Thermal Properties and Fracture Toughness of Epoxy Nanocomposites Loaded with Hyperbranched-Polymers-Based Core/Shell Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
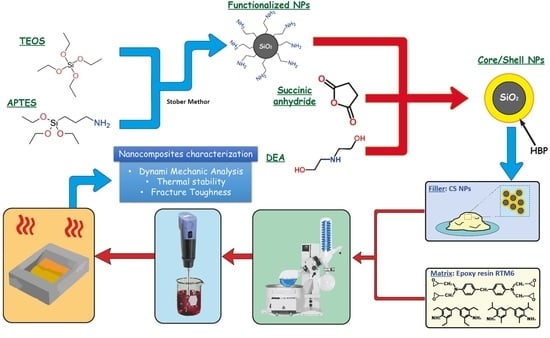
2.2. CSNPs Synthesis
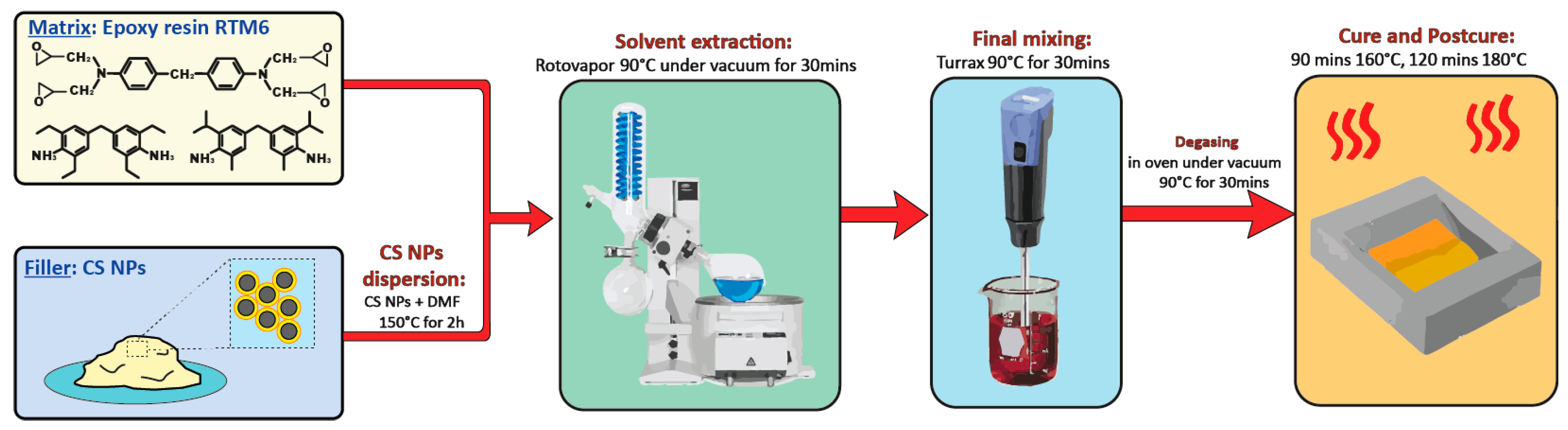
2.3. Nanocomposites Preparation
2.4. Characterization
3. Results and Discussion
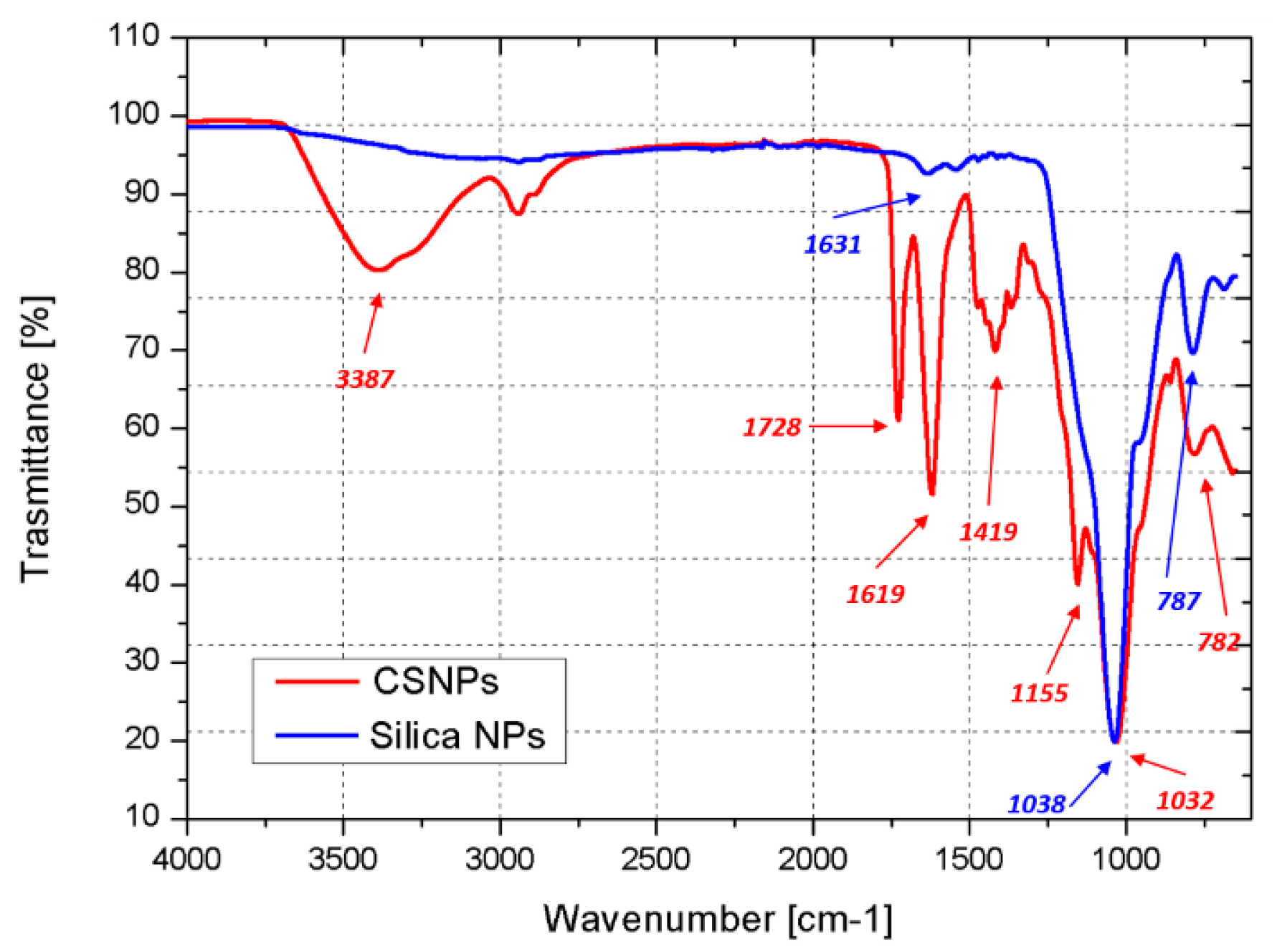
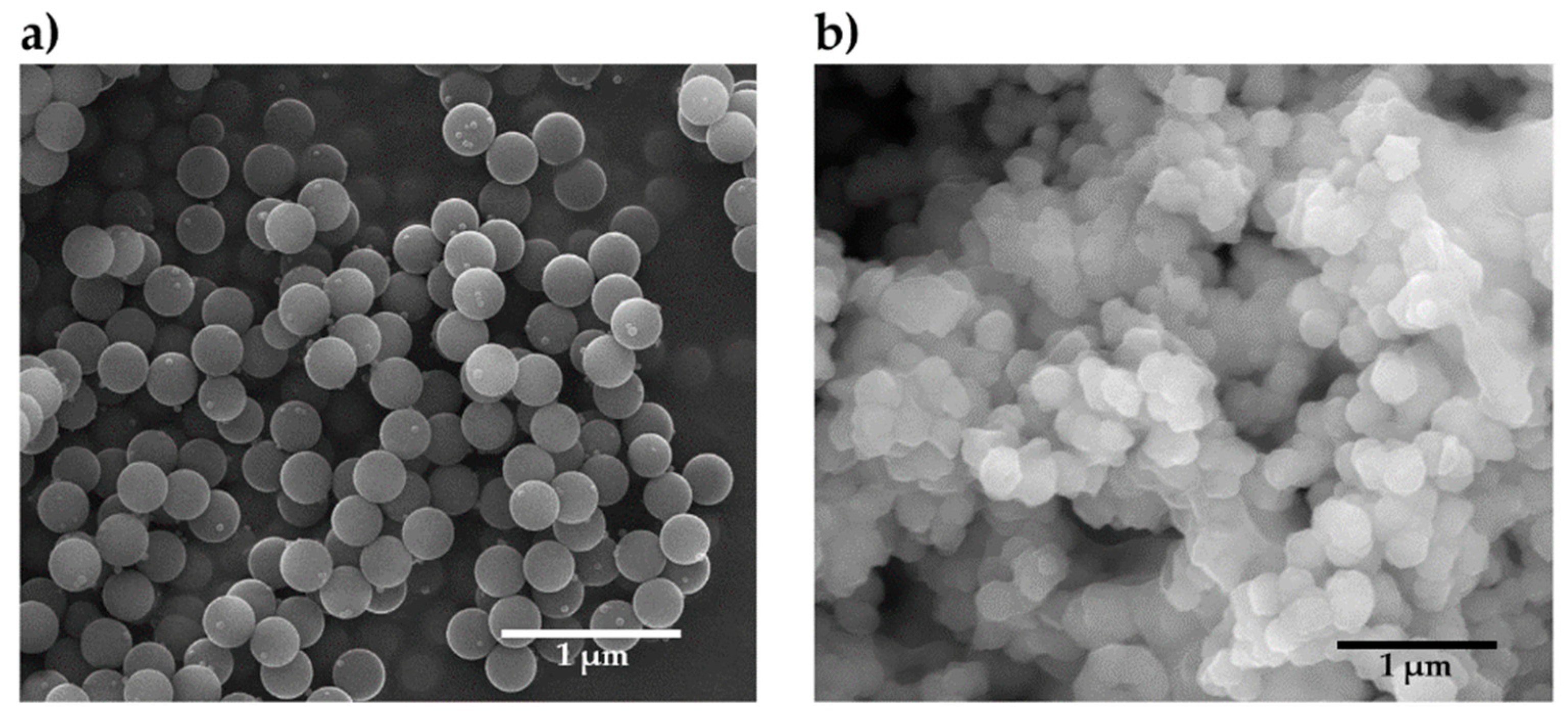
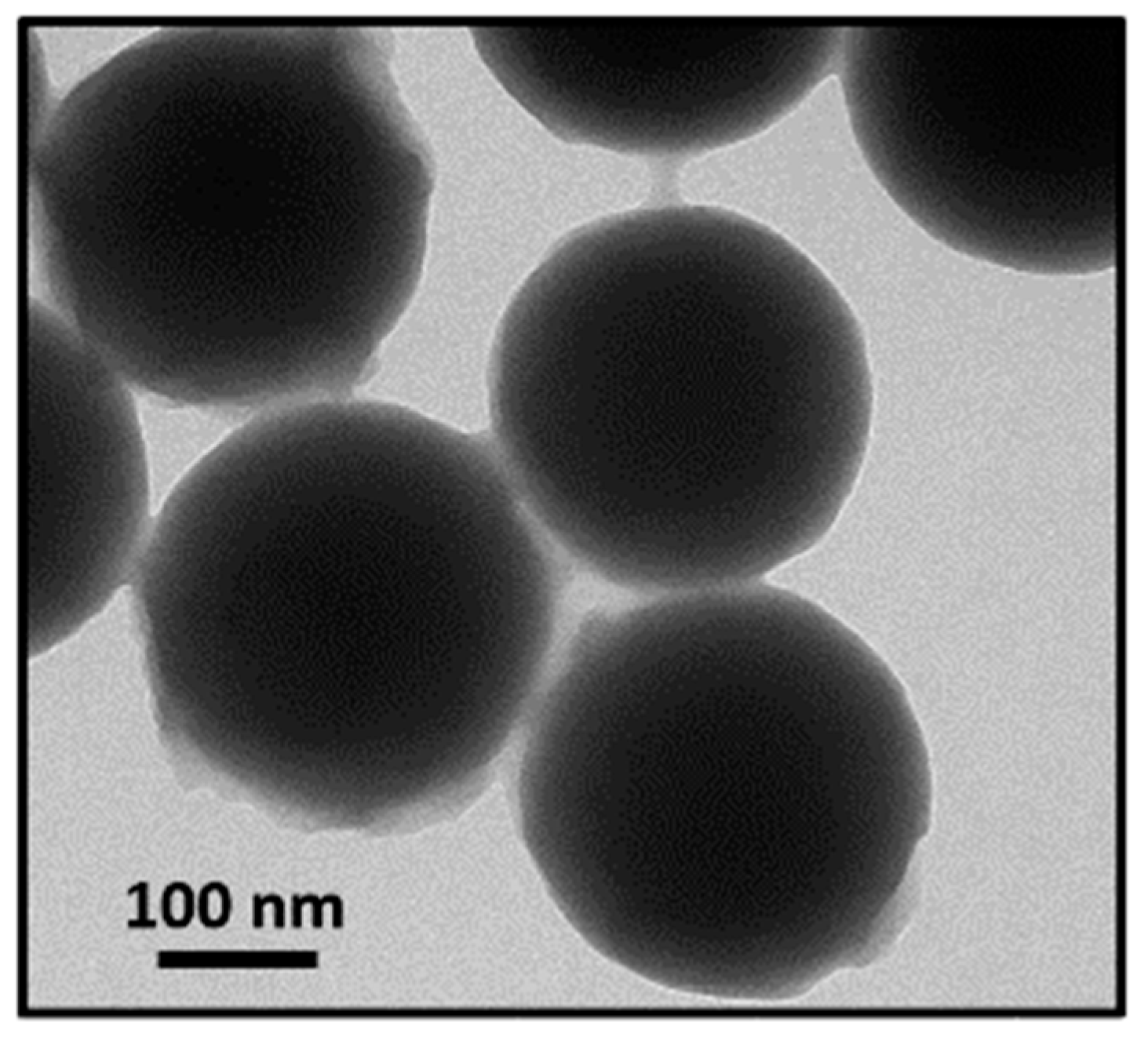
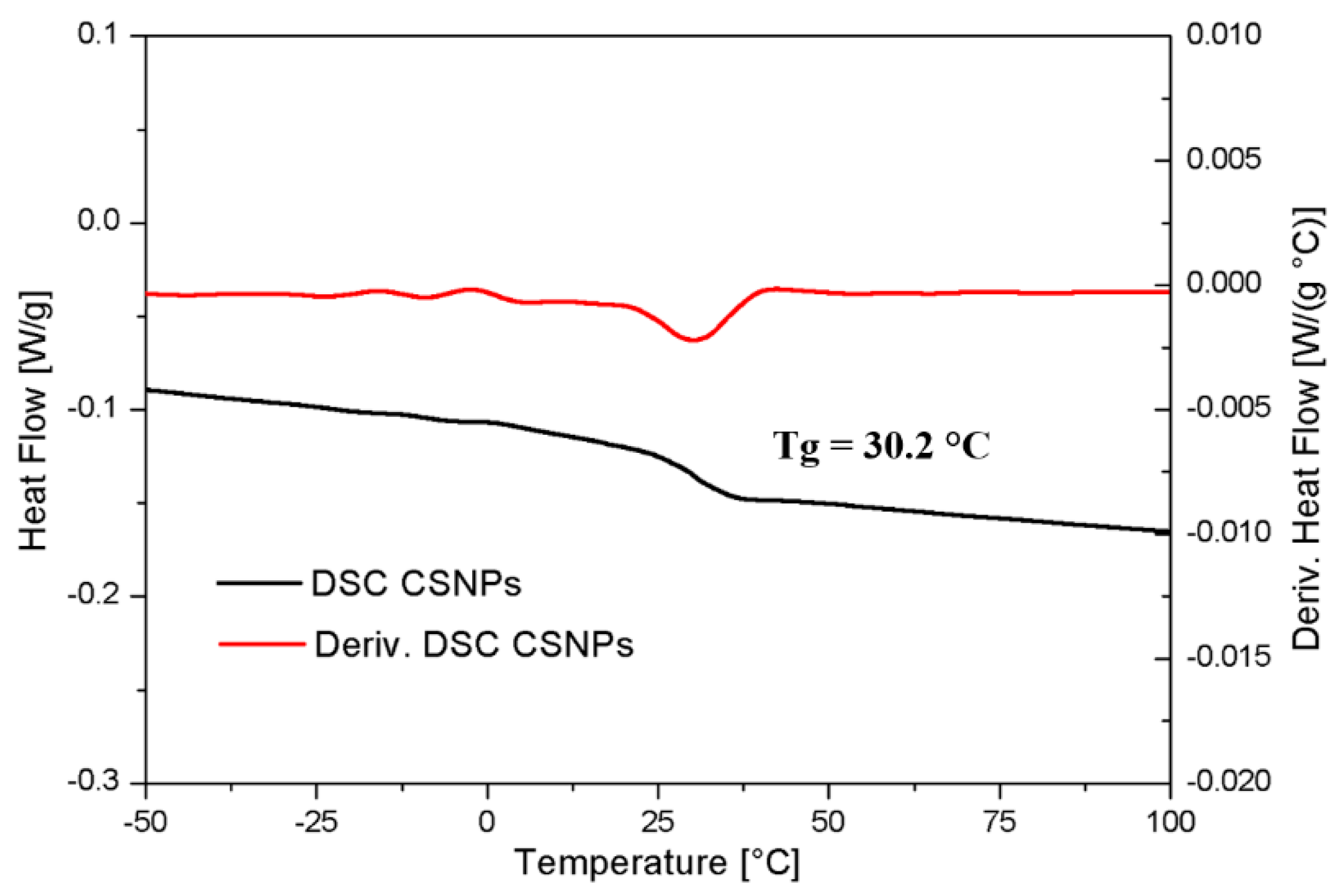
3.1. CSNPs Characterization
3.2. CSNPs/RTM6 Nanocomposites Characterization
3.2.1. Dynamic Mechanical Properties
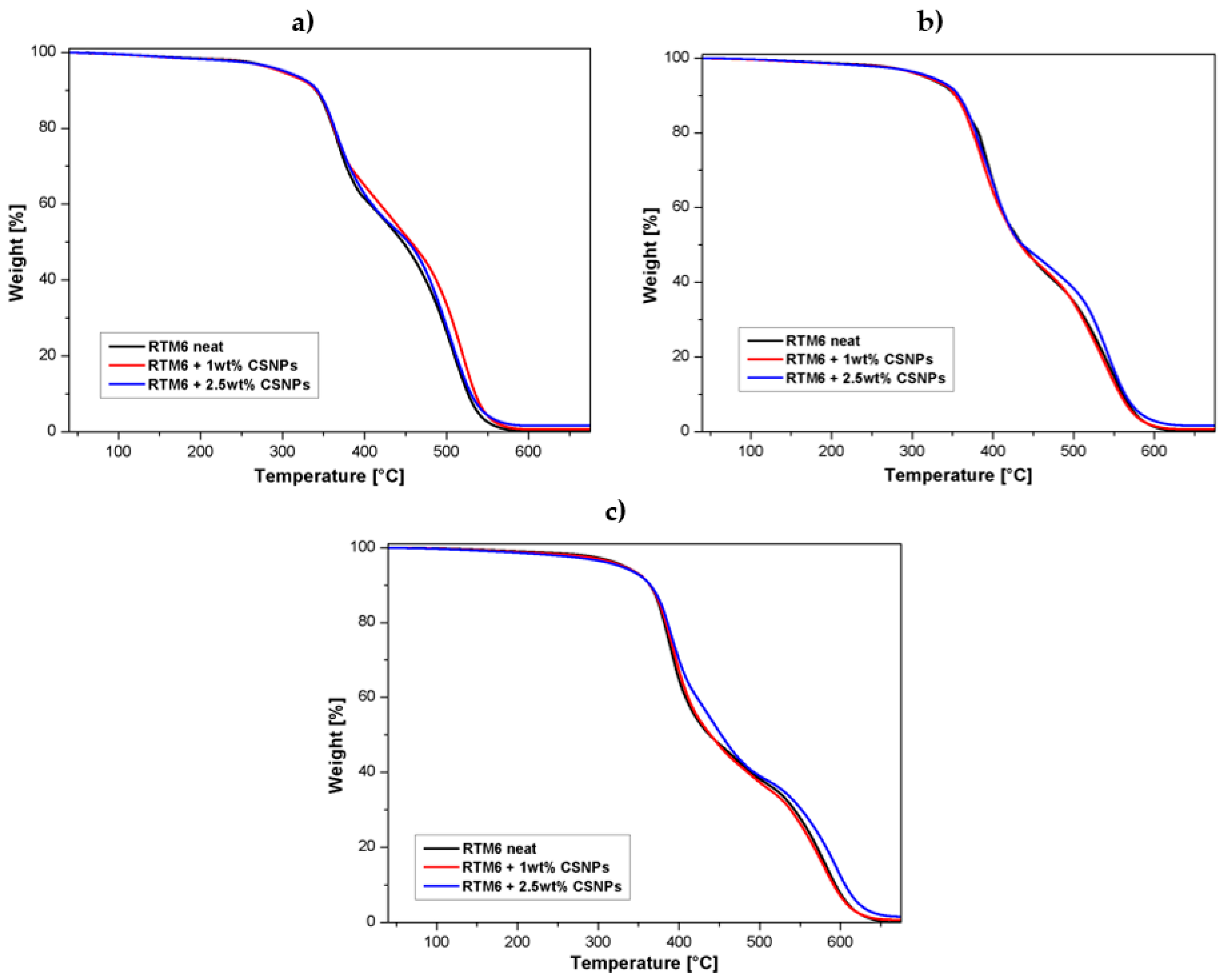
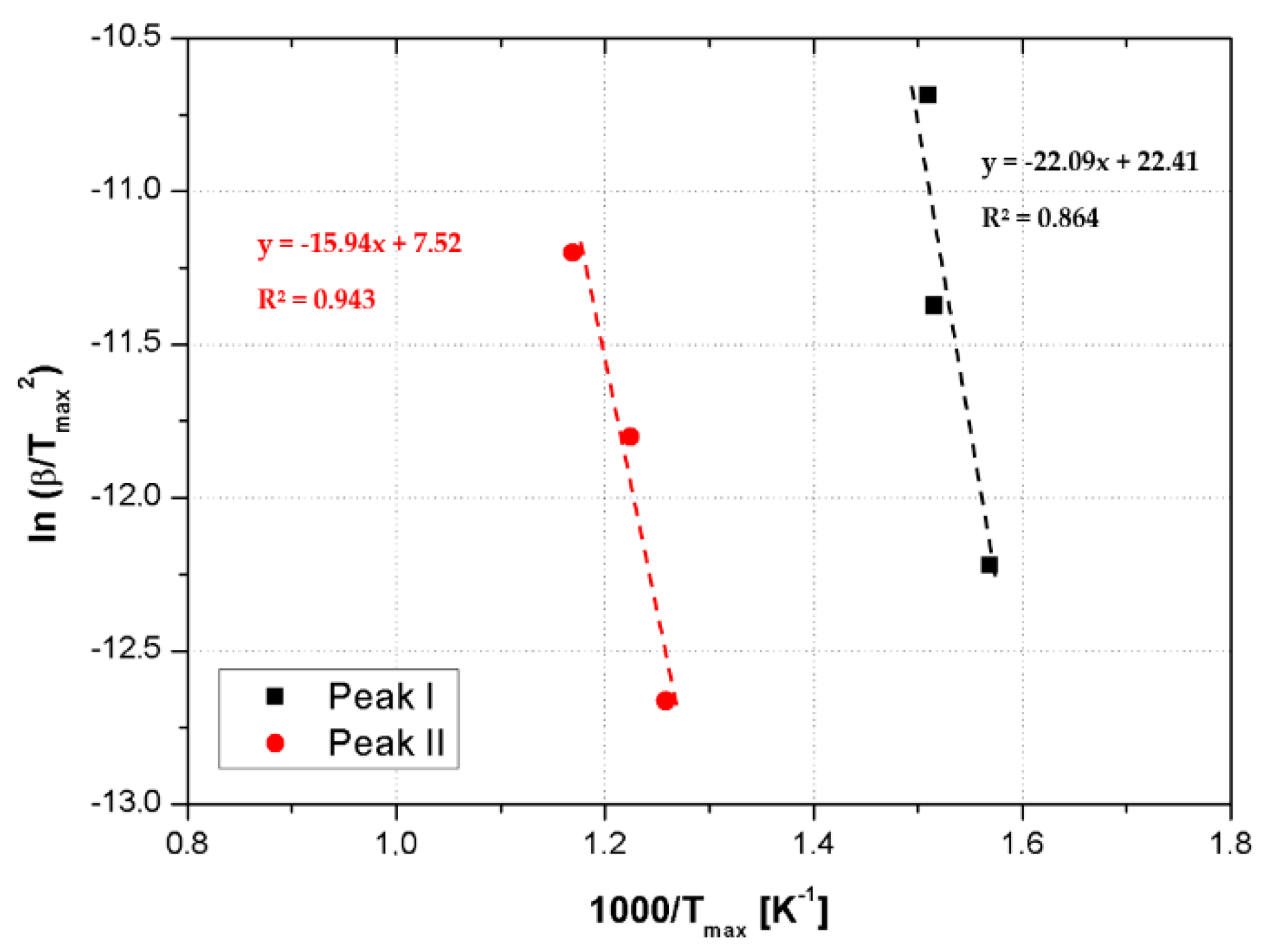
3.2.2. Thermal Stability
- A first degradation step up to 450 °C, which mainly involves dehydration (around 100 °C) of the material and formation of a poly-aromatic structure [22];
- A second step between 500–600 °C, characterized by thermo-oxidative reactions, which lead to the complete degradation of the carbonaceous materials and char formation [22].
- (1)
- At low CSNPs content (1 wt%), the activation energies of both degradation steps increased compared to the neat resin. This behavior can be explained assuming that CSNPs act as physical barriers both to postpone the thermal decomposition of volatile components and to prevent the transport phenomena of volatile decomposed products into the hosting matrix [24]. Therefore, at lower filler contents, the predominant effect associated with the presence of an inorganic core is an increase of the activation energy;
- (2)
- Higher CSNPs content (2.5 wt%) induces a reduction of both activation energies: In accordance with Zhou et al. [25], the HBP shell of the CSNPs can induce a lowering of activation energies associated with the reduction of the crosslink density in the hosting matrix. The energy reduction effect associated with the presence of the polymeric shell became more relevant compared to the sample filled at 1 wt% of CSNPs.
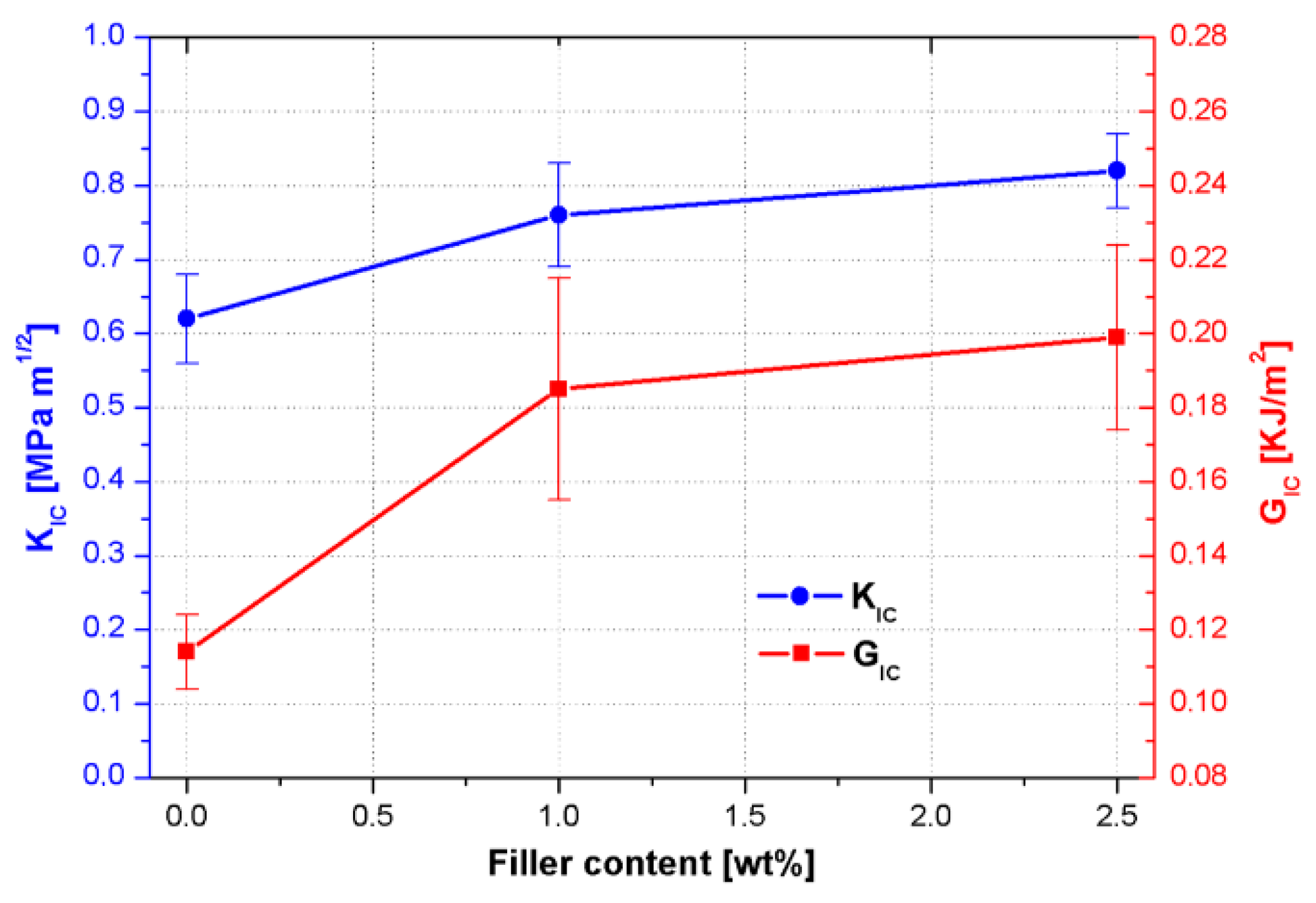
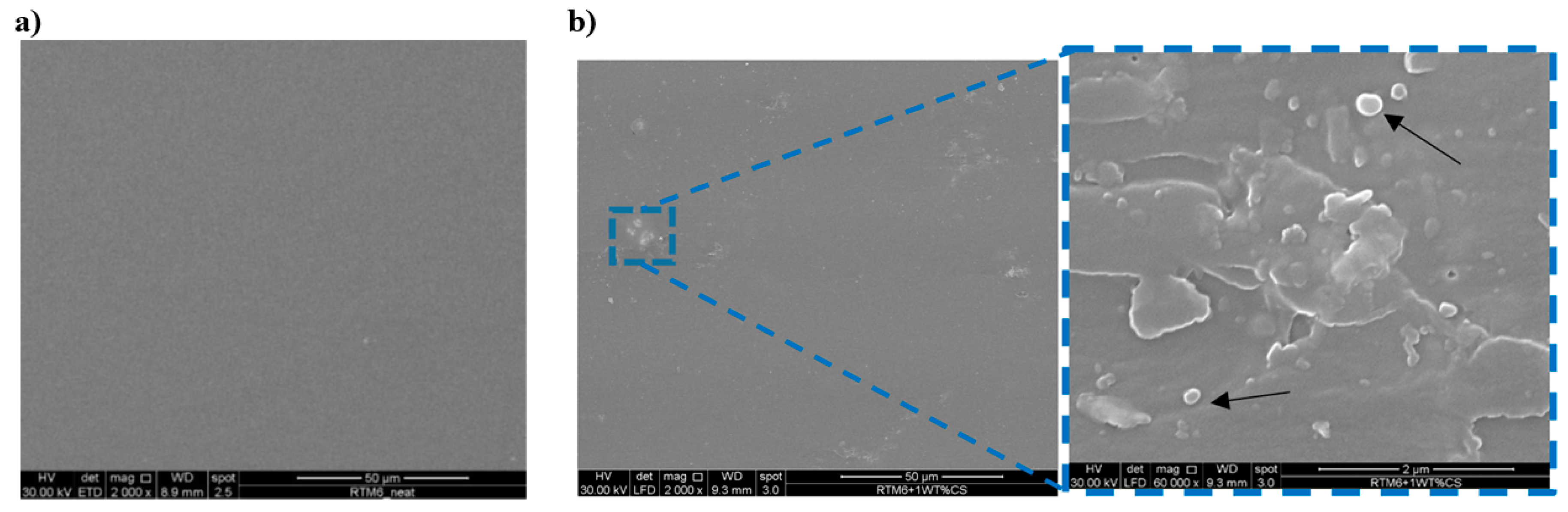
3.2.3. Fracture Toughness Results
- reduction of the crosslink density which affects the internal stress level induced during the epoxy cure [26];
- induction of a triaxial stress state in the matrix around CSNPs due to the presence of the inorganic core and thus promoting the plastic deformation mechanism in the polymeric phase and then crack tip blunting [27];
- further promotion of plastic deformation associated with the presence of the HBP shell [28].
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pascault, J.P.; Sautereau, H.; Verdu, J.; Williams, R.J.J. Thermosetting Polymers; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Ellis, B. Chemistry and Technology of Epoxy Resins; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Anderson, B.J. Thermal stability of high temperature epoxy adhesives by thermogravimetric and adhesive strength measurements. Polym. Degrad. Stab. 2011, 96, 1874–1881. [Google Scholar] [CrossRef]
- Ma, H.Y.; Wei, G.S.; Liu, Y.Q.; Zhang, X.H.; Gao, J.M.; Huang, F.; Tan, B.H.; Song, Z.H.; Qiao, J.L. Effect of elastomeric nanoparticles on properties of phenolic resin. Polymer 2005, 46, 10568–10573. [Google Scholar] [CrossRef]
- Becu, L.; Maazouz, A.; Sautereau, H.; Gerard, J.F. Fracture behavior of epoxy polymers modified with core-shell rubber particles. J. Appl. Polym. Sci. 1997, 65, 2419. [Google Scholar] [CrossRef]
- Zhang, D.; Jia, D. Toughness and strength improvement of diglycidyl ether of bisphenol—A by low viscosity liquid hyperbranched epoxy resin. J. Appl. Polym. Sci. 2006, 101, 2504. [Google Scholar] [CrossRef]
- Macan, J.; Brnardic, I.; Orlic, S.; Ivankovic, H.; Ivankovic, M. Thermal degradation of epoxy-silica organic-inorganic hybrid materials. Polym. Degrad. Stab. 2006, 91, 122–127. [Google Scholar] [CrossRef]
- Samyn, F.; Bourbigot, S.; Jama, C.; Bellayer, S. Fire retardancy of polymer clay nanocomposites: Is there an influence of the nanomorphology? Polym. Degrad. Stab. 2011, 93, 2019–2024. [Google Scholar] [CrossRef]
- Zotti, A.; Borriello, A.; Martone, A.; Antonucci, V.; Giordano, M.; Zarrelli, M. Effect of sepiolite filler on mechanical behaviour of a bisphenol A-based epoxy system. Compos. Part B 2014, 67, 400–409. [Google Scholar] [CrossRef]
- Tarrıo-Saavedra, J.; Lopez-Beceiro, J.; Naya, S.; Artiaga, R. Effect of silica content on thermal stability of fumed silica/epoxy composites. Polym. Degrad. Stab. 2008, 93, 2133–2137. [Google Scholar] [CrossRef]
- Zotti, A.; Borriello, A.; Ricciardi, M.; Antonucci, V.; Giordano, M.; Zarrelli, M. Effects of sepiolite clay on degradation and fire behaviour of a bisphenol A-based epoxy. Compos. Part B 2015, 73, 139–148. [Google Scholar] [CrossRef]
- Nazir, T.; Afzala, A.; Siddiqi, H.M.; Ahmad, Z.; Dumon, M. Thermally and mechanically superior hybrid epoxy–silica polymer films via sol–gel method. Prog. Org. Coat. 2010, 69, 100–106. [Google Scholar] [CrossRef]
- Ragosta, G.; Abbate, M.; Musto, P.; Scarinzi, G.; Mascia, L. Epoxy-silica particulate nanocomposites: Chemical interactions, reinforcement and fracture toughness. Polymer 2005, 46, 10506–10516. [Google Scholar] [CrossRef]
- Fu, J.F.; Shi, L.Y.; Yuan, S.; Zhong, Q.D.; Zhang, D.S.; Chen, Y.; Wu, J. Morphology, toughness mechanism, and thermal properties of hyperbranched epoxy modified diglycidyl ether of bisphenol A (DGEBA) interpenetrating polymer networks. Polym. Adv. Technol. 2008, 19, 1597–1607. [Google Scholar] [CrossRef]
- Jin, F.L.; Park, S.J. Thermal Properties and Toughness Performance of Hyperbranched-Polyimide-Modified Epoxy Resins. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3348–3356. [Google Scholar] [CrossRef]
- Sue, H.J.; Bertram, J.L.; Garcia-Meitin, E.Y.; Wilchester, J.W.; Walker, L.L. Fracture behavior of core-shell rubber-modified crosslinkable epoxy thermoplastics. Colloid Polym. Sci. 1994, 272, 456–466. [Google Scholar] [CrossRef]
- Quan, D.; Ivankovic, A. Effect of coreeshell rubber (CSR) nano-particles on mechanical properties and fracture toughness of an epoxy polymer. Polymer 2015, 66, 16–28. [Google Scholar] [CrossRef]
- Zotti, A.; Borriello, A.; Zuppolini, S.; Antonucci, V.; Giordano, M.; Pomogailo, A.D.; Lesnichaya, V.A.; Golubeva, N.D.; Bychkov, A.N.; Dzhardimalieva, G.I.; et al. Fabrication and characterization of metal-core carbon-shell nanoparticles filling an aeronautical composite matrix. Eur. Polym. J. 2015, 71, 140–151. [Google Scholar] [CrossRef]
- Huang, L.A.; Zhu, P.; Li, G.; Lu, D.D.; Sun, R.; Wong, C. Core–shell SiO2@RGO hybrids for epoxy composites with low percolation threshold and enhanced thermo-mechanical properties. J. Mater. Chem. A 2014, 2, 18246. [Google Scholar] [CrossRef]
- Fang, M.; Volotinen, T.T.; Kulkarni, S.K.; Belova, L.; Rao, K.V. Effect of embedding Fe3O4 nanoparticles in silica spheres on the optical transmission properties of three-dimensional magnetic photonic crystals. J. Appl. Phys. 2010, 108, 103501. [Google Scholar] [CrossRef]
- Li, S.; Lin, Q.; Cui, C. The effect of core–shell particles on the mechanical performance of epoxy resins modified with hyperbranched polymers. J. Mater. Res. 2016, 31, 1393. [Google Scholar] [CrossRef]
- Rose, N.; Le Bras, M.; Bourbigot, S.; Delobel, R. Thermal oxidative degradation of epoxy resins: Evaluation of their heat resistance using invariant kinetic parameters. Polym. Degrad. Stab. 1994, 45, 387–397. [Google Scholar] [CrossRef]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis and Kinetic study of poplar wood pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.K.; Kim, S.H. Thermal Decomposition Behavior of Poly(ethylene 2,6-naphthalate)/Silica Nanocomposites. Polym. Compos. 2009, 30, 1779–1787. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, D.; Li, T.; Zhang, A. Thermal Degradation Properties of Hyperbranched Poly(Trimellitic Anhydride Diethylene Glycol)/DGEBA Epoxy Hybrid Resin. Polym.-Plast. Technol. Eng. 2012, 51, 1149–1154. [Google Scholar] [CrossRef]
- Liu, D.; Wang, H.; Jiang, H.; Zhou, D. Improving the heat-resistance and toughness performance of phenolic resins by adding a rigid aromatic hyperbranched polyester. J. Appl. Polym. Sci. 2015, 133, 42734–42741. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Maxwell, D.; Young, R.J. Micromechanisms of crack propagation in hybrid particulate composites. J. Mater. Sci. Lett. 1985, 4, 1276–1279. [Google Scholar] [CrossRef]
- Wong, S.; Shanks, R.A.; Hodzic, A. Mechanical Behavior and Fracture Toughness of Poly(l-lactic acid)-Natural Fiber Composites Modified with Hyperbranched Polymers. Macromol. Mater. Eng. 2004, 289, 447–456. [Google Scholar] [CrossRef]
- Zotti, A.; Zuppolini, S.; Zarrelli, M.; Borriello, A. Fracture Toughening Mechanisms in Epoxy Adhesives. In Adhesives—Applications and Properties; Rudawska, A., Ed.; InTech: London, UK, 2016; pp. 237–269. [Google Scholar] [CrossRef]


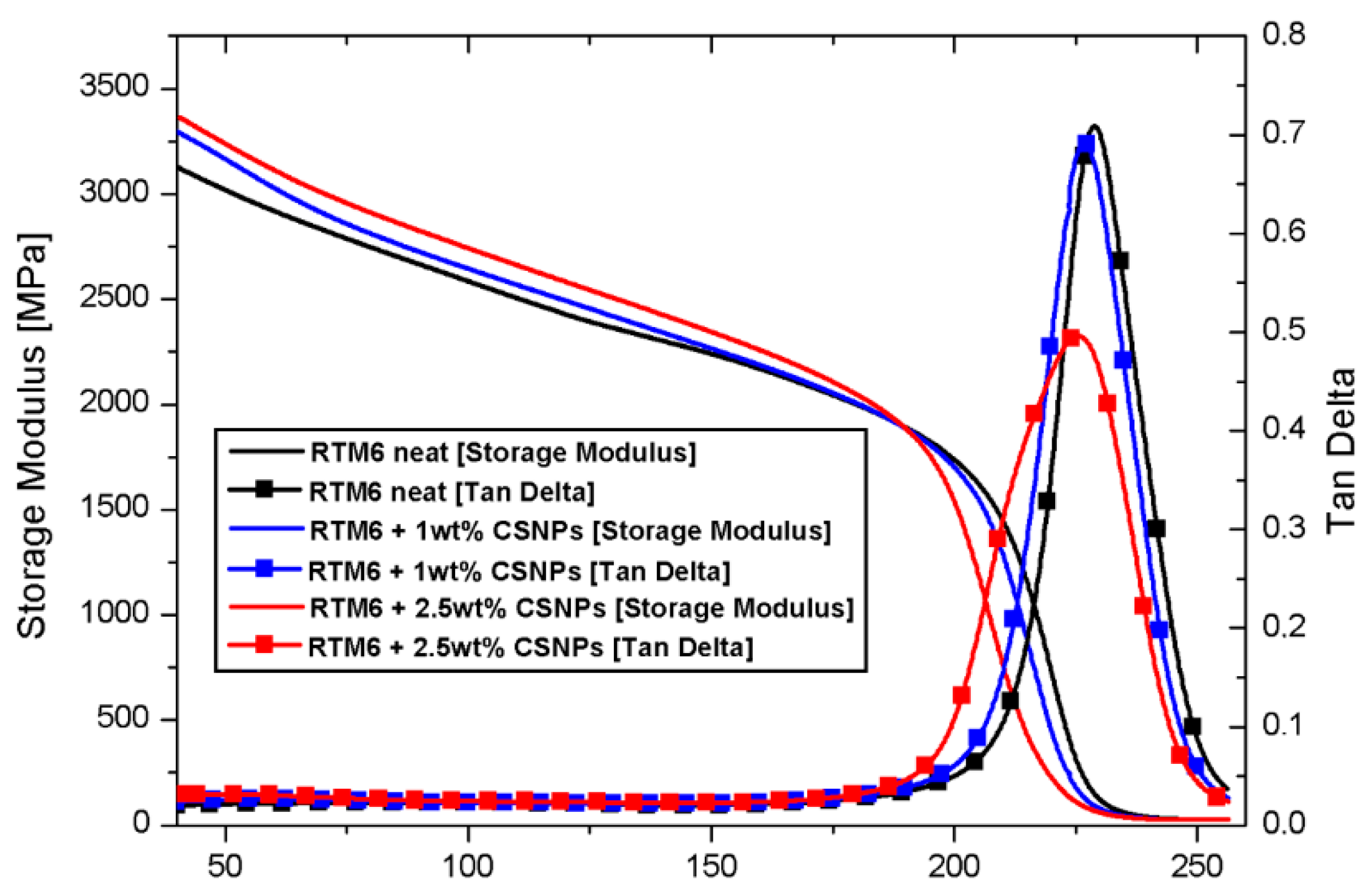

| Sample | Tg [°C] | Storage Modulus at 40 °C [MPa] |
|---|---|---|
| Neat RTM6 | 228.9 | 3107 ± 15 |
| RTM6 + 1 wt% CSNPs | 226.8 | 3296 ± 21 |
| RTM6 + 2.5 wt% CSNPs | 225.4 | 3392 ± 23 |
| Sample | Tend set I (°C) | Tend set II (°C) | TPeak I (°C) | TPeak II (°C) | Char Yield (%) |
|---|---|---|---|---|---|
| Neat RTM6 | 407.9 | 612.7 | 387.0 | 584.1 | 0.06 |
| RTM6 + 1 wt% CSNPs | 408.8 | 611.1 | 389.0 | 581.9 | 0.73 |
| RTM6 + 2.5 wt% CSNPs | 406.7 | 621.8 | 393.0 | 595.9 | 1.61 |
| Sample | EaI (KJ/mol) | EaII (KJ/mol) |
|---|---|---|
| Neat RTM6 | 103.5 | 110.6 |
| RTM6 + 1 wt% CSNPs | 183.7 | 132.6 |
| RTM6 + 2.5 wt% CSNPs | 152.0 | 90.6 |
| Sample | KIC [MPa m1/2] | ΔKIC | % Variation KIC | GIC [KJ/m2] | ΔGIC | % Variation GIC |
|---|---|---|---|---|---|---|
| RTM6 Neat | 0.62 | 0.06 | - | 0.114 | 0.01 | - |
| RTM6 + 1 wt% CSNPs | 0.76 | 0.07 | 22.6 | 0.185 | 0.03 | 62.3 |
| RTM6 + 2.5 wt% CSNPs | 0.82 | 0.05 | 32.3 | 0.199 | 0.025 | 74.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zotti, A.; Zuppolini, S.; Borriello, A.; Zarrelli, M. Thermal Properties and Fracture Toughness of Epoxy Nanocomposites Loaded with Hyperbranched-Polymers-Based Core/Shell Nanoparticles. Nanomaterials 2019, 9, 418. https://doi.org/10.3390/nano9030418
Zotti A, Zuppolini S, Borriello A, Zarrelli M. Thermal Properties and Fracture Toughness of Epoxy Nanocomposites Loaded with Hyperbranched-Polymers-Based Core/Shell Nanoparticles. Nanomaterials. 2019; 9(3):418. https://doi.org/10.3390/nano9030418
Chicago/Turabian StyleZotti, Aldobenedetto, Simona Zuppolini, Anna Borriello, and Mauro Zarrelli. 2019. "Thermal Properties and Fracture Toughness of Epoxy Nanocomposites Loaded with Hyperbranched-Polymers-Based Core/Shell Nanoparticles" Nanomaterials 9, no. 3: 418. https://doi.org/10.3390/nano9030418