Construction of Novel Polymerizable Ionic Liquid Microemulsions and the In Situ Synthesis of Poly(Ionic Liquid) Adsorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization of PILs
3. Results and Discussion
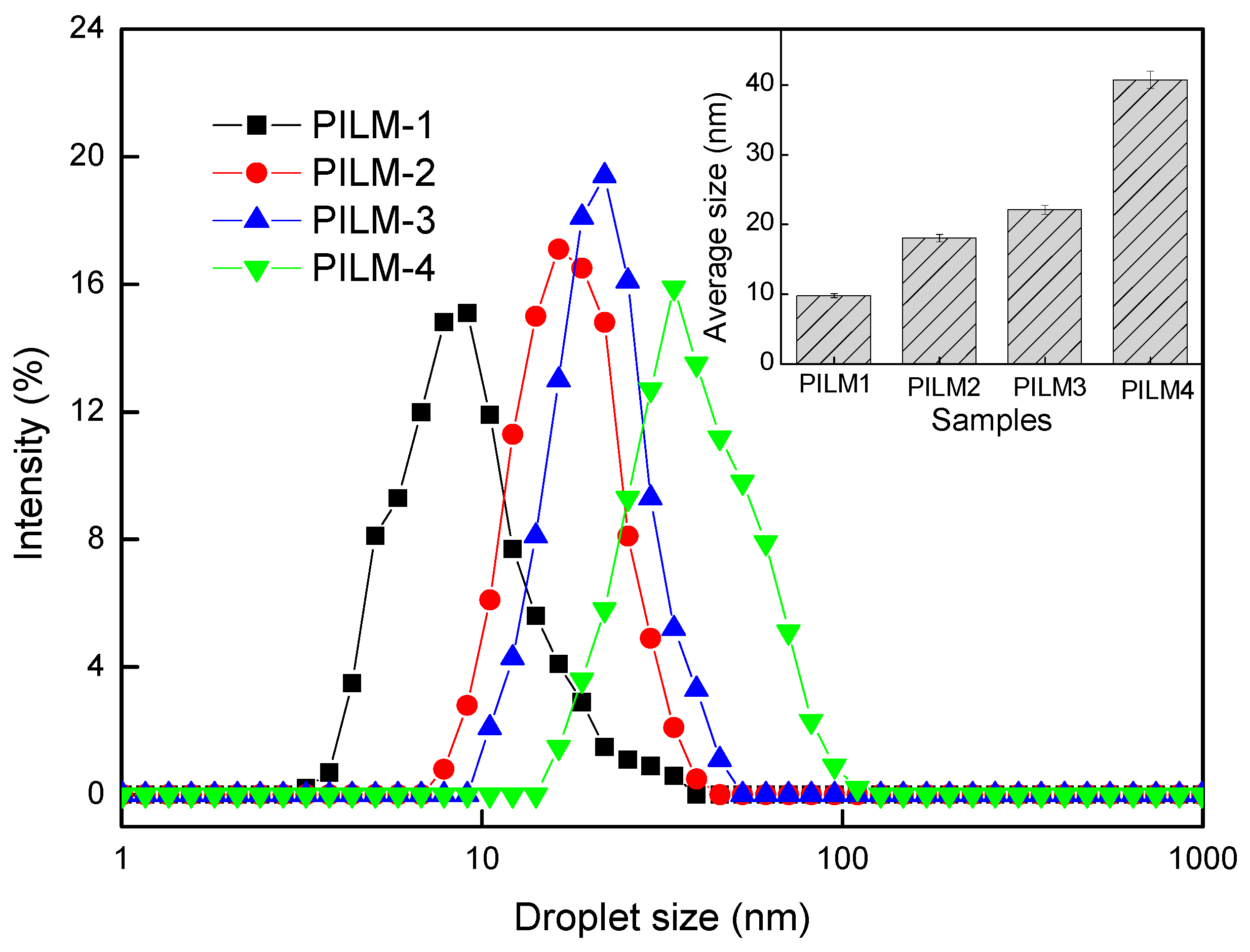
3.1. Droplet Size and Size Distribution
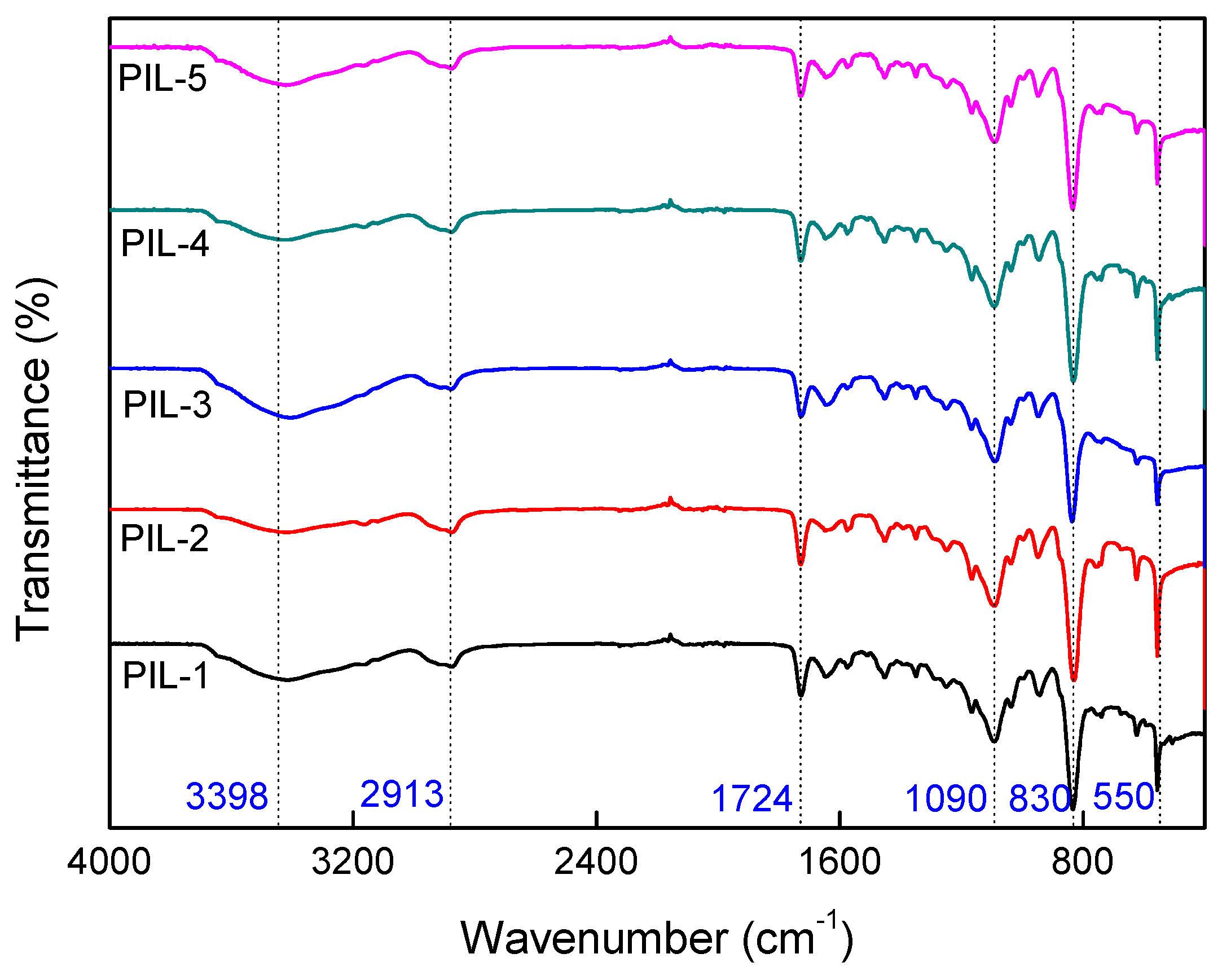
3.2. FTIR Analysis
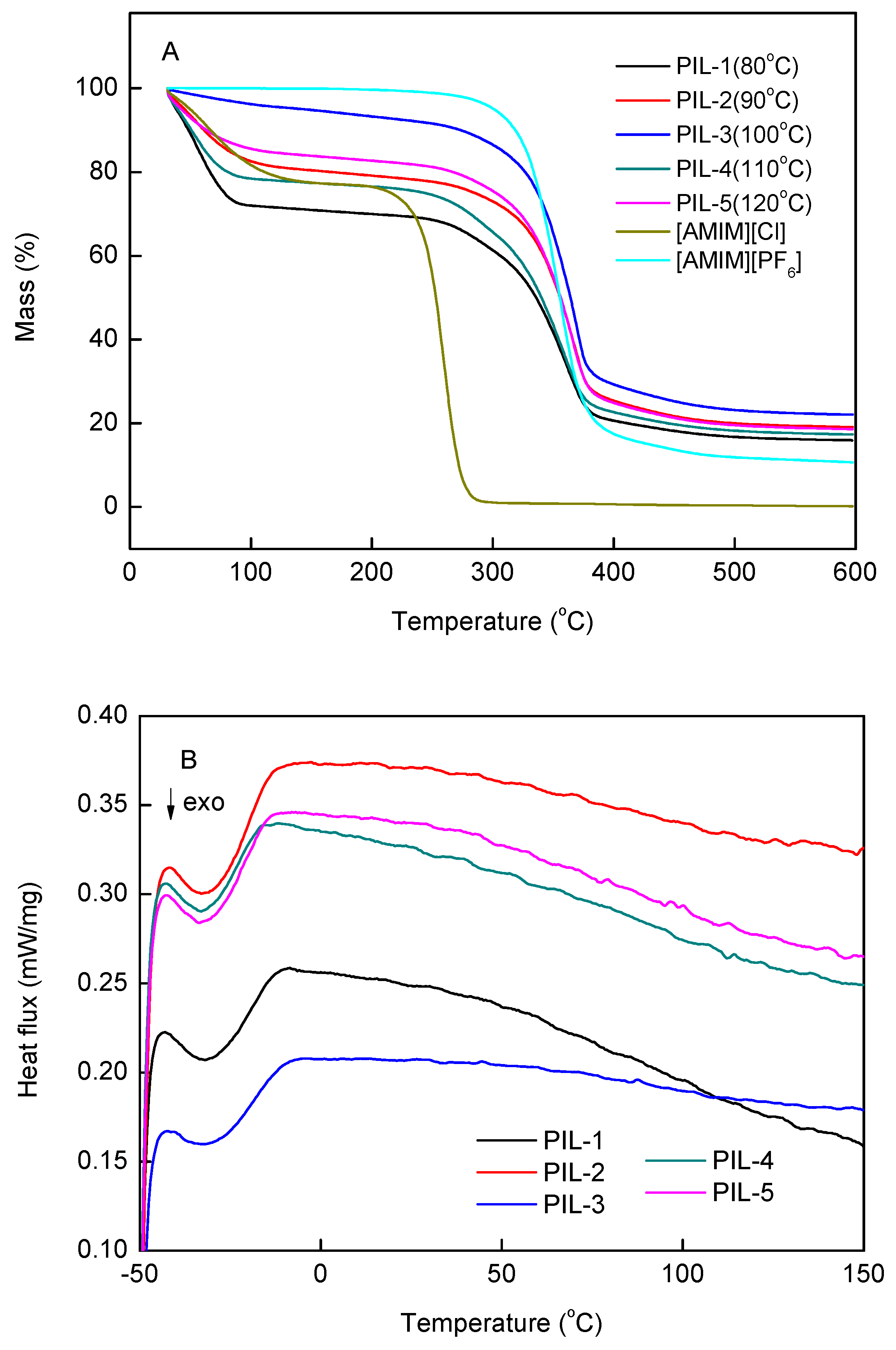
3.3. Thermal Analysis of PILs
3.4. Influence on the Adsorption Potential of PILs
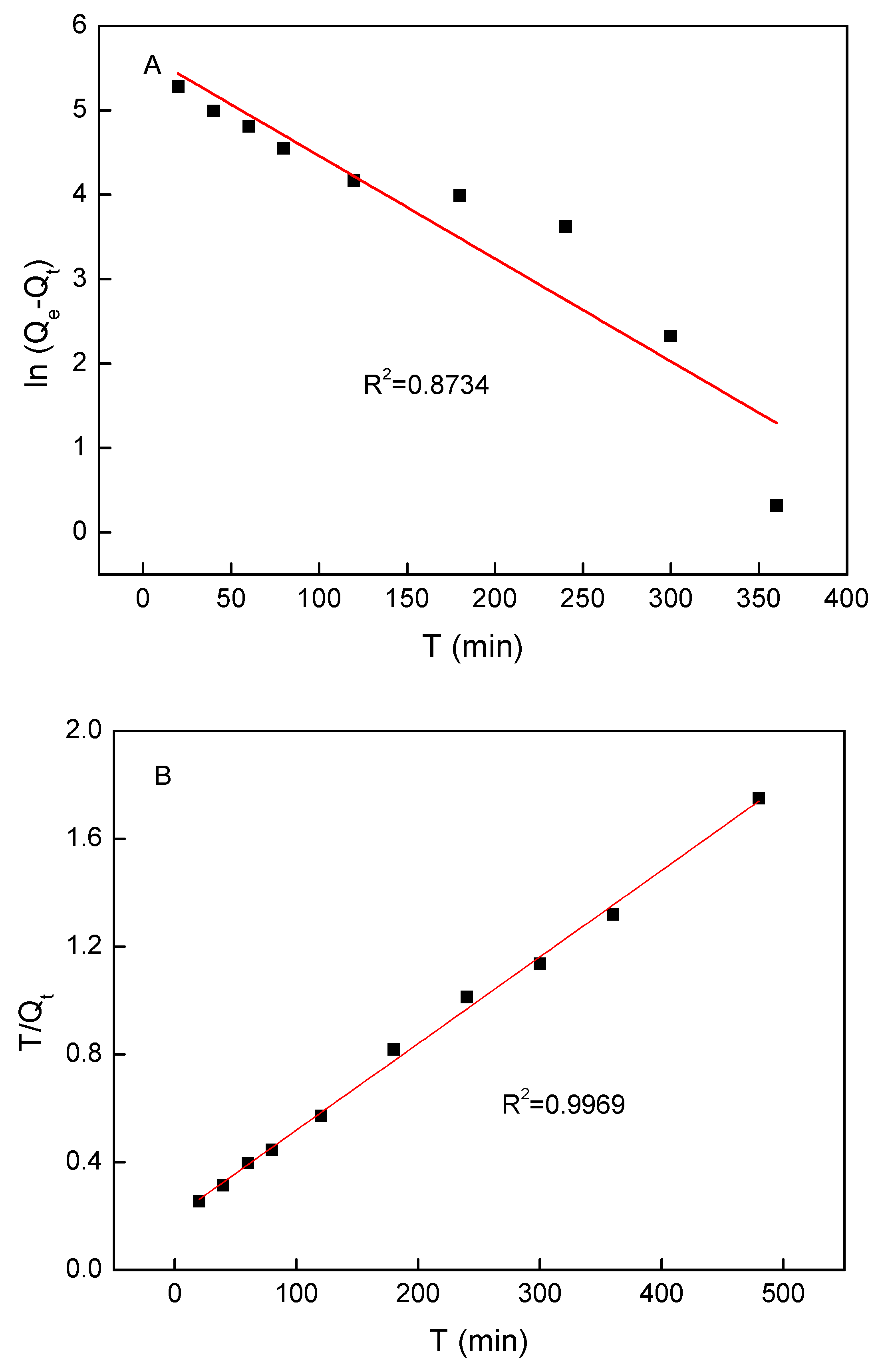
3.5. Adsorption Kinetics
3.6. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, J.Y.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Wang, S.J.; Ma, H.L.; Peng, J.; Zhang, Y.W.; Chen, J.; Wang, L.L.; Xu, L.; Li, J.Q.; Zhai, M.L. Facile synthesis of a novel polymeric ionic liquid gel and its excellent performance for hexavalent chromium removal. Dalton Trans. 2015, 44, 7618–7625. [Google Scholar] [CrossRef] [PubMed]
- Patrascu, C.; Gauffre, F.; Nallet, F.; Bordes, R.; Oberdisse, J.; de Lauth-Viguerie, N.; Mingotaud, C. Micelles in ionic liquids: Aggregation behavior of alkyl poly(ethyleneglycol)-ethers in 1-butyl-3-methyl-imidazolium type ionic liquids. ChemPhysChem 2006, 7, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.Y.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.J.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef] [PubMed]
- Mudraboyina, B.P.; Obadia, M.M.; Allaoua, I.; Sood, R.; Serghei, A.; Drockenmuller, E. 1,2,3-Triazolium-Based Poly(ionic liquid)s with Enhanced Ion Conducting Properties Obtained through a Click Chemistry Polyaddition Strategy. Chem. Mater. 2014, 26, 1720–1726. [Google Scholar] [CrossRef]
- Zhao, W.F.; Tang, Y.S.; Xi, J.; Kong, J. Functionalized graphene sheets with poly(ionic liquid)s and high adsorption capacity of anionic dyes. Appl. Surf. Sci. 2015, 326, 276–284. [Google Scholar] [CrossRef]
- Liu, C.; Liao, Y.M.; Huang, X.J. Extraction of triazole fungicides in environmental waters utilizing poly (ionic liquid)-functionalized magnetic adsorbent. J. Chromatogr. A 2017, 1524, 13–20. [Google Scholar] [CrossRef]
- Mi, H.; Jiang, Z.G.; Kong, J. Hydrophobic Poly(ionic liquid) for Highly Effective Separation of Methyl Blue and Chromium Ions from Water. Polymers 2013, 5, 1203–1214. [Google Scholar] [CrossRef] [Green Version]
- Khiratkar, A.G.; Kumar, S.S.; Bhagat, P.R. Designing a sulphonic acid functionalized benzimidazolium based poly(ionic liquid) for efficient adsorption of hexavalent chromium. RSC Adv. 2016, 6, 37757–37764. [Google Scholar] [CrossRef]
- Wang, A.L.; Chen, L.; Zhang, J.X.; Sun, W.C.; Guo, P.; Ren, C.Y. Ionic liquid microemulsion-assisted synthesis and improved photocatalytic activity of ZnIn2S4. J. Mater. Sci. 2017, 52, 2413–2421. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Lee, S.; Yim, T.; Park, Y.D.; Mun, J. Room Temperature Ionic Liquid-Activated Nafion Polymer Electrolyte for High Temperature Operation. Polym.-Korea 2018, 42, 682–686. [Google Scholar] [CrossRef]
- Wang, A.L.; Chen, L.; Jiang, D.Y.; Zeng, H.Y.; Yan, Z.C. Vegetable oil-based ionic liquid microemulsion biolubricants: Effect of integrated surfactants. Ind. Crops Prod. 2014, 62, 515–521. [Google Scholar] [CrossRef]
- Mirhoseini, F.; Salabat, A. Ionic liquid based microemulsion method for the fabrication of poly(methyl methacrylate)-TiO2 nanocomposite as a highly efficient visible light photocatalyst. RSC Adv. 2015, 5, 12536–12545. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Zhu, X.; Zhou, Y.; Li, D. Synthesis of Tributylphosphate Capped Luminescent Rare Earth Phosphate Nanocrystals in an Ionic Liquid Microemulsion. Chem. Mater. 2009, 21, 3570–3575. [Google Scholar] [CrossRef]
- He, D.L.; Xia, S.B.; Zhou, Z.; Zhong, J.F.; Guo, Y.N.; Yang, R.H. Electropolymerization of polyaniline in ionic liquid (bmim PF6)/water microemulsion. J. Exp. Nanosci. 2013, 8, 103–112. [Google Scholar] [CrossRef]
- Wang, A.; Li, S.; Zhang, L.; Chen, H.; Li, Y.; Hu, L.; Peng, X. Ionic liquid microemulsion-mediated in situ thermosynthesis of poly(ionic liquid)s and their adsorption properties for Zn(II). Polym. Eng. Sci. 2019. [Google Scholar] [CrossRef]
- Ennigrou, D.J.; Ali, M.B.; Dhahbi, M. Copper and Zinc removal from aqueous solutions by polyacrylic acid assisted-ultrafiltration. Desalination 2014, 343, 82–87. [Google Scholar] [CrossRef]
- Warisnoicharoen, W.; Lansley, A.B.; Lawrence, M.J. Nonionic oil-in-water microemulsions: The effect of oil type on phase behaviour. Int. J. Pharm. 2000, 198, 7–27. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.Y.; Bai, T.C. Phase Diagrams, Density, and Viscosity for the Pseudoternary System of {Propan-2-yl Tetradecanoate (IPM) (1) plus Tween 80 (21) plus Propan-1-ol (22) (2) plus Water (3)}. J. Chem. Eng. Data 2012, 57, 2023–2029. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Sreejalekshmi, K.G.; Vimexen, V.; Dev, V.V. Evaluation of adsorption properties of sulphurised activated carbon for the effective and economically viable removal of Zn(II) from aqueous solutions. Ecotoxicol. Environ. Saf. 2016, 124, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Musso, T.B.; Parolo, M.E.; Pettinari, G.; Francisca, E.M. Cu(II) and Zn(II) adsorption capacity of three different clay liner materials. J. Environ. Manag. 2014, 146, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.J.; Parvini, M.; Ghorbani, M. Experimental design data for the zinc ions adsorption based on mesoporous modified chitosan using central composite design method. Carbohydr. Polym. 2018, 188, 197–212. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tomita, M.; Kato, K. Synthesis of amino-functionalized mesoporous silica sheets and their application for metal ion capture. J. Asian Ceram. Soc. 2015, 3, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.Y.; Tang, L.H.; Li, K.; Ning, P.; Peng, J.H.; Guo, H.B.; Zhu, T.T.; Liu, Y. Highly selective removal of Zn(II) ion from hot-dip galvanizing pickling waste with amino-functionalized Fe3O4@SiO2 magnetic nano-adsorbent. J. Colloid Interface Sci. 2016, 462, 235–242. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A. Preparation of cross-linked magnetic chitosan-phenylthiourea resin for adsorption of Hg(II), Cd(II) and Zn(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 209, 240–249. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Chen, D.H. Fast removal of copper ions by gum arabic modified magnetic nano-adsorbent. J. Hazard. Mater. 2007, 147, 792–799. [Google Scholar] [CrossRef]
- Da’na, E.; Sayari, A. Adsorption of copper on amine-functionalized SBA-15 prepared by co-condensation: Equilibrium properties. Chem. Eng. J. 2011, 166, 445–453. [Google Scholar] [CrossRef]
- Oke, I.A.; Olarinoye, N.O.; Adewusi, S.R.A. Adsorption kinetics for arsenic removal from aqueous solutions by untreated powdered eggshell. Adsorpt.-J. Int. Adsorpt. Soc. 2008, 14, 73–83. [Google Scholar] [CrossRef]
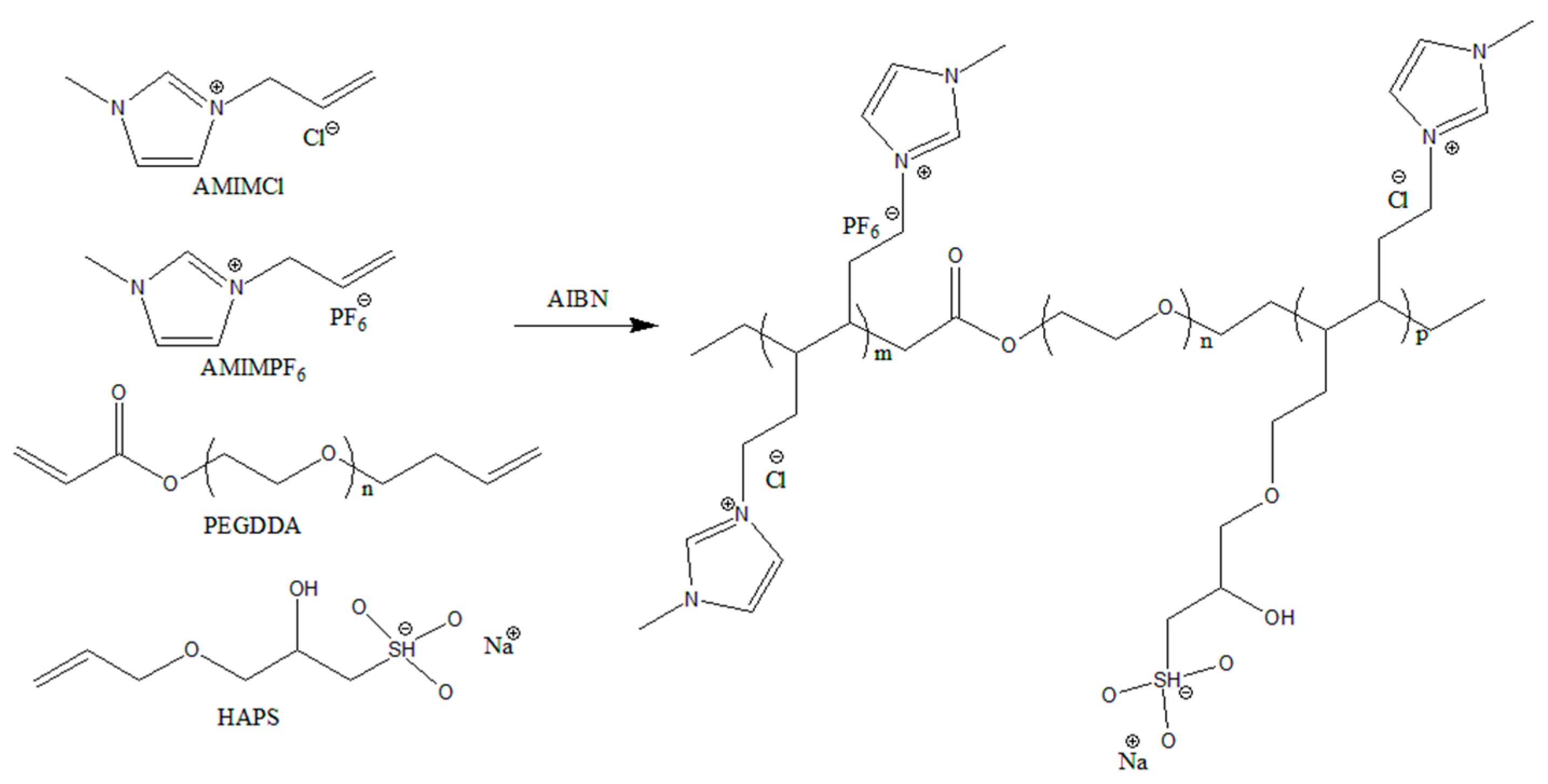


| Sample Names | m(AMIMCl) /g | m(AMIMPF6) /g | m(HAPS) /g | m(PEGDA) /g | m(AIBN) /g | Temp. /°C |
|---|---|---|---|---|---|---|
| PILM-1 | 3.0611 | 4.0031 | 6.0065 | 0.7221 | \ | 60 |
| PILM-2 | 3.0618 | 4.0243 | 5.9999 | 1.0731 | \ | 60 |
| PILM-3 | 3.0481 | 4.0031 | 6.0145 | 1.7653 | \ | 60 |
| PILM-4 | 3.0814 | 4.0005 | 6.0062 | 2.8480 | \ | 60 |
| PIL-1 | 3.0615 | 4.0207 | 6.0125 | 2.8482 | 1.1188 | 80 |
| PIL-2 | 3.0588 | 4.0515 | 6.0003 | 2.8548 | 1.1196 | 90 |
| PIL-3 | 3.0492 | 4.0054 | 6.0082 | 2.8395 | 1.1172 | 100 |
| PIL-4 | 3.0630 | 4.0943 | 6.0168 | 2.8704 | 1.1361 | 110 |
| PIL-5 | 3.0346 | 4.0721 | 6.0270 | 2.8555 | 1.1231 | 120 |
| PIL | PIL-1 | PIL-2 | PIL-3 | PIL-4 | PIL-5 |
|---|---|---|---|---|---|
| Es | 21.37 | 21.01 | 20.82 | 21.18 | 20.85 |
| Qe,exp mg/g | Pseudo-First Order | Pseudo-Second Order | |||||
|---|---|---|---|---|---|---|---|
| Qe,cal mg/g | k1 | R2 | Qe,cal mg/g | k2 × 104 | H mg/(g min) | R2 | |
| 273.03 | 196.17 | 0.0120 | 0.8743 | 312.5 | 0.5172 | 5.0505 | 0.9969 |
| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| Qmax mg/g | kL L/mg | R2 | Kf L/g | n | R2 |
| 0.1505 | 0.6801 | 0.1056 | 8.9991 | 1.1325 | 0.9970 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Li, S.; Chen, H.; Liu, Y.; Peng, X. Construction of Novel Polymerizable Ionic Liquid Microemulsions and the In Situ Synthesis of Poly(Ionic Liquid) Adsorbents. Nanomaterials 2019, 9, 454. https://doi.org/10.3390/nano9030454
Wang A, Li S, Chen H, Liu Y, Peng X. Construction of Novel Polymerizable Ionic Liquid Microemulsions and the In Situ Synthesis of Poly(Ionic Liquid) Adsorbents. Nanomaterials. 2019; 9(3):454. https://doi.org/10.3390/nano9030454
Chicago/Turabian StyleWang, Aili, Shuhui Li, Hou Chen, Ying Liu, and Xiong Peng. 2019. "Construction of Novel Polymerizable Ionic Liquid Microemulsions and the In Situ Synthesis of Poly(Ionic Liquid) Adsorbents" Nanomaterials 9, no. 3: 454. https://doi.org/10.3390/nano9030454