation. ChemCatChem 2016, 8, 3663–3671. [Google Scholar] [CrossRef]
- Ciftci, A.; Peng, B.; Jentys, A.; Lercher, J.A.; Hensen, E.J.M. Support effects in the aqueous phase reforming of glycerol over supported platinum catalysts. Appl. Catal. A Gen. 2012, 431–432, 113–119. [Google Scholar] [CrossRef]
- Choi, Y.; Park, H.; Yun, Y.S.; Yi, J. Effects of Catalyst Pore Structure and Acid Properties on the Dehydration of Glycerol. ChemSusChem 2015, 8, 974–979. [Google Scholar] [CrossRef]
- Dam, J.T.; Kapteijn, F.; Djanashvili, K.; Hanefeld, U. Tuning selectivity of Pt/CaCO3 in glycerol hydrogenolysis-A Design of Experiments approach. Catal. Commun. 2011, 13, 1–5. [Google Scholar]
- Montassier, C.; Dumas, J.M.; Granger, P.; Barbie, J. Deactivation of supported copper based catalysts during polyol conversion in aqueous phase. Appl. Catal. A Gen. 1995, 121, 231–244. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Kim, J.; Fernando, W.J.N. Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Appl. Catal. A Gen. 2009, 363, 1–10. [Google Scholar] [CrossRef]
- Osatiashtiani, A.; Lee, A.F.; Brown, D.R.; Melero, J.A.; Morales, G.; Wilson, K. Bifunctional SO4/ZrO2 catalysts for 5-hydroxymethylfufural (5-HMF) production from glucose. Catal. Sci. Technol. 2014, 4, 333–342. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Pal, P.; Tarafdar, A.; Bajaj, H.C.; Panda, A.B. Mesoporous Borated Zirconia: A Solid Acid-Base Bifunctional Catalyst. ChemCatChem 2013, 5, 331–338. [Google Scholar] [CrossRef]
- Pizzio, L.; Vázquez, P.; Cáceres, C.; Blanco, M. Tungstophosphoric and molybdophosphoric acids supported on zirconia as esterification catalysts. Catal. Lett. 2001, 77, 233–239. [Google Scholar] [CrossRef]
- Zhou, W.; Luo, J.; Wang, Y.; Liu, J.; Zhao, Y.; Wang, S.; Ma, X. WOx domain size, acid properties and mechanistic aspects of glycerol hydrogenolysis over Pt/WOx/ZrO2. Appl. Catal. B 2019, 242, 410–421. [Google Scholar] [CrossRef]
- Fan, Y.; Cheng, S.; Wang, H.; Tian, J.; Xie, S.; Pei, Y.; Qiao, M.; Zong, B. Pt–WOx on monoclinic or tetrahedral ZrO2: Crystal phase effect of zirconia on glycerol hydrogenolysis to 1,3-propanediol. Appl. Catal. B 2017, 217, 331–341. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Zhu, Y.; Xiang, X.; Hu, C.; Li, Y. Alkaline metals modified Pt–H4SiW12O40/ZrO2 catalysts for the selective hydrogenolysis of glycerol to 1,3-propanediol. Appl. Catal. B 2013, 140–141, 60–67. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, Y.; Hao, S.; Zheng, H.; Mo, T.; Li, Y. One-step hydrogenolysis of glycerol to biopropanols over Pt-H4SiW12O40/ZrO2 catalysts. Green Chem. 2012, 14, 2607–2616. [Google Scholar] [CrossRef]
- Gong, L.; Lu, Y.; Ding, Y.; Lin, R.; Li, J.; Dong, W.; Wang, T.; Chen, W. Selective hydrogenolysis of glycerol to 1,3-propanediol over a Pt/WO3/TiO2/SiO2 catalyst in aqueous media. Appl. Catal. A Gen. 2010, 390, 119–126. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Y.; Zheng, H.; Ding, G.; Li, Y. Direct conversion of glycerol into 1,3-propanediol over Cu-H4SiW12O40/SiO2 in vapor phase. Catal. Lett. 2009, 131, 312–320. [Google Scholar] [CrossRef]
- Arundhathi, R.; Mizugaki, T.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Highly selective hydrogenolysis of glycerol to 1,3-propanediol over a boehmite-supported platinum/tungsten catalyst. ChemSusChem 2013, 6, 1345–1349. [Google Scholar] [CrossRef]
- Zhu, S.; Qiu, Y.; Zhu, Y.; Hao, S.; Zheng, H.; Li, Y. Hydrogenolysis of glycerol to 1,3-propanediol over bifunctional catalysts containing Pt and heteropolyacids. Catal. Today 2013, 212, 120–126. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Porras, A.; Urbano, F.J. Synthesis and characterization of ZrO2 as an acid-base catalyst: Dehydration-dehydrogenation of propan-2-ol. J. Chem. Soc. Faraday Trans. 1997, 93, 1431–1438. [Google Scholar] [CrossRef]
- Haffad, D.; Chambellan, A.; Lavalley, J.C. Propan-2-ol transformation on simple metal oxides TiO2, ZrO2 and CeO2. J. Mol. Catal. A Chem. 2001, 168, 153–164. [Google Scholar] [CrossRef]
- Alves-Rosa, M.A.; Martins, L.; Hammer, P.; Pulcinelli, S.H.; Santilli, C.V. Structure and catalytic properties of sulfated zirconia foams. J. Sol-Gel Sci. Technol. 2014, 72, 252–259. [Google Scholar] [CrossRef]
- Consonni, M.; Jokic, D.; Murzin, D.Y.; Touroude, R. High performances of Pt/ZnO catalysts in selective hydrogenation of crotonaldehyde. J. Catal. 1999, 188, 165–175. [Google Scholar] [CrossRef]
- Miranda, M.; Ramírez, S.A.; Jurado, S.G.; Vera, C.R. Superficial effects and catalytic activity of ZrO2–SO42− as a function of the crystal structure. J. Mol. Catal. A Chem. 2015, 398, 325–335. [Google Scholar] [CrossRef]
- Seretis, A.; Tsiakaras, P. Aqueous phase reforming (APR) of glycerol over platinum supported on Al2O3 catalyst. Renew. Energy 2016, 85, 1116–1126. [Google Scholar] [CrossRef]
- Priya, S.S.; Kumar, V.P.; Kantam, M.L.; Bhargava, S.K.; Periasamy, S.; Chary, K.V.R. Metal–acid bifunctional catalysts for selective hydrogenolysis of glycerol under atmospheric pressure: A highly selective route to produce propanols. Appl. Catal. A Gen. 2015, 498, 88–98. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Requies, J.; Güemez, M.B.; Fierro, J.L.G. Hydrogenolysis of glycerol to propanediols over a Pt/ASA catalyst: The role of acid and metal sites on product selectivity and the reaction mechanism. Appl. Catal. B Environ. 2010, 97, 248–256. [Google Scholar] [CrossRef]
- Suprun, W.; Lutecki, M.; Haber, T.; Papp, H. Acidic catalysts for the dehydration of glycerol: Activity and deactivation. J. Mol. Catal. A Chem. 2009, 309, 71–78. [Google Scholar] [CrossRef]
- Du, H.; Chen, S.; Wang, H.; Lu, J. Acidic alumina overcoating on platinum nanoparticles: Close metal–acid proximity enhances bifunctionality for glycerol hydrogenolysis. Chin. J. Catal. 2017, 38, 1237–1244. [Google Scholar] [CrossRef]
- García-Fernández, S.; Gandarias, I.; Requies, J.; Soulimani, F.; Arias, P.L.; Weckhuysen, B.M. The role of tungsten oxide in the selective hydrogenolysis of glycerol to 1,3-propanediol over Pt/WOx/Al2O3. Appl. Catal. B Environ. 2017, 204, 260–272. [Google Scholar] [CrossRef]
- Liao, X.; Li, K.; Xiang, X.; Wang, S.G.; She, X.; Zhu, Y.; Li, Y. Mediatory role of K, Cu and Mo over Ru/SiO2 catalysts for glycerol hydrogenolysis. J. Ind. Eng. Chem. 2012, 18, 818–821. [Google Scholar] [CrossRef]











| Catalyst | SBET (m2·g−1) | Metal a Content (at.%) ICP-MS | Pt Content (wt.%) ICP-MS | Pt Particle Size (nm) H2 Chemisorp. | XPS (at.%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Zr (%) | M a (%) | Cl (%) | O (%) | Pt (%) | |||||
| Pt/ZrO2 | 109 | - | 4.8 | 4 | 27.76 | - | 11.74 | 59.26 | 1.24 |
| Pt//B/ZrO2 | 93 | 11 | 4.6 | 7 | 25.61 | 7.70 | 11.74 | 53.85 | 1.10 |
| Pt//Mo/ZrO2 | 86 | 7.0 | 4.6 | 13 | 28.11 | 2.31 | 11.37 | 57.45 | 0.76 |
| Pt//W/ZrO2 | 86 | 8.3 | 4.6 | 8 | 27.42 | 1.55 | 10.00 | 59.96 | 1.07 |
| Catalyst | Temp. | Conv. | Products Selectivity (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| i-PrOH | n-PrOH | Acetol | 1,2-PDO | 1,3-PDO | EG | Others | Gas Phase | |||
| Pt//Mo/ZrO2-200 | 200 | 8.4 | 0.0 | 5.1 | 7.6 | 6.6 | 0.0 | 0.9 | 60.5 | 19.3 |
| 180 | 6.7 | 2.3 | 8.1 | 2.0 | 48.9 | 0.0 | 4.3 | 13.3 | 21.2 | |
| 160 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Pt//W/ZrO2-200 | 200 | 53.8 | 8.0 | 40.5 | 1.2 | 7.2 | 12.3 | 0.3 | 14.7 | 15.8 |
| 180 | 34.5 | 5.1 | 52.4 | 0.8 | 5.2 | 23.0 | 0.3 | 7.1 | 6.1 | |
| 160 | 19.7 | 2.9 | 34.5 | 0.3 | 4.2 | 42.1 | 0.1 | 6.4 | 9.5 | |
| Pt//B/ZrO2-200 | 200 | 8.3 | 3.8 | 12.2 | 5.9 | 14.0 | 0.0 | 11.5 | 23.3 | 29.2 |
| 180 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 160 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Pt/ZrO2-200 | 200 | 5.4 | 3.8 | 10.7 | 8.9 | 18.9 | 0.0 | 18.0 | 39.8 | >1 |
| 180 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 160 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Pt (wt.%) | W (wt.%) | |
|---|---|---|
| Pt//W/ZrO2-Fresh | 4.60 | 8.32 |
| Pt//W/ZrO2-Used | 4.58 | 7.46 |
| Aqueous Phase | 0.01 | 0.80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checa, M.; Montes, V.; Hidalgo-Carrillo, J.; Marinas, A.; Urbano, F.J. Influence of Boron, Tungsten and Molybdenum Modifiers on Zirconia Based Pt Catalyst for Glycerol Valorization. Nanomaterials 2019, 9, 509. https://doi.org/10.3390/nano9040509
Checa M, Montes V, Hidalgo-Carrillo J, Marinas A, Urbano FJ. Influence of Boron, Tungsten and Molybdenum Modifiers on Zirconia Based Pt Catalyst for Glycerol Valorization. Nanomaterials. 2019; 9(4):509. https://doi.org/10.3390/nano9040509
Chicago/Turabian StyleCheca, Manuel, Vicente Montes, Jesús Hidalgo-Carrillo, Alberto Marinas, and Francisco J. Urbano. 2019. "Influence of Boron, Tungsten and Molybdenum Modifiers on Zirconia Based Pt Catalyst for Glycerol Valorization" Nanomaterials 9, no. 4: 509. https://doi.org/10.3390/nano9040509