Fabrication of Cu2ZnSnS4 (CZTS) Nanoparticle Inks for Growth of CZTS Films for Solar Cells
Abstract
:1. Introduction
2. Experiment Details
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
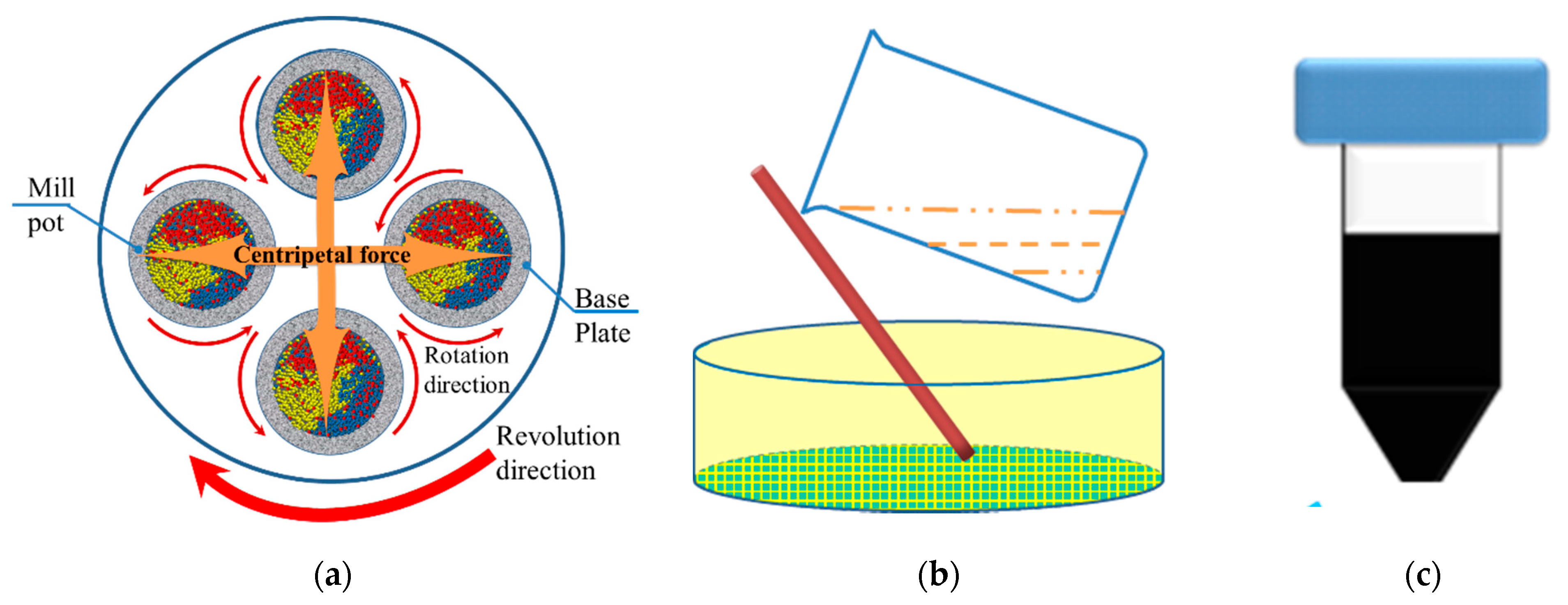
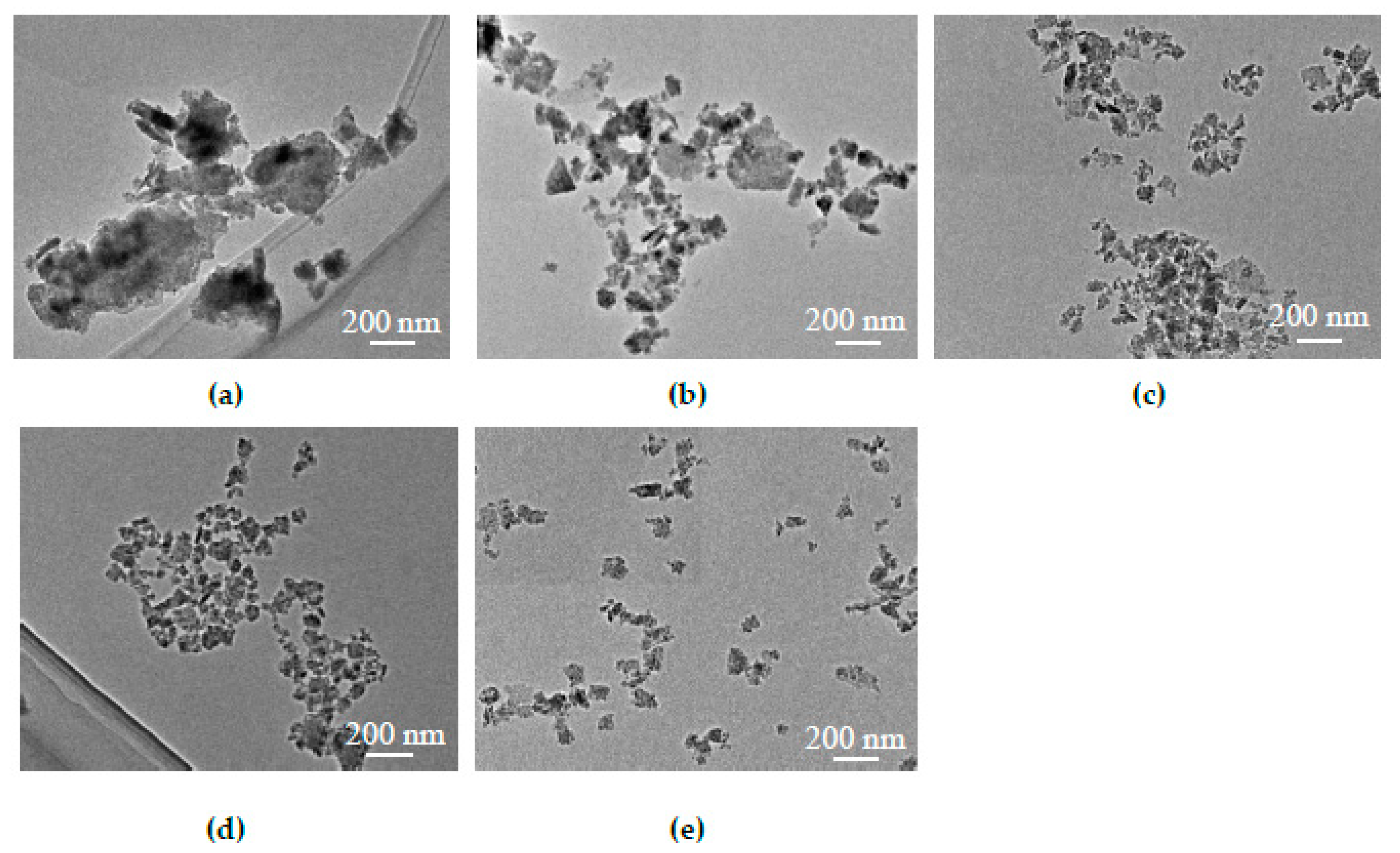
3.1. Centrifugation to Obtain CZTS Nanoparticle Ink
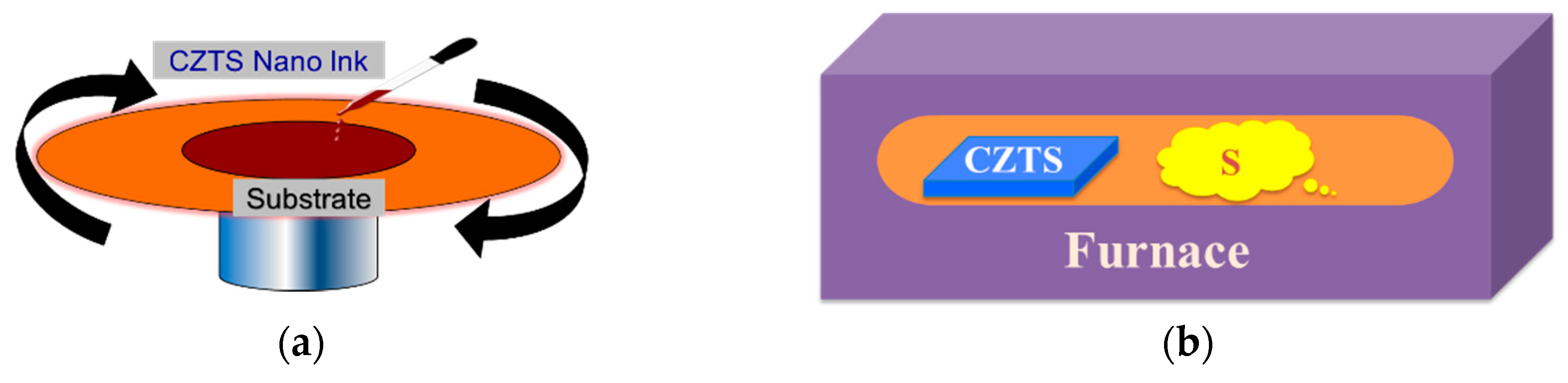
3.2. Deposition of CZTS Precursors
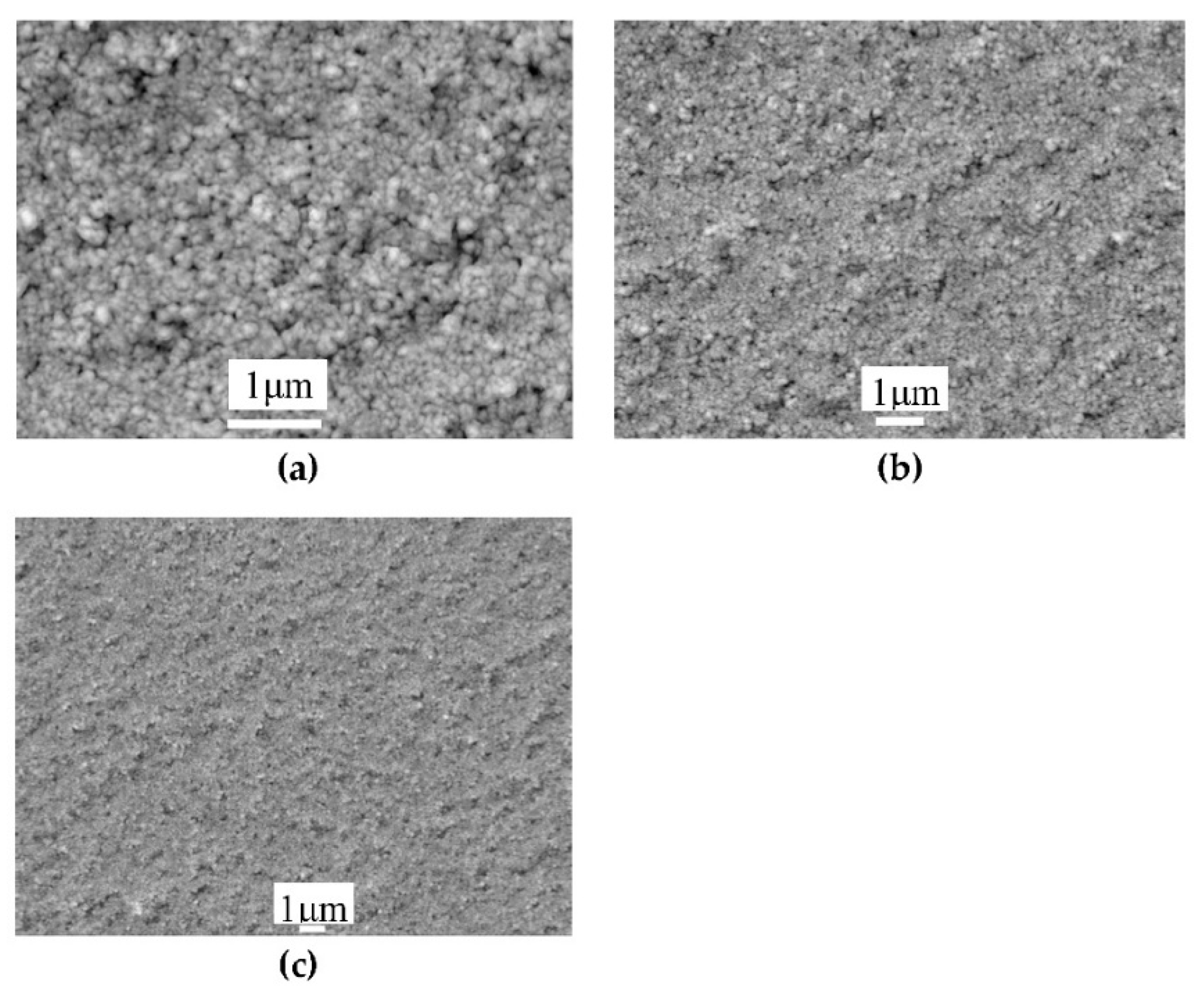
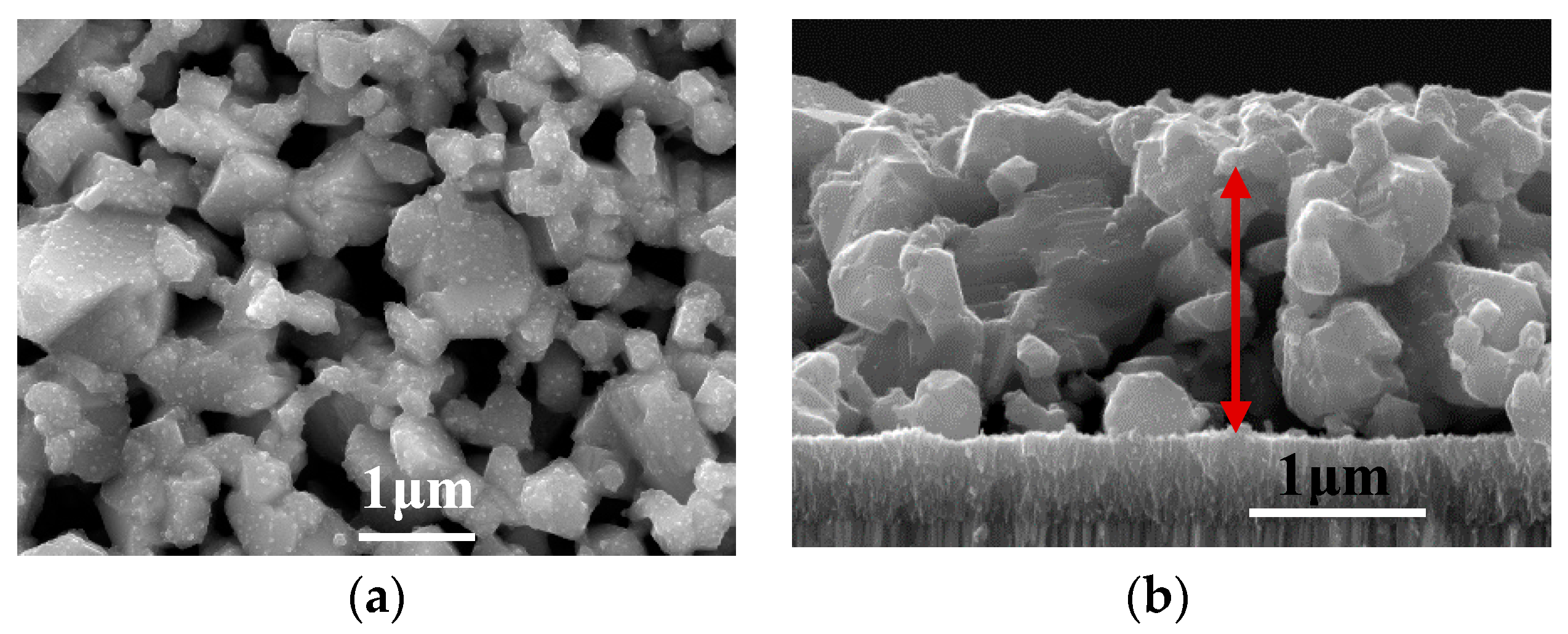
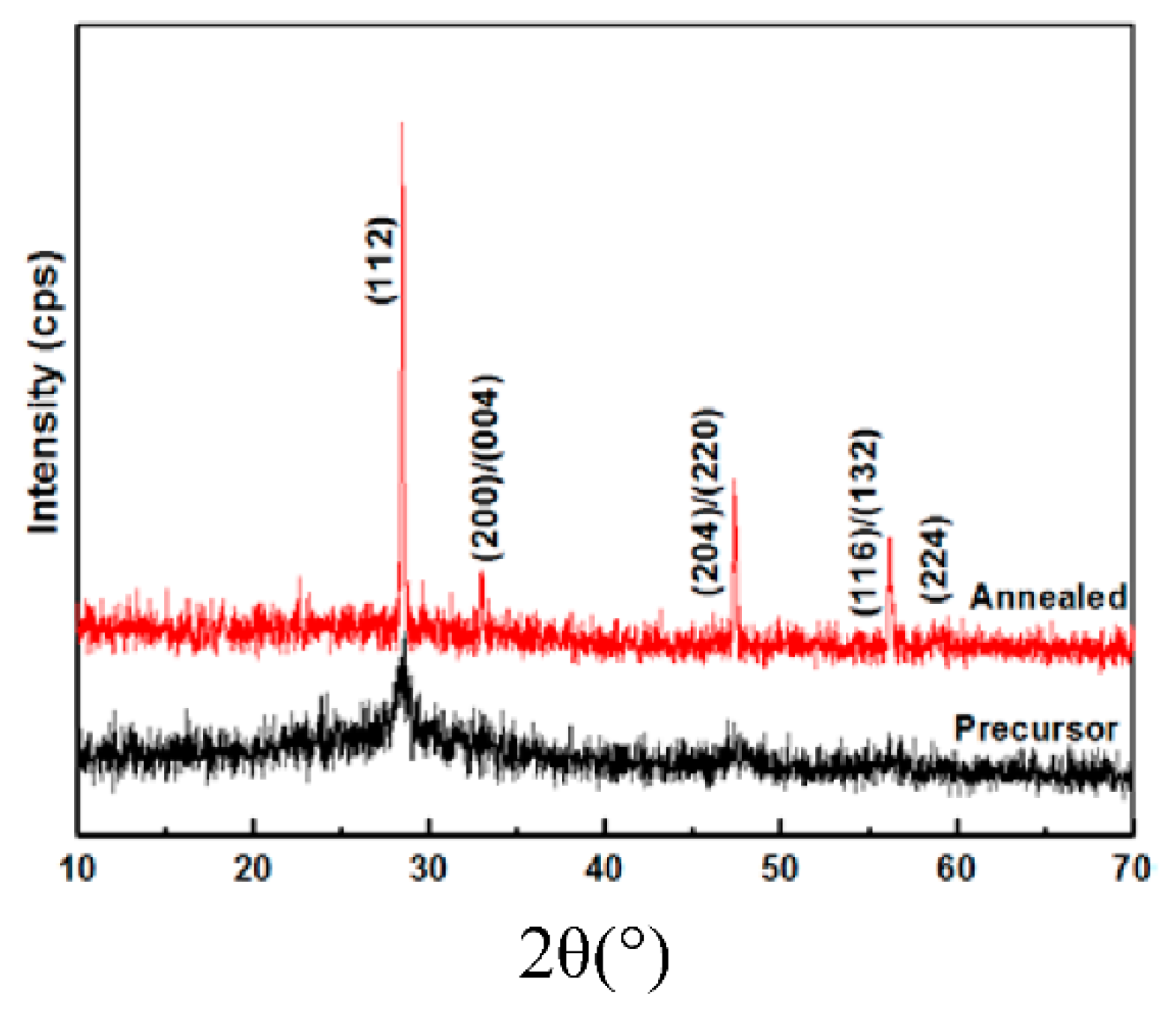
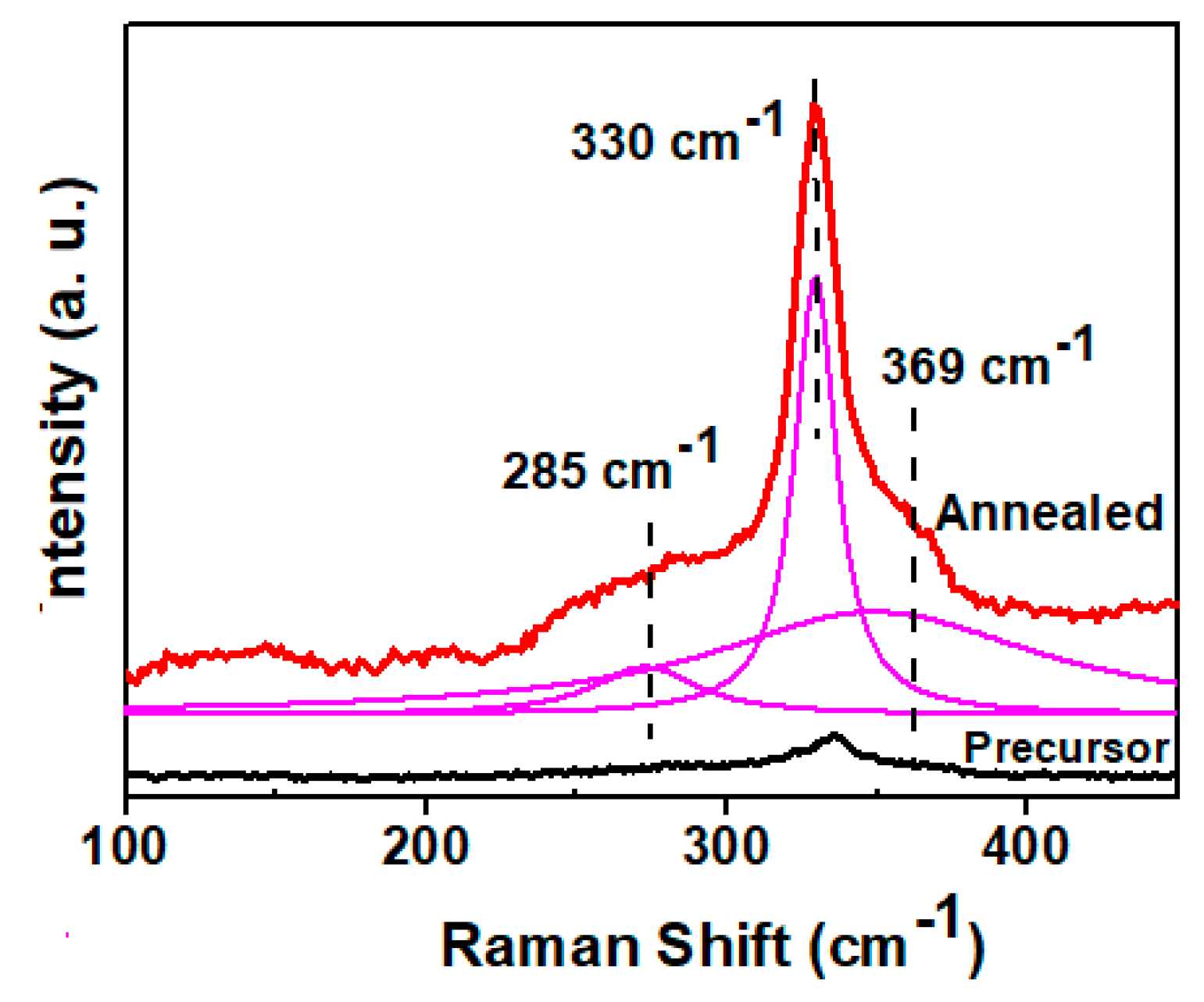
3.3. Annealing of the Precursor
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Roelofs, K.E.; Guo, Q.J.; Subramoney, S.; Caspar, J.V. Investigation of local compositional uniformity in Cu2ZnSn(S,Se)4 thin film solar cells prepared from nanoparticle inks. J. Mater. Chem. A 2014, 2, 13464–13470. [Google Scholar] [CrossRef]
- Ravindiran, M.; Praveenkuma, C. Status review and the future prospects of CZTS based solar cell—A novel approach on the device structure and material modeling for CZTS based photovoltaic device. Renew. Sustain. Energy Rev. 2018, 94, 317–329. [Google Scholar] [CrossRef]
- Fella, C.M.; Uhl, A.R.; Romanyuk, Y.E.; Tiwari, A.N. Cu2ZnSnSe4 absorbers processed from solution deposited metal salt precursors under different selenization conditions. Phys. Status Solidi A 2012, 209, 1043–1048. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device Characteristics of CZTSSe Thin-Film Solar Cells with 12.6% Efficiency. Adv. Energy Mater. 2014, 4, 1–5. [Google Scholar] [CrossRef]
- Seol, J.S.; Lee, S.Y.; Lee, J.C.; Nam, H.D.; Kim, K.H. Electrical and optical properties of Cu2ZnSnS4 thin films prepared by rf magnetron sputtering process. Sol. Energy Mater. Sol. C 2003, 75, 155–162. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawasaki, D.; Nishio, M.; Gu, Q.X.; Ogawal, H. Fabrication of Cu2ZnSnS4 thin films by co-evaporation. Phys. Status Solidi C 2006, 3, 2844–2847. [Google Scholar] [CrossRef]
- Song, N.; Young, M.; Liu, F.Y.; Erslev, P.; Wilson, S.; Harvey, S.P.; Teeter, G.; Huang, Y.D.; Hao, X.J.; Green, M.A. Epitaxial Cu2ZnSnS4 thin film on Si (111) 4 degrees substrate. Appl. Phys. Lett. 2015, 106, 1–9. [Google Scholar] [CrossRef]
- Tanaka, K.; Oonuki, M.; Moritake, N.; Uchiki, H. Cu2ZnSnS4 thin film solar cells prepared by non-vacuum processing. Sol. Energy Mater. Sol. C 2009, 93, 583–587. [Google Scholar] [CrossRef]
- Woo, K.; Kim, Y.; Moon, J. A non-toxic, solution-processed, earth abundant absorbing layer for thin-film solar cells. Energy Environ. Sci. 2012, 5, 5340–5345. [Google Scholar] [CrossRef]
- Patel, M.; Mukhopadhyay, I.; Ray, A. Structural, optical and electrical properties of spray-deposited CZTS thin films under a non-equilibrium growth condition. J. Phys. D Appl. Phys. 2012, 45, 1–10. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Denny, M.S.; Caspar, J.V.; Farneth, W.E.; Guo, Q.J.; Ionkin, A.S.; Johnson, L.K.; Lu, M.J.; Malajovich, I.; Radu, D.; et al. High-Efficiency Solution-Processed Cu2ZnSn(S,Se)4 Thin-Film Solar Cells Prepared from Binary and Ternary Nanoparticles. J. Am. Chem. Soc. 2012, 134, 15644–15647. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Gunawan, O.; Zhu, Y.; Bojarczuk, N.A.; Chey, S.J.; Guha, S. Thin film solar cell with 8.4% power conversion efficiency using an earth-abundant Cu2ZnSnS4 absorber. Prog. Photovolt. 2013, 21, 72–76. [Google Scholar] [CrossRef]
- Todorov, T.K.; Reuter, K.B.; Mitzi, D.B. High-Efficiency Solar Cell with Earth-Abundant Liquid-Processed Absorber. Adv. Mater. 2010, 22, E156–E159. [Google Scholar] [CrossRef] [PubMed]
- Pawar, B.S.; Pawar, S.M.; Shin, S.W.; Choi, D.S.; Park, C.J.; Kolekar, S.S.; Kim, J.H. Effect of complexing agent on the properties of electrochemically deposited Cu2ZnSnS4 (CZTS) thin films. Appl. Surf. Sci. 2010, 257, 1786–1791. [Google Scholar] [CrossRef]
- Akhavan, V.A.; Goodfellow, B.W.; Panthani, M.G.; Steinhagen, C.; Harvey, T.B.; Stolle, C.J.; Korgel, B.A. Colloidal CIGS and CZTS nanocrystals: A precursor route to printed photovoltaics. J. Solid State Chem. 2012, 189, 2–12. [Google Scholar] [CrossRef]
- Woo, K.; Kim, Y.; Yang, W.; Kim, K.; Kim, I.; Oh, Y.; Kim, J.Y.; Moon, J. Band-gap-graded Cu2ZnSn(S1−x,Sex)(4) Solar Cells Fabricated by an Ethanol-based, Particulate Precursor Ink Route. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scragg, J.J.; Ericson, T.; Kubart, T.; Edoff, M.; Platzer-Bjorkman, C. Chemical Insights into the Instability of Cu2ZnSnS4 Films during Annealing. Chem. Mater. 2011, 23, 4625–4633. [Google Scholar] [CrossRef]
- Fairbrother, A.; Garcia-Hemme, E.; Izquierdo-Roca, V.; Fontane, X.; Pulgarin-Agudelo, F.A.; Vigil-Galan, O.; Perez-Rodriguez, A.; Saucedo, E. Development of a Selective Chemical Etch to Improve the Conversion Efficiency of Zn-Rich Cu2ZnSnS4 Solar Cells. J. Am. Chem. Soc. 2012, 134, 8018–8021. [Google Scholar] [CrossRef] [PubMed]
- Ilari, G.M.; Fella, C.M.; Ziegler, C.; Uhl, A.R.; Romanyuk, Y.E.; Tiwari, A.N. Cu2ZnSnS4 solar cell absorbers spin-coated from amine-containing ether solutions. Sol. Energy Mater. Sol. Cells 2012, 104, 125–130. [Google Scholar] [CrossRef]
- Redinger, A.; Berg, D.M.; Dale, P.J.; Siebentritt, S. The Consequences of Kesterite Equilibria for Efficient Solar Cells. J. Am. Chem. Soc. 2011, 133, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Khare, A.; Himmetoglu, B.; Johnson, M.; Norris, D.J.; Cococcioni, M.; Aydil, E.S. Calculation of the lattice dynamics and Raman spectra of copper zinc tin chalcogenides and comparison to experiments. J. Appl. Phys. 2012, 111, 083708. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Salome, P.M.P.; da Cunha, A.F.; Schubert, B.A. Cu2ZnSnS4 solar cells prepared with sulphurized dc-sputtered stacked metallic precursors. Thin Solid Films 2011, 519, 7382–7385. [Google Scholar] [CrossRef]
- Wada, T.; Kohara, N.; Nishiwaki, S.; Negami, T. Characterization of the Cu(In,Ga)Se2/Mo interface in CIGS solar cells. Thin Solid Films 2001, 387, 118–122. [Google Scholar] [CrossRef]
- Ramanathan, K.; Contreras, M.A.; Perkins, C.L.; Asher, S.; Hasoon, F.S.; Keane, J.; Young, D.; Romero, M.; Metzger, W.; Noufi, R.; et al. Properties of 19.2% efficiency ZnO/CdS/CuInGaSe2 thin-film solar cells. Prog. Photovolt. 2003, 11, 225–230. [Google Scholar] [CrossRef]
- Nelson, J. The Physics of Solar Cells; Imperial College Press: London, UK; World Scientific Pub. Co.: River Edge, NJ, USA, 2003; p. 363. [Google Scholar]
- Christians, J.A.; Manser, J.S.; Kamat, P.V. Best Practices in Perovskite Solar Cell Efficiency Measurements. Avoiding the Error of Making Bad Cells Look Good. J. Phys. Chem. Lett. 2015, 6, 852–857. [Google Scholar] [CrossRef] [PubMed]


| Cu (%) | Zn (%) | Sn (%) | S (%) | Zn/Sn | Cu/(Zn + Sn) | |
|---|---|---|---|---|---|---|
| Precursor | 25.4 | 9.9 | 15.6 | 49.1 | 0.63 | 1.00 |
| Annealed CZTS film | 24.7 | 9.8 | 15.1 | 50.5 | 0.65 | 0.99 |
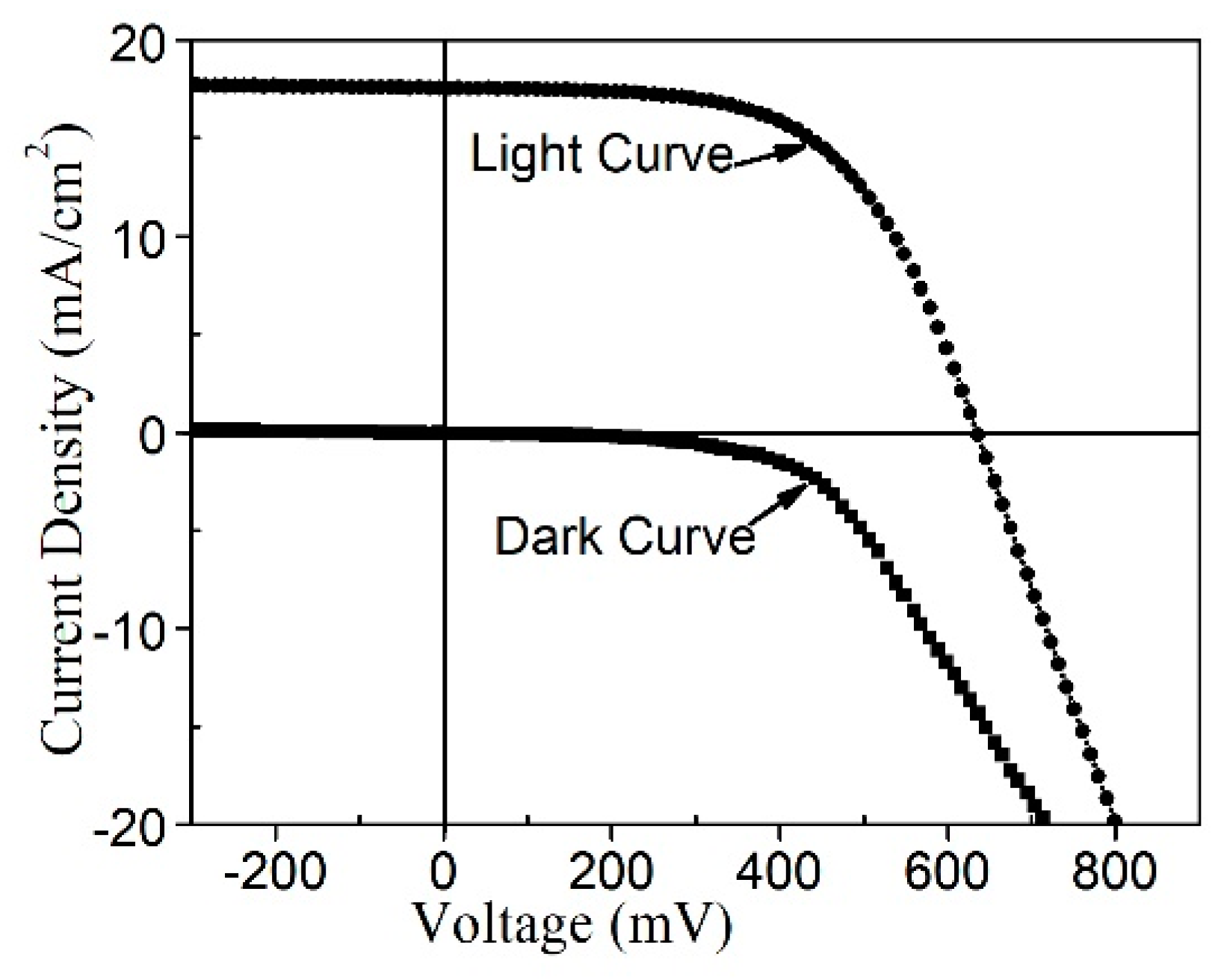
| Sample No. | Eff | Voc (mV) | Jsc (mA/cm2) | FF (%) |
|---|---|---|---|---|
| 1 | 6.2 | 633.3 | 17.6 | 55.8 |
| 2 | 4.3 | 578.2 | 15.3 | 48.6 |
| 3 | 2.5 | 497.1 | 12.2 | 41.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fu, E.; Wang, Y.; Zhang, C. Fabrication of Cu2ZnSnS4 (CZTS) Nanoparticle Inks for Growth of CZTS Films for Solar Cells. Nanomaterials 2019, 9, 336. https://doi.org/10.3390/nano9030336
Zhang X, Fu E, Wang Y, Zhang C. Fabrication of Cu2ZnSnS4 (CZTS) Nanoparticle Inks for Growth of CZTS Films for Solar Cells. Nanomaterials. 2019; 9(3):336. https://doi.org/10.3390/nano9030336
Chicago/Turabian StyleZhang, Xianfeng, Engang Fu, Yuehui Wang, and Cheng Zhang. 2019. "Fabrication of Cu2ZnSnS4 (CZTS) Nanoparticle Inks for Growth of CZTS Films for Solar Cells" Nanomaterials 9, no. 3: 336. https://doi.org/10.3390/nano9030336






