Transparent and Robust All-Cellulose Nanocomposite Packaging Materials Prepared in a Mixture of Trifluoroacetic Acid and Trifluoroacetic Anhydride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of All-Cellulose Nanocomposites
2.3. Morphological Characterization
2.4. Optical Characterization
2.5. Structural Characterization
2.6. Mechanical Characterization
2.7. Thermal Characterization
2.8. Water Uptake and Permeability
3. Results and Discussion
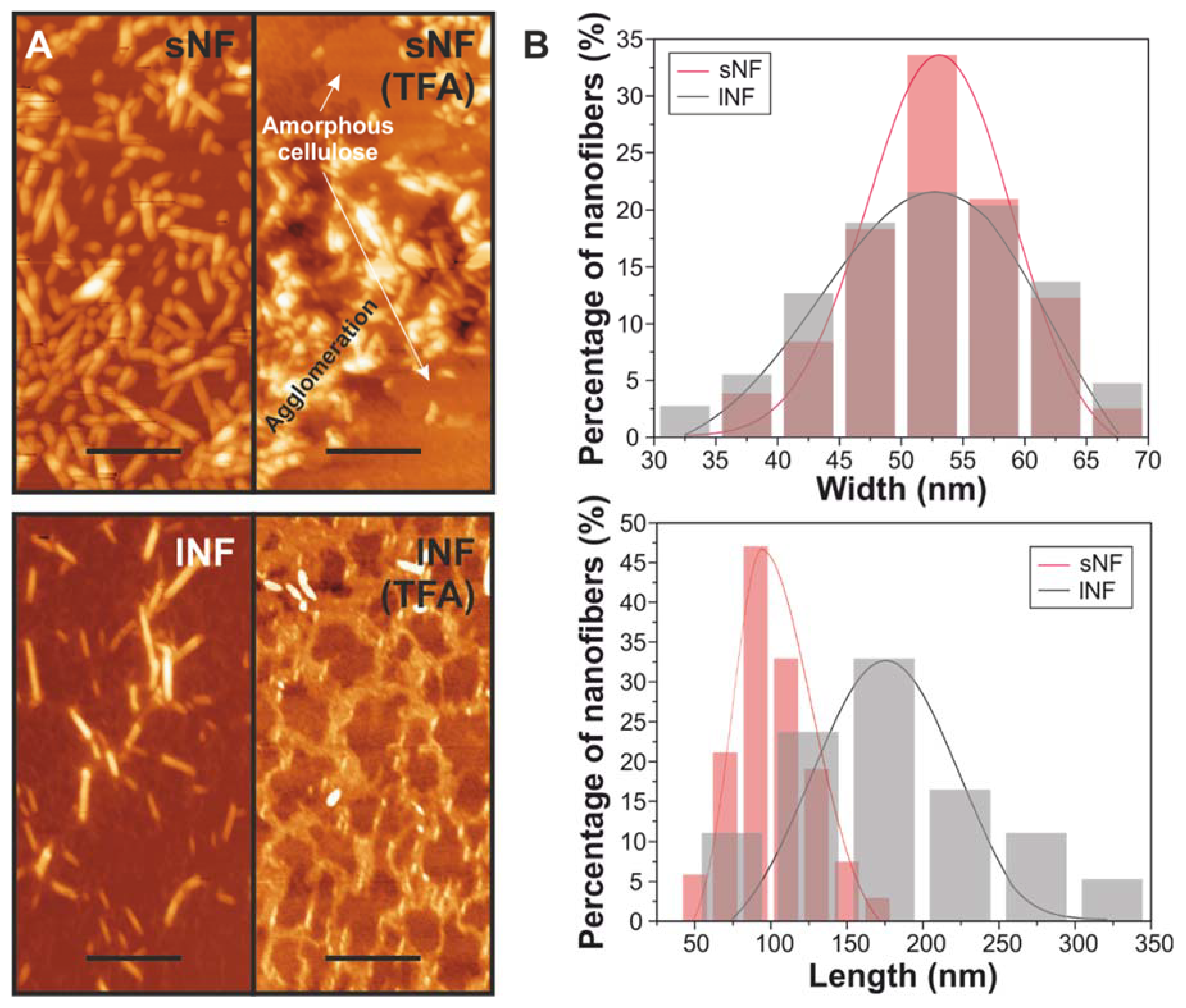
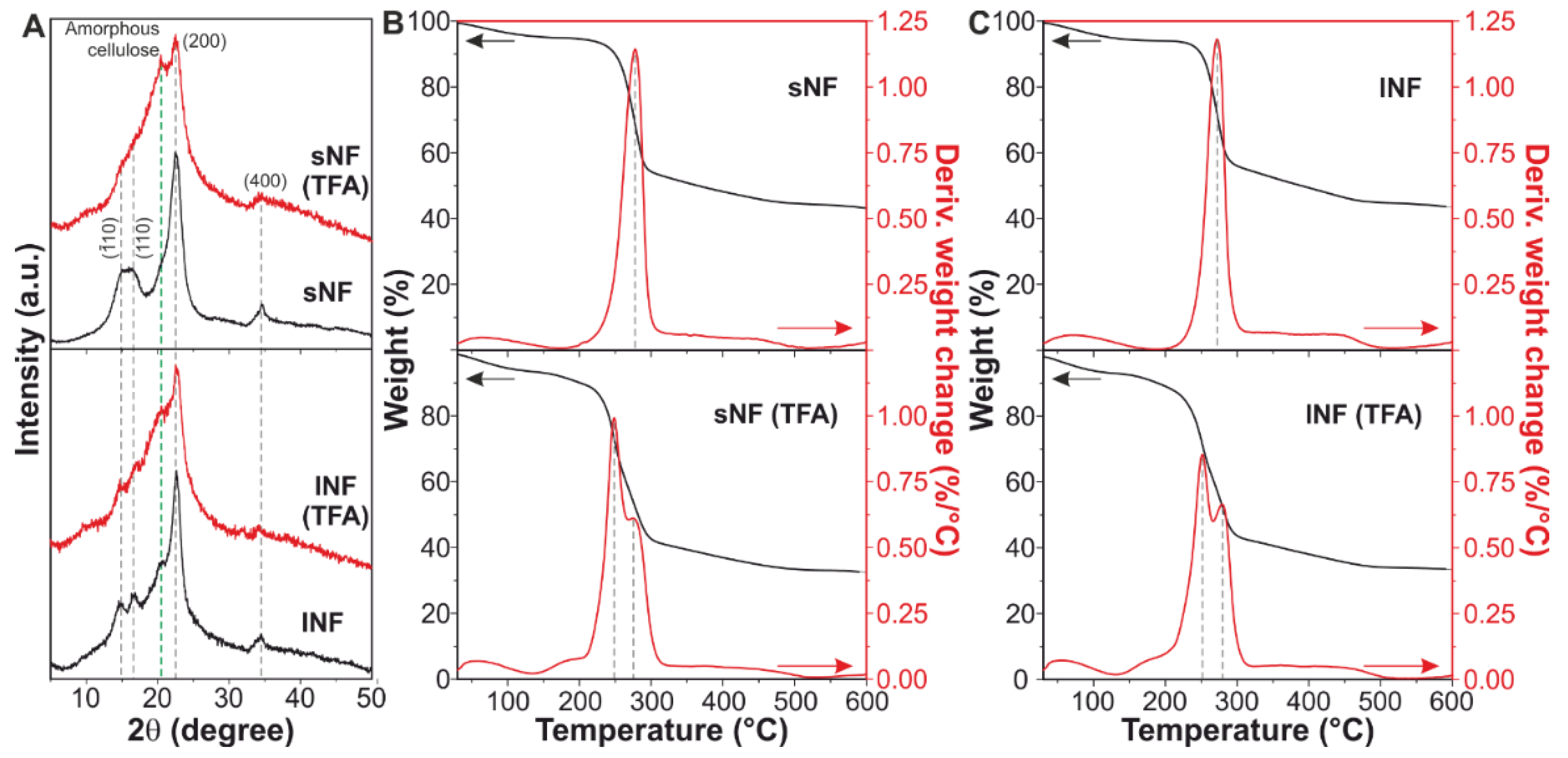
3.1. Effect of TFA/TFAA Mixture on the Cellulose Nanofibers
3.2. Optical and Morphological Characterization of the Nanocomposites
3.3. Mechanical Characterization of the Nanocomposites
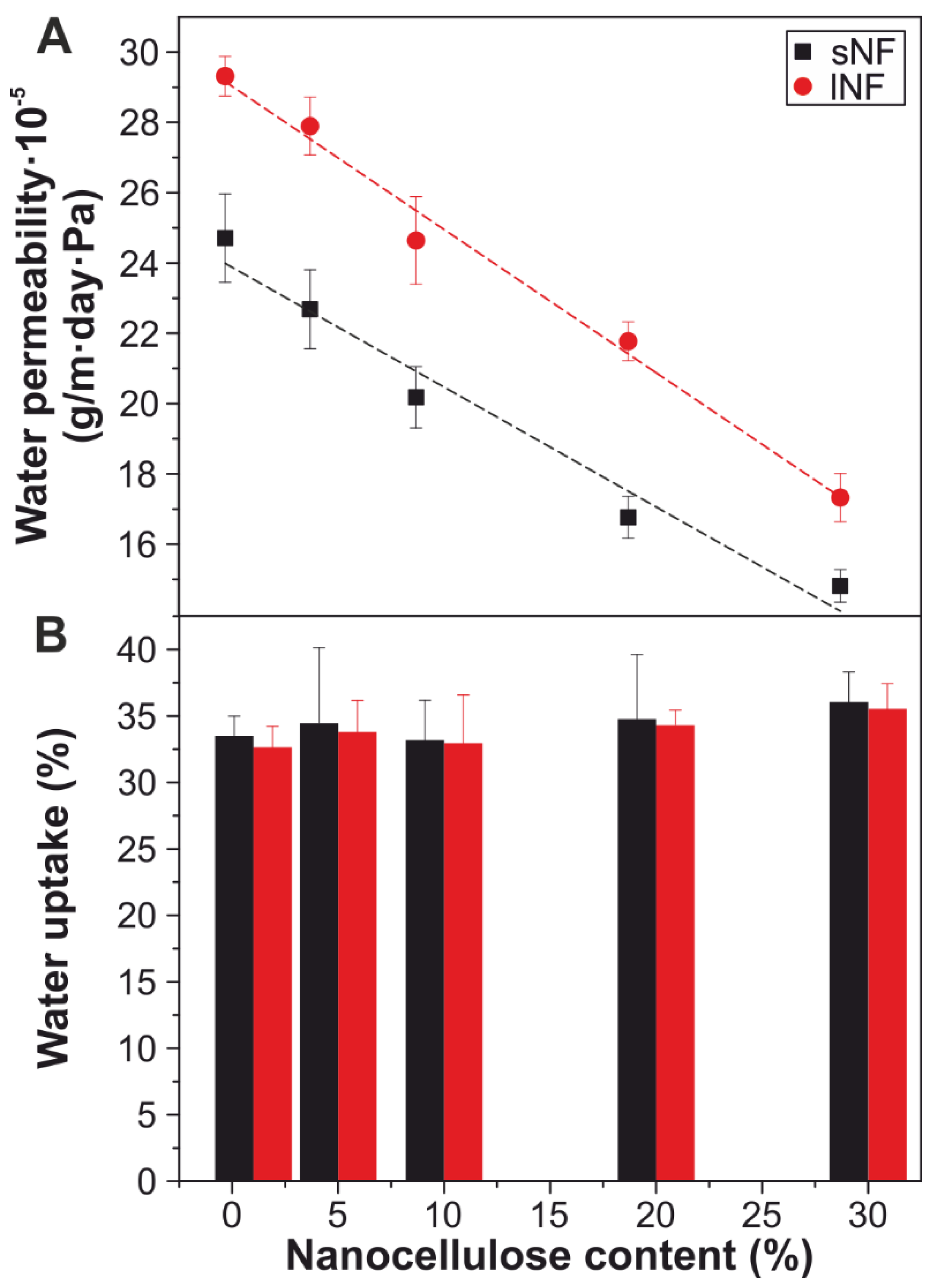
3.4. Water Permeability and Uptake of the Nanocomposites
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Blettler, M.C.M.; Abrial, E.; Khan, F.R.; Sivri, N.; Espinola, L.A. Freshwater plastic pollution: Recognizing research biases and identifying knowledge gaps. Water Res. 2018, 143, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Hamid, F.S.; Bhatti, M.S.; Anuar, N.; Anuar, N.; Mohan, P.; Periathamby, A. Worldwide distribution and abundance of microplastic: How dire is the situation? Waste Manag. Res. 2018, 36, 873–897. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L.; Annual, R. Plastics in the Marine Environment. In Annual Review of Marine Science; Annual Reviews; Palo Alto: Santa Clara, CA, USA, 2017; Volume 9, pp. 205–229. [Google Scholar]
- Heredia-Guerrero, J.A.; Athanassiou, A. Editorial: Non-polysaccharide Plant Polymeric Materials. Front. Mater. 2016, 3, 15. [Google Scholar] [CrossRef]
- Penca, J. European Plastics Strategy: What promise for global marine litter? Mar. Pol. 2018, 97, 197–201. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of bioplastics: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef]
- Tedeschi, G.; Benitez, J.J.; Ceseracciu, L.; Dastmalchi, K.; Itin, B.; Stark, R.E.; Heredia, A.; Athanassiou, A.; Heredia-Guerrero, J.A. Sustainable Fabrication of Plant Cuticle-Like Packaging Films from Tomato Pomace Agro-Waste, Beeswax, and Alginate. ACS Sustain. Chem. Eng. 2018, 6, 14955–14966. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benitez, J.J.; Cataldi, P.; Paul, U.C.; Contardi, M.; Cingolani, R.; Bayer, I.S.; Heredia, A.; Athanassiou, A. All-Natural Sustainable Packaging Materials Inspired by Plant Cuticles. Adv. Sustain. Syst. 2017, 1, 1600024. [Google Scholar] [CrossRef]
- Bayer, I.S.; Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Pignatelli, F.; Ruffilli, R.; Cingolani, R.; Athanassiou, A. Direct Transformation of Edible Vegetable Waste into Bioplastics. Macromolecules 2014, 47, 5135–5143. [Google Scholar] [CrossRef]
- Delgado, M.; Felix, M.; Bengoechea, C. Development of bioplastic materials: From rapeseed oil industry by products to added-value biodegradable biocomposite materials. Ind. Crop. Prod. 2018, 125, 401–407. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Hilliou, L.; Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Madalena, D.; Cabedo, L.; Covas, J.A.; Vicente, A.A.; Lagaron, J.M. Melt processability, characterization, and antibacterial activity of compression-molded green composite sheets made of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) reinforced with coconut fibers impregnated with oregano essential oil. Food Packag. Shelf Life 2018, 17, 39–49. [Google Scholar] [CrossRef]
- Benítez, J.J.; Castillo, P.M.; del Río, J.C.; León-Camacho, M.; Domínguez, E.; Heredia, A.; Guzmán-Puyol, S.; Athanassiou, A.; Heredia-Guerrero, J.A. Valorization of Tomato Processing by-Products: Fatty Acid Extraction and Production of Bio-Based Materials. Materials 2018, 11, 2211. [Google Scholar] [CrossRef] [PubMed]
- Perotto, G.; Ceseracciu, L.; Simonutti, R.; Paul, U.C.; Guzman-Puyol, S.; Tran, T.-N.; Bayer, I.S.; Athanassiou, A. Bioplastics from vegetable waste via an eco-friendly water-based process. Green Chem. 2018, 20, 894–902. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem.-Int. Edit. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Siro, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Ceseracciu, L.; Heredia-Guerrero, J.A.; Anyfantis, G.C.; Cingolani, R.; Athanassiou, A.; Bayer, I.S. Effect of trifluoroacetic acid on the properties of polyvinyl alcohol and polyvinyl alcohol-cellulose composites. Chem. Eng. J. 2015, 277, 242–251. [Google Scholar] [CrossRef]
- Nishino, T.; Peijs, T. All-cellulose Composites. In Handbook of Green Materials, Vol 2: Bionanocomposites: Processing, Characterization and Properties; Oksman, K., Mathew, A.P., Bismarck, A., Rojas, O., Sain, M., Qvintus, P., Eds.; World Scientific Publ Co Pte Ltd.: Singapore, 2014; Volume 5, pp. 201–216. [Google Scholar]
- Huber, T.; Mussig, J.; Curnow, O.; Pang, S.S.; Bickerton, S.; Staiger, M.P. A critical review of all-cellulose composites. J. Mater. Sci. 2012, 47, 1171–1186. [Google Scholar] [CrossRef]
- Wei, X.; Wei, W.; Cui, Y.H.; Lu, T.J.; Jiang, M.; Zhou, Z.W.; Wang, Y. All-cellulose composites with ultra-high mechanical properties prepared through using straw cellulose fiber. RSC Adv. 2016, 6, 93428–93435. [Google Scholar] [CrossRef]
- Li, J.Y.; Nawaz, H.; Wu, J.; Zhang, J.M.; Wan, J.Q.; Mi, Q.Y.; Yu, J.; Zhang, J. All-cellulose composites based on the self-reinforced effect. Compos. Commun. 2018, 9, 42–53. [Google Scholar] [CrossRef]
- Yousefi, H.; Nishino, T.; Faezipour, M.; Ebrahimi, G.; Shakeri, A. Direct fabrication of all-cellulose nanocomposite from cellulose microfibers using ionic liquid-based nanowelding. Biomacromolecules 2011, 12, 4080–4085. [Google Scholar] [CrossRef] [PubMed]
- Tervahartiala, T.; Hildebrandt, N.C.; Piltonen, P.; Schabel, S.; Valkama, J.P. Potential of all-cellulose composites in corrugated board applications: Comparison of chemical pulp raw materials. Packag. Technol. Sci. 2018, 31, 173–183. [Google Scholar] [CrossRef]
- Hu, W.; Chen, G.; Liu, Y.; Liu, Y.Y.; Li, B.; Fang, Z.G. Transparent and Hazy All-Cellulose Composite Films with Superior Mechanical Properties. ACS Sustain. Chem. Eng. 2018, 6, 6974–6980. [Google Scholar] [CrossRef]
- Ghaderi, M.; Mousavi, M.; Yousefi, H.; Labbafi, M. All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packaging application. Carbohydr. Polym. 2014, 104, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Soykeabkaew, N.; Arimoto, N.; Nishino, T.; Peijs, T. All-cellulose composites by surface selective dissolution of aligned ligno-cellulosic fibres. Compos. Sci. Technol. 2008, 68, 2201–2207. [Google Scholar] [CrossRef] [Green Version]
- Mathew, A.P.; Oksman, K.; Pierron, D.; Harmand, M.F. Fibrous cellulose nanocomposite scaffolds prepared by partial dissolution for potential use as ligament or tendon substitutes. Carbohydr. Polym. 2012, 87, 2291–2298. [Google Scholar] [CrossRef]
- He, X.; Xiao, Q.; Lu, C.H.; Wang, Y.R.; Zhang, X.F.; Zhao, J.Q.; Zhang, W.; Zhang, X.M.; Deng, Y.L. Uniaxially Aligned Electrospun All-Cellulose Nanocomposite Nanofibers Reinforced with Cellulose Nanocrystals: Scaffold for Tissue Engineering. Biomacromolecules 2014, 15, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yun, S.; Ounaies, Z. Discovery of cellulose as a smart material. Macromolecules 2006, 39, 4202–4206. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Nogi, M.; Yano, H. Displays from Transparent Film of Natural Nanofibers. Mrs Bull. 2010, 35, 214–218. [Google Scholar] [CrossRef]
- Khan, A.; Abas, Z.; Kim, H.S.; Kim, J. Recent Progress on Cellulose-Based Electro-Active Paper, Its Hybrid Nanocomposites and Applications. Sensors 2016, 16, 1172. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, D.; Syre, P.; Niska, K.O. All cellulose nanocomposites produced by extrusion. J. Biobased Mater. Bioenergy 2007, 1, 367–371. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Ferrer, A.; Tyagi, P.; Yin, Y.Y.; Salas, C.; Pal, L.; Rojas, O.J. Nanocellulose in Thin Films, Coatings, and Plies for Packaging Applications: A Review. Bioresources 2017, 12, 2143–2233. [Google Scholar] [CrossRef]
- Li, F.; Mascheroni, E.; Piergiovanni, L. The Potential of NanoCellulose in the Packaging Field: A Review. Packag. Technol. Sci. 2015, 28, 475–508. [Google Scholar] [CrossRef]
- Kumar, T.S.M.; Rajini, N.; Reddy, K.O.; Rajulu, A.V.; Siengchin, S.; Ayrilmis, N. All-cellulose composite films with cellulose matrix and Napier grass cellulose fibril fillers. Int. J. Biol. Macromol. 2018, 112, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Geddes, A.L. Interaction of trifluoroacetic acid with cellulose and related compounds. J. Polym. Sci. 1956, 22, 31–39. [Google Scholar] [CrossRef]
- Hasegawa, M.; Isogai, A.; Onabe, F.; Usuda, M. Dissolving states of cellulose and chitosan in trifluoroacetic-acid. J. Appl. Polym. Sci. 1992, 45, 1857–1863. [Google Scholar] [CrossRef]
- Liebert, T.; Schnabelrauch, M.; Klemm, D.; Erler, U. Readily hydrolyzable cellulose esters as intermediates for the regioselective derivatization of cellulose. 2. soluble, highly substituted cellulose trifluoroacetates. Cellulose 1994, 1, 249–258. [Google Scholar] [CrossRef]
- Zhao, H.B.; Holladay, J.E.; Kwak, J.H.; Zhang, Z.C. Inverse temperature-dependent pathway of cellulose decrystallization in trifluoroacetic acid. J. Phys. Chem. B 2007, 111, 5295–5300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Holladay, J.E.; Kwak, J.H.; Zhang, Z.C. A new route to improved glucose yields in cellulose hydrolysis. J. Biobased Mater. Bioenergy 2007, 1, 210–214. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Liebert, T. Cellulose Solvents—Remarkable History, Bright Future. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; Liebert, T.F., Heinze, T.J., Edgar, K.J., Eds.; American Chemical Society: Washington, DC, USA, 2010; pp. 3–54. [Google Scholar]
- Scott, B.F.; Macdonald, R.W.; Kannan, K.; Fisk, A.; Witter, A.; Yamashita, N.; Durham, L.; Spencer, C.; Muir, D.C.G. Trifluoroacetate profiles in the Arctic, Atlantic, and Pacific Oceans. Environ. Sci. Technol. 2005, 39, 6555–6560. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; Suidan, M.T.; Wallington, T.J.; Du, X. Biodegradability of trifluoroacetic acid. Environ. Eng. Sci. 2000, 17, 337–342. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Russo, D.; Penna, I.; Ceseracciu, L.; Palazon, F.; Scarpellini, A.; Cingolani, R.; Bertorelli, R.; Bayer, I.S.; Heredia-Guerrero, J.A.; et al. Facile production of seaweed-based biomaterials with antioxidant and anti-inflammatory activities. Algal Res. 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Hajiali, H.; Canale, C.; Scarpellini, A.; Cingolani, R.; Bayer, I.S.; Athanassiou, A.; Mele, E. Low-Cost and Effective Fabrication of Biocompatible Nanofibers from Silk and Cellulose-Rich Materials. ACS Biomater. Sci. Eng. 2016, 2, 526–534. [Google Scholar] [CrossRef]
- Papadopoulou, E.L.; Heredia-Guerrero, J.A.; Vázquez, M.I.; Benavente, J.; Athanassiou, A.; Bayer, I.S. Self-organized microporous cellulose-nylon membranes. Polymer 2017, 120, 255–263. [Google Scholar] [CrossRef]
- Tran, T.N.; Paul, U.; Heredia-Guerrero, J.A.; Liakos, I.; Marras, S.; Scarpellini, A.; Ayadi, F.; Athanassiou, A.; Bayer, I.S. Transparent and flexible amorphous cellulose-acrylic hybrids. Chem. Eng. J. 2016, 287, 196–204. [Google Scholar] [CrossRef]
- Tedeschi, G.; Guzman-Puyol, S.; Paul, U.C.; Barthel, M.J.; Goldoni, L.; Caputo, G.; Ceseracciu, L.; Athanassiou, A.; Heredia-Guerrero, J.A. Thermoplastic cellulose acetate oleate films with high barrier properties and ductile behaviour. Chem. Eng. J. 2018, 348, 840–849. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Goldoni, L.; Benitez, J.J.; Davis, A.; Ceseracciu, L.; Cingolani, R.; Bayer, I.S.; Heinze, T.; Koschella, A.; Heredia, A.; et al. Cellulose-polyhydroxylated fatty acid ester-based bioplastics with tuning properties: Acylation via a mixed anhydride system. Carbohydr. Polym. 2017, 173, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASTM. Standard Test Method for Transparency of Plastic Sheeting; ASTM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Savadekar, N.R.; Mhaske, S.T. Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr. Polym. 2012, 89, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Bedane, A.H.; Eic, M.; Farmahini-Farahani, M.; Xiao, H.N. Water vapor transport properties of regenerated cellulose and nanofibrillated cellulose films. J. Membr. Sci. 2015, 493, 46–57. [Google Scholar] [CrossRef]
- Nishino, T.; Matsuda, I.; Hirao, K. All-Cellulose Composite. Macromolecules 2004, 37, 7683–7687. [Google Scholar] [CrossRef]
- Duchemin, B.J.C.; Newman, R.H.; Staiger, M.P. Structure–property relationship of all-cellulose composites. Compos. Sci. Technol. 2009, 69, 1225–1230. [Google Scholar] [CrossRef]
- Hideno, A. Comparison of the Thermal Degradation Properties of Crystalline and Amorphous Cellulose, as well as Treated Lignocellulosic Biomass. Bioresources 2016, 11, 6309–6319. [Google Scholar] [CrossRef]
- Wang, Z.H.; McDonald, A.G.; Westerhof, R.J.M.; Kersten, S.R.A.; Cuba-Torres, C.M.; Ha, S.; Pecha, B.; Garcia-Perez, M. Effect of cellulose crystallinity on the formation of a liquid intermediate and on product distribution during pyrolysis. J. Anal. Appl. Pyrolysis 2013, 100, 56–66. [Google Scholar] [CrossRef]
- Cazon, P.; Vazquez, M.; Velazquez, G. Cellulose-glycerol-polyvinyl alcohol composite films for food packaging: Evaluation of water adsorption, mechanical properties, light-barrier properties and transparency. Carbohydr. Polym. 2018, 195, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Mallick, P.K. Fiber-Reinforced Composites: Materials, Manufacturing, and Design, 2nd ed.; Taylor & Francis: Didcot, UK, 1993. [Google Scholar]
- Guhados, G.; Wan, W.K.; Hutter, J.L. Measurement of the elastic modulus of single bacterial cellulose fibers using atomic force microscopy. Langmuir 2005, 21, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.A.; Robertson, R.E. The effect of fiber orientation on the toughening of short fiber-reinforced polymers. J. Appl. Polym. Sci. 2003, 90, 2740–2751. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]


| Label | MCC (wt.%) | Nanocellulose | |
|---|---|---|---|
| Short Nanofibers (wt.%) | Long Nanofibers (wt.%) | ||
| Cellulose | 100 | - | - |
| sNF1 | 99 | 1 | - |
| sNF5 | 95 | 5 | - |
| sNF10 | 91 | 9 | - |
| sNF20 | 83 | 17 | - |
| sNF30 | 77 | 23 | - |
| lNF1 | 99 | - | 1 |
| lNF5 | 95 | - | 5 |
| lNF10 | 91 | - | 9 |
| lNF20 | 83 | - | 17 |
| lNF30 | 77 | - | 23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzman-Puyol, S.; Ceseracciu, L.; Tedeschi, G.; Marras, S.; Scarpellini, A.; Benítez, J.J.; Athanassiou, A.; Heredia-Guerrero, J.A. Transparent and Robust All-Cellulose Nanocomposite Packaging Materials Prepared in a Mixture of Trifluoroacetic Acid and Trifluoroacetic Anhydride. Nanomaterials 2019, 9, 368. https://doi.org/10.3390/nano9030368
Guzman-Puyol S, Ceseracciu L, Tedeschi G, Marras S, Scarpellini A, Benítez JJ, Athanassiou A, Heredia-Guerrero JA. Transparent and Robust All-Cellulose Nanocomposite Packaging Materials Prepared in a Mixture of Trifluoroacetic Acid and Trifluoroacetic Anhydride. Nanomaterials. 2019; 9(3):368. https://doi.org/10.3390/nano9030368
Chicago/Turabian StyleGuzman-Puyol, Susana, Luca Ceseracciu, Giacomo Tedeschi, Sergio Marras, Alice Scarpellini, José J. Benítez, Athanassia Athanassiou, and José A. Heredia-Guerrero. 2019. "Transparent and Robust All-Cellulose Nanocomposite Packaging Materials Prepared in a Mixture of Trifluoroacetic Acid and Trifluoroacetic Anhydride" Nanomaterials 9, no. 3: 368. https://doi.org/10.3390/nano9030368
APA StyleGuzman-Puyol, S., Ceseracciu, L., Tedeschi, G., Marras, S., Scarpellini, A., Benítez, J. J., Athanassiou, A., & Heredia-Guerrero, J. A. (2019). Transparent and Robust All-Cellulose Nanocomposite Packaging Materials Prepared in a Mixture of Trifluoroacetic Acid and Trifluoroacetic Anhydride. Nanomaterials, 9(3), 368. https://doi.org/10.3390/nano9030368








