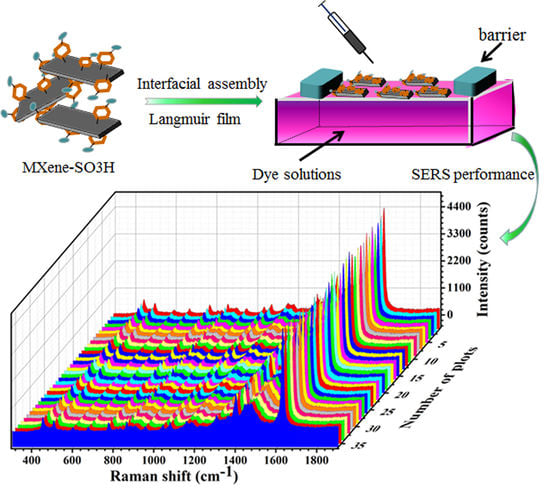
Preparation of Self-Assembled Composite Films Constructed by Chemically-Modified MXene and Dyes with Surface-Enhanced Raman Scattering Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of MXene
2.3. Preparation of Composite Langmuir Films
2.4. SERS Measurements
2.5. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, G.C.; Liu, M.H. Interfacial assemblies of Cyanine dyes and gemini amphiphiles with rigid spacers: Regulation and interconversion of the aggregates. J. Phys. Chem. B 2008, 112, 7430–7437. [Google Scholar] [CrossRef] [PubMed]
- Hansda, C.; Chakraborty, U.; Hussain, S.A.; Hussain, S.A.; Bhattacharjee, D.; Paul, P.K. Layer-by-layer films and colloidal dispersions of graphene oxide nanosheets for efficient control of the fluorescence and aggregation properties of the cationic dye acridine orange. Spectrochim. Acta A 2016, 157, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, T.; Watanabe, T.; Fujishima, A.; Honda, K. Light energy conversion with chlorophyll monolayer electrodes. In vitro electrochemical simulation of photosynthetic primary processes. J. Am. Chem. Soc. 1978, 100, 6657–6665. [Google Scholar] [CrossRef]
- Jiao, T.; Zhao, H.; Zhou, J.; Zhang, Q.; Luo, X.; Hu, J.; Peng, Q.; Yan, X. Self-assembly reduced graphene oxide nanosheet hydrogel fabrication by anchorage of chitosan/silver and its potential efficient application toward dyes degradation for wastewater treatments. ACS Sustain. Chem. Eng. 2015, 3, 3130–3139. [Google Scholar] [CrossRef]
- Yao, H.; Kobayashi, S. Self-assembly of acridine orange dye at a mica/solution interface: Formation of nanostripe supramolecular architectures. J. Colloid Interface Sci. 2007, 307, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Dähne, L.; Biller, E. Color variation in highly oriented dye layers by polymorphism of dye aggregates. Adv. Mater. 1998, 10, 241–245. [Google Scholar] [CrossRef]
- Yao, H.; Domoto, K.; Isohashi, T.; Kimura, K. In situ detection of birefringent mesoscopic H and J aggregates of thiacarbocyanine dye in solution. Langmuir 2005, 21, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Muenter, A.A.; Brumbaugh, D.V.; Apolito, J.; Horn, L.A.; Spano, F.C.; Mukamel, S. Size dependence of excited-state dynamics for J-aggregates at silver bromide interfaces. J. Phys. Chem. 1992, 96, 2783–2790. [Google Scholar] [CrossRef]
- Kashida, H.; Asanuma, H.; Komiyama, M. Interstrand H-aggregation of cationic dyes for narrowing the absorption spectra and stabilizing the duplex. Supramol. Chem. 2004, 16, 459–464. [Google Scholar] [CrossRef]
- Song, J.W.; Ma, K.; Jiao, T.F.; Xing, R.R.; Zhang, L.X.; Zhou, J.X.; Peng, Q.M. Preparation and self-assembly of graphene oxide-dye composite Langmuir films: Nanostructures and aggregations. Colloid Surf. A-Physicochem. Eng. Asp. 2017, 529, 793–800. [Google Scholar] [CrossRef]
- Rubtsov, I.V.; Ebina, K.; Satou, F.; Oh, J.W.; Kumazaki, S.; Suzumoto, T.; Tani, T.; Yoshihara, K.J. Spectral sensitization and supersensitization of AgBr nanocrystals studied by ultrafast fluorescence spectroscopy. J. Phys. Chem. A 2002, 106, 2795–2802. [Google Scholar] [CrossRef]
- Sinoforoglu, M.; Gür, B.; Arık, M.; Onganer, Y.; Meral, K. Graphene oxide sheets as a template for dye assembly: Graphene oxide sheets induce H-aggregates of pyronin (Y) dye. RSC Adv. 2013, 3, 11832–11838. [Google Scholar] [CrossRef]
- Ng, V.M.H.; Huang, H.; Zhou, K.; Lee, P.S.; Que, W.X.; Xu, Z.C.; Kong, L.B. (MXenes) and their composites: Synthesis and applications. J. Mater. Chem. A 2017, 5, 3039–3068. [Google Scholar]
- Eklund, P.; Rosen, J.; Persson, P.O.A. Layered ternary Mn+1AXn phases and their 2D derivative MXene: An overview from a thin-film perspective. J. Phys. D-Appl. Phys. 2017, 50, 113001. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.T.; Chen, F.F.; Zhu, Y.J.; Zhang, Y.G.; Jiang, Y.Y.; Ma, M.G.; Chen, F. Binary strengthening and toughening of MXene/Cellulose nanofiber composite paper with nacre-inspired structure and superior electromagnetic interference shielding properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef]
- Xu, L.D.; Zhu, D.G.; Liu, Y.L.; Suzuki, T.S.; Kim, B.; Sakka, Y.; Grasso, S.; Hu, C.F. Effect of texture on oxidation resistance of Ti3AlC2. J. Eur. Ceram. Soc. 2018, 38, 3417–3423. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Wang, H.B.; Zhang, J.F.; Wu, Y.P.; Huang, H.J.; Li, G.Y.; Zhang, X.; Wang, Z.Y. Surface modified MXene Ti3C2 multilayers by aryl diazonium salts leading to large-scale delamination. Appl. Surf. Sci. 2016, 384, 287–293. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, P.G.; Tian, W.B.; Qiu, X.; Zhang, Y.M.; Sun, Z.M. Alkali treated Ti3C2Tx MXenes and their dye adsorption performance. Mater. Chem. Phys. 2018, 206, 270–276. [Google Scholar]
- Kang, K.M.; Kim, D.W.; Ren, C.E.; Cho, K.M.; Kim, S.J.; Choi, J.H.; Nam, Y.T.; Gogotsi, Y.; Jung, H.T. Selective molecular separation on Ti3C2Tx-graphene oxide membranes during pressure-driven filtration: Comparison with graphene oxide and MXenes. ACS Appl. Mater. Interface 2017, 9, 44687–44694. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.M.; Guo, J.X.; Zhang, Q.R.; Xiang, J.Y.; Liu, B.Z.; Zhou, A.; Liu, R.P.; Tian, Y.J. Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.M.; Tao, X.Y.; Zhang, J.; Xia, Y.; Huang, H.; Zhang, L.Y.; Gan, Y.P.; Liang, C.; Zhang, W.K. Sn4+ ion decorated highly conductive Ti3C2 MXene: Promising lithium-ion anodes with enhanced volumetric capacity and cyclic performance. ACS Nano 2016, 10, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D Titanium carbide (MXene). Adv. Mater. 2015, 28, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, M.; Li, Z.; Fan, G.; Fan, L.Z.; Zhou, A. Two-dimensional Ti3C2 as anode material for Li-ion batteries. Electrochem. Commun. 2014, 47, 80–83. [Google Scholar] [CrossRef]
- Gronwald, O.; Snip, E.; Shinkai, S. Gelators for organic liquids based on self-assembly: A new facet of supramolecular and combinatorial chemistry. J. Colloid Interface Sci. 2002, 7, 148–156. [Google Scholar] [CrossRef]
- Zhang, G.C.; Zhai, X.D.; Liu, M.H.; Tang, Y.L.; Zhang, Y.Z. Regulation of aggregation and morphology of cyanine dyes on monolayers via gemini amphiphiles. J. Phys. Chem. B 2007, 111, 9301–9308. [Google Scholar] [CrossRef] [PubMed]
- Gamot, T.D.; Bhattacharyya, A.R.; Sridhar, T.; Beach, F.; Tabor, R.F.; Majumber, M. Synthesis and stability of water-in-oil emulsion using partially reduced graphene oxide as a tailored surfactant. Langmuir 2017, 33, 10311–10321. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, K.; Kumar, J.; Taneja, P.; Gupta, R.K.; Manjuladevi, V. Langmuir–Blodgett films of stearic acid deposited on substrates at different orientations relative to compression direction: Alignment layer for nematic liquid crystal. Liq. Cryst. 2017, 44, 1592–1599. [Google Scholar] [CrossRef]
- Banik, S.; Saha, M.; Hussain, S.A.; Bhattacharjee, D. pH induced interaction of DPPC with a fluorescent dye in Langmuir and Langmuir Blodgett (LB) films. Mol. Cryst. Liq. Cryst. 2017, 643, 255–265. [Google Scholar] [CrossRef]
- Ru, E.C.L.; Blackie, E.; Meyer, A.M.; Etchegoin, P.G. Surface enhanced Raman scattering enhancement factors: a comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar]
- Sarycheva, A.; Makaryan, T.; Maleski, K.; Satheeshkumar, E.; Melikyan, A.; Minassian, H.; Yoshimura, M.; Gogotsi, Y. Two-dimensional titanium carbide (MXene) as surface-enhanced Raman scattering substrate. J. Phys. Chem. C 2017, 121, 19983–19988. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y.F. Guidelines for synthesis and processing of two-dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Zhou, J.; Zha, X.; Chen, F.Y.; Ye, Q.; Eklund, P.; Du, S.Y.; Huang, Q. A two-dimensional zirconium carbide by selective etching of Al3C3 from nanolaminated Zr3Al3C5. Angew. Chem. Int. Ed. 2016, 55, 5008–5013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zha, X.H.; Zhou, X.B.; Chen, F.Y.; Gao, G.L.; Wang, S.W.; Shen, C.; Chen, T.; Zhi, C.Y.; Eklund, P.; et al. Synthesis and electrochemical properties of two-dimensional hafnium carbide. ACS Nano 2017, 11, 3841–3850. [Google Scholar] [CrossRef]
- Orler, E.B.; Yontz, D.J.; Moore, R.B. Sulfonation of syndiotactic polystyrene for model semicrystalline ionomer investigations. Macromolecules 1993, 26, 5157–5160. [Google Scholar] [CrossRef]
- Liu, F.J.; Sun, J.; Zhu, L.F.; Meng, X.G.; Qi, C.Z.; Xiao, F.S. Sulfated graphene as an efficient solid catalyst for acid-catalyzed liquid reactions. J. Mater. Chem. 2012, 22, 5495–5502. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Wang, Y.; Chen, H.; Caro, J.; Wang, H. A two-dimensional lamellar membrane: MXene nanosheet stacks. Angew. Chem. Int. Ed. 2017, 56, 1825–1829. [Google Scholar] [CrossRef]
- Yeo, J.S.; Yun, J.M.; Jung, Y.S.; Kim, D.Y.; Noh, Y.D.; Kim, S.S.; Na, S.I. Sulfonic acid-functionalized, reduced graphene oxide as an advanced interfacial material leading to donor polymer-independent high-performance polymer solar cells. J. Mater. Chem. A 2014, 2, 292–298. [Google Scholar] [CrossRef]
- Guo, R.; Jiao, T.F.; Li, R.F.; Chen, Y.; Guo, W.; Zhang, L.; Zhou, J.; Zhang, Q.; Peng, Q. Sandwiched Fe3O4/carboxylate graphene oxide nanostructures constructed by layer-by-layer assembly for highly efficient and magnetically recyclable dye removal. ACS Sustain. Chem. Eng. 2018, 6, 1279–1288. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhang, L.T.; Wang, Y. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Ge, M.; Huang, J.; Lai, Y.; Lin, C.; Zhang, K.; Meng, K.; Tang, Y. Constructing multifunctional MOF@rGO hydro-/aerogels by the self-assembly process for customized water remediation. J. Mater. Chem. A 2017, 5, 11873–11881. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, C.; Jiao, T.; Song, J.; Zhang, X.; Xing, R.; Zhou, J.; Zhang, L.; Peng, Q. Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment. Nanomaterials 2018, 8, 35. [Google Scholar] [CrossRef]
- Huo, S.; Duan, P.; Jiao, T.; Peng, Q.; Liu, M. Self-assembled luminescent quantum dots to generate full-color and white circularly polarized light. Angew. Chem. Int. Ed. 2017, 56, 12174–12178. [Google Scholar] [CrossRef] [PubMed]
- Canning, J.; Huyang, G.; Ma, M.; Beavis, A.; Bishop, D.; Cook, K.; McDonagh, A.; Shi, D.Q.; Peng, G.D.; Crossley, M.J. Percolation diffusion into self-assembled mesoporous silica microfibres. Nanomaterials 2014, 4, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ma, K.; Jiao, T.; Xing, R.; Ma, X.; Hu, J.; Huang, H.; Zhang, L.; Yan, X. Fabrication of hierarchical layer-by-layer assembled diamond based core-shell nanocomposites as highly efficient dye absorbents for wastewater treatment. Sci. Rep. 2017, 7, 44076. [Google Scholar] [CrossRef] [PubMed]
- Derkus, B.; Emregul, K.C.; Emregul, E. Evaluation of protein immobilization capacity on various carbon nanotube embedded hydrogel biomaterials. Mater. Sci. Eng. C 2015, 56, 132–140. [Google Scholar] [CrossRef]
- Jiao, T.F.; Liu, Y.Z.; Wu, Y.T.; Zhang, Q.R.; Yan, X.H.; Gao, F.M.; Bauer, A.J.P.; Liu, J.Z.; Zeng, T.Y.; Li, B.B. Facile and scalable preparation of graphene oxide-based magnetic hybrids for fast and highly efficient removal of organic dyes. Sci. Rep. 2015, 5, 12451. [Google Scholar] [CrossRef]
- Li, K.K.; Jiao, T.F.; Xing, R.R.; Zou, G.Y.; Zhou, J.X.; Zhang, L.X.; Peng, Q.M. Fabrication of tunable hierarchical MXene@AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. Sci. China Mater. 2018, 61, 728–736. [Google Scholar] [CrossRef]
- Xing, R.; Wang, W.; Jiao, T.; Ma, K.; Zhang, Q.; Hong, W.; Qiu, H.; Zhou, J.; Zhang, L.; Peng, Q. Bioinspired polydopamine sheathed nanofibers containing carboxylate graphene oxide nanosheet for high-efficient dyes scavenger. ACS Sustain. Chem. Eng. 2017, 5, 4948–4956. [Google Scholar] [CrossRef]
- Guo, H.; Jiao, T.; Zhang, Q.; Guo, W.; Peng, Q.; Yan, X. Preparation of graphene oxide-based hydrogels as efficient dye adsorbents for wastewater treatment. Nanoscale Res. Lett. 2015, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, S.; Zhang, L.; Yin, J.; Jiao, T.; Zhang, L.; Xu, Y.; Zhou, J.; Peng, Q. Facile preparation and catalytic performance characterization of AuNPs-loaded hierarchical electrospun composite fibers by solvent vapor annealing treatment. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 561, 283–291. [Google Scholar] [CrossRef]
- Sun, S.; Wang, C.; Han, S.; Jiao, T.; Wang, R.; Yin, J.; Li, Q.; Wang, Y.; Geng, L.; Yu, X.; Peng, Q. Interfacial nanostructures and acidichromism behaviors in self-assembRecent progress in layered transition metal carbides and/or nitrides led terpyridine derivatives Langmuir-Blodgett films. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 564, 1–9. [Google Scholar] [CrossRef]
- Huang, X.; Jiao, T.; Liu, Q.; Zhang, L.; Zhou, J.; Li, B.; Peng, Q. Hierarchical electrospun nanofibers treated by solvent vapor annealing as air filtration mat for high-efficiency PM2.5 capture. Sci. China Mater. 2019, 62, 423–436. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, B.; Wang, R.; Zhang, L.; Jiao, T.; Liu, Z. Facile preparation of rod-like MnO nanomixtures via hydrothermal approach and highly efficient removal of methylene blue for wastewater treatment. Nanomaterials 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, J.; Zhang, L.; Xing, R.; Jiao, T.; Gao, F.; Peng, Q. Facile synthesis of self-assembled carbon nanotubes/dye composite films for sensitive electrochemical determination of Cd(II) ions. Nanotechnology 2018, 29, 445603. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, F.; Jiao, T.; Xing, R.; Zhang, L.; Zhang, Q.; Peng, Q. Selective Cu(II) ion removal from wastewater via surface charged self-assembled polystyrene-Schiff base nanocomposites. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 545, 60–67. [Google Scholar] [CrossRef]
- Luo, X.; Ma, K.; Jiao, T.; Xing, R.; Zhang, L.; Zhou, J.; Li, B. Graphene oxide-polymer composite Langmuir films constructed by interfacial thiol-ene photopolymerization. Nanoscale Res. Lett. 2017, 12, 99. [Google Scholar] [CrossRef]
- Song, J.; Xing, R.; Jiao, T.; Peng, Q.; Yuan, C.; Möhwald, H.; Yan, X. Crystalline dipeptide nanobelts based on solid-solid phase transformation self-assembly and their polarization imaging of cells. ACS Appl. Mater. Interfaces 2018, 10, 2368–2376. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Xing, R.R.; Jiao, T.F.; Ma, K.; Chen, C.J.; Ma, G.H.; Yan, X.H. Synergistic in vivo photodynamic and photothermal antitumor therapy based on collagen-gold hybrid hydrogels with inclusion of photosensitive drugs Colloids and Surfaces A: Physicochemical and Engineering Aspects. ACS Appl. Mater. Interfaces 2016, 8, 13262–13269. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Jiao, T.; Liu, Y.; Ma, K.; Zou, Q.; Ma, G.; Yan, X. Co-assembly of graphene oxide and albumin/photosensitizer nanohybrids towards enhanced photodynamic therapy. Polymers 2016, 8, 181. [Google Scholar] [CrossRef]
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016, 28, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.W.; Wang, M.Q.; Li, L.; Li, J.J.; Yang, Z.; Cao, M.H. Hierarchical MoS2 -microspheres decorated with 3D AuNPs arrays for high-efficiency SERS sensing. Sens. Actuat. B-Chem. 2017, 255, 1407–1414. [Google Scholar] [CrossRef]
- Yang, L.; Wang, W.H.; Jiang, H.Y.; Zhang, Q.H.; Shan, H.H.; Zhang, M.; Zhu, K.R.; Lv, J.G.; He, G.; Sun, Z.Q. Improved SERS performance of single-crystalline TiO2, nanosheet arrays with coexposed {001} and {101} facets decorated with Ag nanoparticles. Sens. Actuat. B-Chem. 2017, 242, 932–939. [Google Scholar] [CrossRef]
- Shi, G.C.; Wang, M.L.; Zhu, Y.Y.; Wang, Y.H.; Ma, W.L. Synthesis of flexible and stable SERS substrate based on Au nanofilms/cicada wing array for rapid detection of pesticide residues. Opt. Commun. 2018, 425, 49–57. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Shen, L.; Sun, X.; Shi, G.; Ma, W.; Yan, X. High-performance flexible surface-enhanced Raman scattering substrates fabricated by depositing Ag nanoislands on the dragonfly wing. Appl. Surf. Sci. 2018, 436, 391–397. [Google Scholar] [CrossRef]
- Satheeshkumar, E.; Makaryan, T.; Melikyan, A.; Minassian, H.; Gogotsi, Y.; Yoshimura, M. One-step solution processing of Ag, Au and Pd@ MXene hybrids for SERS. Sci. Rep. 2016, 6, 32049. [Google Scholar] [CrossRef]
- Jiao, T.; Guo, H.; Zhang, Q.; Peng, Q.; Tang, Y.; Yan, X.; Li, B. Reduced graphene oxide-based silver nanoparticle-containing composite hydrogel as highly efficient dye catalysts for wastewater treatment. Sci. Rep. 2015, 5, 11873. [Google Scholar] [CrossRef]
- Zhan, F.; Wang, R.; Yin, J.; Han, Z.; Zhang, L.; Jiao, T.; Zhou, J.; Zhang, L.; Peng, Q. Facile solvothermal preparation of Fe3O4–Ag nanocomposite with excellent catalytic performance. RSC Adv. 2019, 9, 878–883. [Google Scholar] [CrossRef]
- Wang, C.; Yin, J.; Wang, R.; Jiao, T.; Huang, H.; Zhou, J.; Zhang, L.; Peng, Q. Facile preparation of self-assembled polydopamine-modified electrospun fibers for highly effective removal of organic dyes. Nanomaterials 2019, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, R.; Yin, J.; Jiao, T.; Huang, H.; Zhao, X.; Zhang, L.; Li, Q.; Zhou, J.; Peng, Q. Fabrication and highly efficient dye removal characterization of beta-cyclodextrin-based composite polymer fibers by electrospinning. Nanomaterials 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, R.; Jiao, T.; Zou, G.; Zhan, F.; Yin, J.; Zhang, L.; Zhou, J.; Peng, Q. Facile preparation of hierarchical AgNP-loaded MXene/Fe3O4/polymer nanocomposites by electrospinning with enhanced catalytic performance for wastewater treatment. ACS Omega 2019, 4, 1897–1906. [Google Scholar] [CrossRef]
- Yin, Y.; Ma, N.; Xue, J.; Wang, G.; Liu, S.; Li, H.; Guo, P. Insights into the role of poly(vinylpyrrolidone) in the synthesis of palladium nanoparticles and their electrocatalytic properties. Langmuir 2019, 35, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yuan, C.Q.; Zou, Q.L.; Xie, Z.C.; Yan, X.H. Self-assembled Zinc/cystine-based chloroplast mimics capable of photoenzymatic reactions for sustainable fuel synthesis. Angew. Chem. Int. Ed. 2017, 56, 7876–7880. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xing, R.R.; Li, Y.X.; Zou, Q.L.; Möhwald, H.; Yan, X.H. Mimicking primitive photobacteria: Sustainable hydrogen evolution based on peptide-porphyrin co-assemblies with self-mineralized reaction center. Angew. Chem. Int. Ed. 2016, 55, 12503–12507. [Google Scholar] [CrossRef]
- Liu, K.; Xing, R.R.; Chen, C.J.; Shen, G.Z.; Yan, L.Y.; Zou, Q.L.; Ma, G.H.; Möhwald, H.; Yan, X.H. Peptide-induced hierarchical long-range order and photocatalytic activity of porphyrin assemblies. Angew. Chem. Int. Ed. 2015, 54, 500–505. [Google Scholar] [CrossRef]












| Element | C | Ti | F | N | S | O |
|---|---|---|---|---|---|---|
| Atomic percentage (%) | 71.30 | 4.39 | 1.65 | 5.53 | 2.24 | 14.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Yan, X.; Li, J.; Jiao, T.; Cai, C.; Zou, G.; Wang, R.; Wang, M.; Zhang, L.; Peng, Q. Preparation of Self-Assembled Composite Films Constructed by Chemically-Modified MXene and Dyes with Surface-Enhanced Raman Scattering Characterization. Nanomaterials 2019, 9, 284. https://doi.org/10.3390/nano9020284
Chen K, Yan X, Li J, Jiao T, Cai C, Zou G, Wang R, Wang M, Zhang L, Peng Q. Preparation of Self-Assembled Composite Films Constructed by Chemically-Modified MXene and Dyes with Surface-Enhanced Raman Scattering Characterization. Nanomaterials. 2019; 9(2):284. https://doi.org/10.3390/nano9020284
Chicago/Turabian StyleChen, Kaiyue, Xiaoya Yan, Junkai Li, Tifeng Jiao, Chong Cai, Guodong Zou, Ran Wang, Mingli Wang, Lexin Zhang, and Qiuming Peng. 2019. "Preparation of Self-Assembled Composite Films Constructed by Chemically-Modified MXene and Dyes with Surface-Enhanced Raman Scattering Characterization" Nanomaterials 9, no. 2: 284. https://doi.org/10.3390/nano9020284