Oil-in-Water Emulsions Stabilized by Cellulose Nanofibrils—The Effects of Ionic Strength and pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Enzymatically Pretreated CNFs (CNF-E)
2.1.2. TEMPO-Oxidized CNFs (CNF-T)
2.2. Characterization of Rapeseed Oil and CNFs
2.2.1. Characterization of Rapeseed Oil
2.2.2. Characterization of CNF Film
2.3. Preparation of O/W Model Emulsions
2.4. Characterization of O/W Emulsions
3. Results and Discussion
3.1. Characterization of CNF Samples
3.2. Characterization of the Rapeseed Oil
3.3. The Effect of Addition of Salt and Acid on CNF-Stabilized Emulsions
3.3.1. Apparent Viscosity and pH
3.3.2. Visual Stability
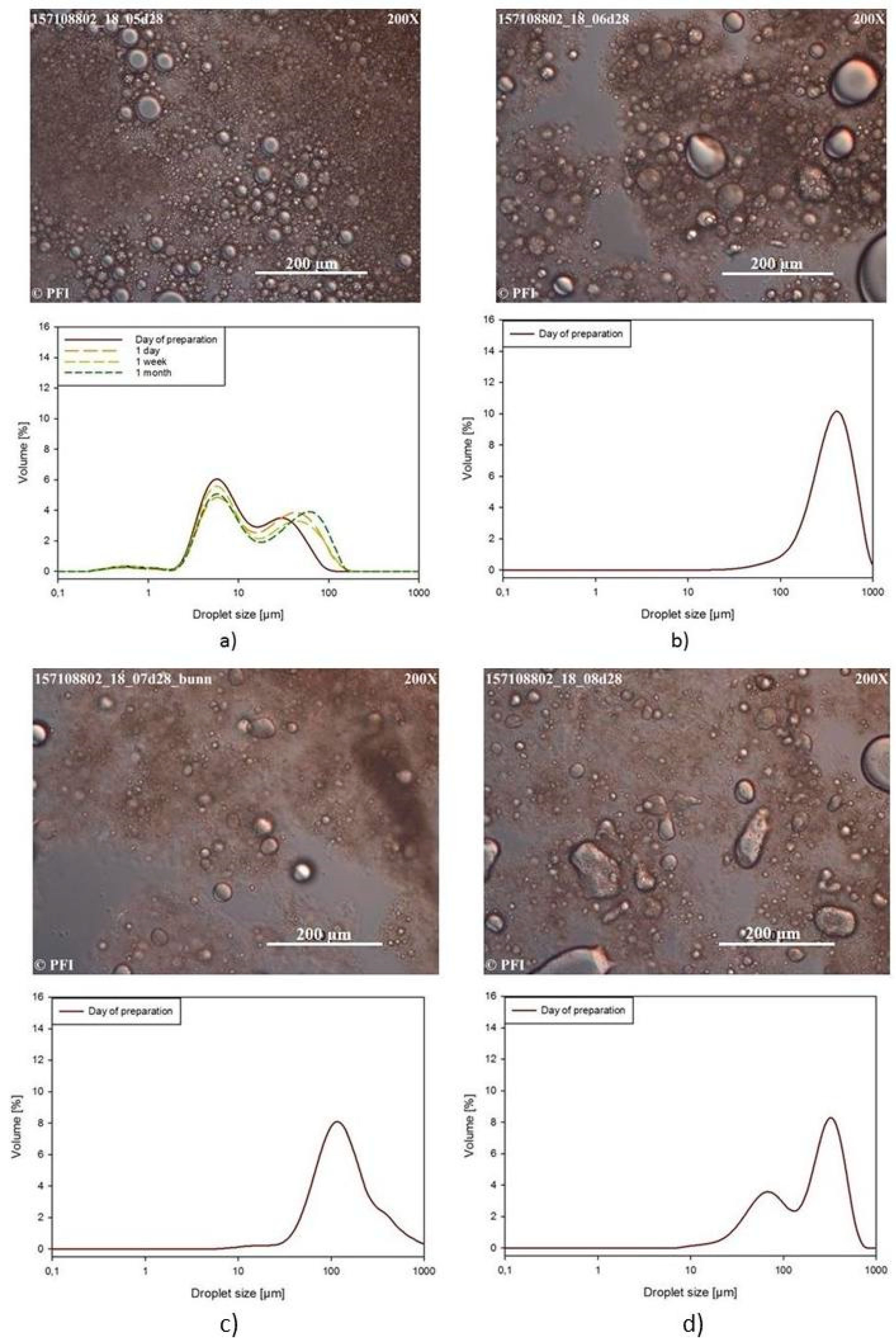
3.3.3. Light Microscopy Images and Droplet Size Distribution
3.3.4. Accelerated Stability Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arslan-Alaton, I.; Erdinc, E. Effect of photochemical treatment on the biocompatibility of a commercial nonionic surfactant used in the textile industry. Water Res. 2006, 40, 3409–3418. [Google Scholar] [CrossRef] [PubMed]
- Gomez, V.; Ferreres, L.; Pocurull, E.; Borrull, F. Determination of non-ionic and anionic surfactants in environmental water matrices. Talanta 2011, 84, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Jardak, K.; Drogui, P.; Daghrir, R. Surfactants in aquatic and terrestrial environment: Occurrence, behavior, and treatment processes. Environ. Sci. Pollut. Res. 2016, 23, 3195–3216. [Google Scholar] [CrossRef]
- Pickering, S.U. CXCVI.-Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Ramsden, W. Separation of solids in the surface-layers of solutions and ’suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation).—Preliminary account. Proc. R. Soc. Lond. 1903, 72, 156–164. [Google Scholar]
- Binks, B.P. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Denkov, N.; Ivanov, I.; Kralchevsky, P.; Wasan, D. A possible mechanism of stabilization of emulsions by solid particles. J. Colloid Interface Sci. 1992, 150, 589–593. [Google Scholar] [CrossRef]
- Horozov, T.S.; Binks, B.P. Particle-stabilized emulsions: A bilayer or a bridging monolayer? Angew. Chem. 2006, 118, 787–790. [Google Scholar] [CrossRef]
- Abend, S.; Bonnke, N.; Gutschner, U.; Lagaly, G. Stabilization of emulsions by heterocoagulation of clay minerals and layered double hydroxides. Colloid Polym. Sci. 1998, 276, 730–737. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Blaker, J.J.; Murakami, R.; Heng, J.Y.Y.; Bismarck, A. Phase behavior of medium and high internal phase water-in-oil emulsions stabilized solely by hydrophobized bacterial cellulose nanofibrils. Langmuir 2014, 30, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.G.; Mougel, J.-B.; Cathala, B.; Berglund, L.A.; Capron, I. Preparation of double Pickering emulsions stabilized by chemically tailored nanocelluloses. Langmuir 2014, 30, 9327–9335. [Google Scholar] [CrossRef] [PubMed]
- Gestranius, M.; Stenius, P.; Kontturi, E.; Sjöblom, J.; Tammelin, T. Phase behaviour and droplet size of oil-in-water Pickering emulsions stabilised with plant-derived nanocellulosic materials. Colloids Surf. A: Physicochem. Eng. Asp. 2017, 519, 60–70. [Google Scholar] [CrossRef]
- Ougiya, H.; Watanabe, K.; Morinaga, Y.; Yoshinaga, F. Emulsion-stabilizing effect of bacterial cellulose. Biosci. Biotechnol. Biochem. 1997, 61, 1541–1545. [Google Scholar] [CrossRef]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Facile route to transparent, strong, and thermally stable nanocellulose/polymer nanocomposites from an aqueous Pickering emulsion. Biomacromolecules 2016, 18, 266–271. [Google Scholar] [CrossRef]
- Mikulcová, V.; Bordes, R.; Kašpárková, V. On the preparation and antibacterial activity of emulsions stabilized with nanocellulose particles. Food Hydrocoll. 2016, 61, 780–792. [Google Scholar] [CrossRef]
- Paximada, P.; Tsouko, E.; Kopsahelis, N.; Koutinas, A.A.; Mandala, I. Bacterial cellulose as stabilizer of o/w emulsions. Food Hydrocoll. 2016, 53, 225–232. [Google Scholar] [CrossRef]
- Ström, G.; Öhgren, C.; Ankerfors, M. Nanocellulose as an additive in foodstuff. Innventia Rep. 2013, 403, 1–25. [Google Scholar]
- Svagan, A.J.; Musyanovych, A.; Kappl, M.; Bernhardt, M.; Glasser, G.; Wohnhaas, C.; Berglund, L.A.; Risbo, J.; Landfester, K. Cellulose nanofiber/nanocrystal reinforced capsules: A fast and facile approach toward assembly of liquid-core capsules with high mechanical stability. Biomacromolecules 2014, 15, 1852–1859. [Google Scholar] [CrossRef]
- Winuprasith, T.; Suphantharika, M. Microfibrillated cellulose from mangosteen (Garcinia mangostana L.) rind: Preparation, characterization, and evaluation as an emulsion stabilizer. Food Hydrocoll. 2013, 32, 383–394. [Google Scholar] [CrossRef]
- Jiménez Saelices, C.; Capron, I. Design of Pickering micro-and nanoemulsions based on the structural characteristics of nanocelluloses. Biomacromolecules 2018, 19, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Capron, I.; Cathala, B. Surfactant-free high internal phase emulsions stabilized by cellulose nanocrystals. Biomacromolecules 2013, 14, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Cherhal, F.; Cousin, F.; Capron, I. Structural description of the interface of Pickering emulsions stabilized by cellulose nanocrystals. Biomacromolecules 2016, 17, 496–502. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromolecules 2011, 13, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Sèbe, G.; Ham-Pichavant, F.d.r.; Pecastaings, G. Dispersibility and emulsion-stabilizing effect of cellulose nanowhiskers esterified by vinyl acetate and vinyl cinnamate. Biomacromolecules 2013, 14, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Xhanari, K.; Syverud, K.; Chinga-Carrasco, G.; Paso, K.; Stenius, P. Structure of nanofibrillated cellulose layers at the o/w interface. J. Colloid Interface Sci. 2011, 356, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Xhanari, K.; Syverud, K.; Stenius, P. Emulsions stabilized by microfibrillated cellulose: The effect of hydrophobization, concentration and o/w ratio. J. Dispers. Sci. Technol. 2011, 32, 447–452. [Google Scholar] [CrossRef]
- Agoda-Tandjawa, G.; Durand, S.; Berot, S.; Blassel, C.; Gaillard, C.; Garnier, C.; Doublier, J.-L. Rheological characterization of microfibrillated cellulose suspensions after freezing. Carbohydr. Polym. 2010, 80, 677–686. [Google Scholar] [CrossRef]
- Fall, A.B.; Lindström, S.B.; Sundman, O.; Ödberg, L.; Wågberg, L. Colloidal stability of aqueous nanofibrillated cellulose dispersions. Langmuir 2011, 27, 11332–11338. [Google Scholar] [CrossRef]
- Lowys, M.-P.; Desbrieres, J.; Rinaudo, M. Rheological characterization of cellulosic microfibril suspensions. Role of polymeric additives. Food Hydrocoll. 2001, 15, 25–32. [Google Scholar] [CrossRef]
- Saarikoski, E.; Saarinen, T.; Salmela, J.; Seppälä, J. Flocculated flow of microfibrillated cellulose water suspensions: An imaging approach for characterisation of rheological behaviour. Cellulose 2012, 19, 647–659. [Google Scholar] [CrossRef]
- Naderi, A.; Lindström, T.; Sundström, J.; Flodberg, G.; Erlandsson, J. A comparative study of the properties of three nanofibrillated cellulose systems that have been produced at about the same energy consumption levels in the mechanical delamination step. Nord. Pulp. Pap. Res. J. 2016, 31, 364–371. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Chinga-Carrasco, G.; Averianova, N.; Kondalenko, O.; Garaeva, M.; Petrov, V.; Leinsvang, B.; Karlsen, T. The effect of residual fibres on the micro-topography of cellulose nanopaper. Micron 2014, 56, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/search/list?home=true: (accessed on 31 January 2019).
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Venditti, R.A.; Rojas, O.J. Pickering emulsions stabilized by cellulose nanocrystals grafted with thermo-responsive polymer brushes. J. Colloid Interface Sci. 2012, 369, 202–209. [Google Scholar] [CrossRef]
- Dong, H.; Snyder, J.F.; Williams, K.S.; Andzelm, J.W. Cation-induced hydrogels of cellulose nanofibrils with tunable moduli. Biomacromolecules 2013, 14, 3338–3345. [Google Scholar] [CrossRef]
- Jowkarderis, L.; van de Ven, T.G. Intrinsic viscosity of aqueous suspensions of cellulose nanofibrils. Cellulose 2014, 21, 2511–2517. [Google Scholar] [CrossRef]
- Saito, T.; Uematsu, T.; Kimura, S.; Enomae, T.; Isogai, A. Self-aligned integration of native cellulose nanofibrils towards producing diverse bulk materials. Soft Matter 2011, 7, 8804–8809. [Google Scholar] [CrossRef]
- Fujisawa, S.; Okita, Y.; Fukuzumi, H.; Saito, T.; Isogai, A. Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohydr. Polym. 2011, 84, 579–583. [Google Scholar] [CrossRef]
- Wågberg, L.; Decher, G.; Norgren, M.; Lindström, T.; Ankerfors, M.; Axnäs, K. The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 2008, 24, 784–795. [Google Scholar] [CrossRef] [PubMed]




| Sample | NaCl | AcOH |
|---|---|---|
| (wt %) | (wt %) | |
| CNF-E | - | - |
| CNF-E + AcOH | - | 0.2 |
| CNF-E + NaCl | 1.0 | - |
| CNF-E + NaCl + AcOH | 1.0 | 0.2 |
| CNF-T | - | - |
| CNF-T + AcOH | - | 0.2 |
| CNF-T + NaCl | 1.0 | - |
| CNF-T + NaCl + AcOH | 1.0 | 0.2 |
| CNF-E (5 Passes) | CNF-T (1 Pass) | |
|---|---|---|
| Total charge (mmol/g) | 0.044 ± 0.003 | 1.49 ± 0.02 |
| Residual fiber content (%) | 0.1 ± 0.0 | 5.5 ± 0.2 |
| CNF Sample | 0.5 wt % CNF-E | 0.5 wt % CNF-T | ||||||
|---|---|---|---|---|---|---|---|---|
| Medium | Ref | AcOH | NaCl | AcOH + NaCl | Ref | AcOH | NaCl | AcOH + NaCl |
| Average viscosity in mPa∙s | 5324 | 7277 | 2809 | 5217 | 38305 | 9122 | 1311 | 4013 |
| Cv | 5.5% | 2.2% | 2.8% | 5.6% | 0.6% | 3.8% | 35% | 21% |
| CNF Sample | 0.5 wt % CNF-E | 0.5 wt % CNF-T | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dispersion | Emulsions | Dispersion | Emulsions | |||||||
| Medium | CNF dispersion | Ref | AcOH | NaCl | AcOH + NaCl | CNF dispersion | Ref | AcOH | NaCl | AcOH + NaCl |
| pH | 7.52 | 7.31 | 3.22 | 5.55 | 3.02 | 6.91 | 6.04 | 3.95 | 6.42 | 3.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aaen, R.; Brodin, F.W.; Simon, S.; Heggset, E.B.; Syverud, K. Oil-in-Water Emulsions Stabilized by Cellulose Nanofibrils—The Effects of Ionic Strength and pH. Nanomaterials 2019, 9, 259. https://doi.org/10.3390/nano9020259
Aaen R, Brodin FW, Simon S, Heggset EB, Syverud K. Oil-in-Water Emulsions Stabilized by Cellulose Nanofibrils—The Effects of Ionic Strength and pH. Nanomaterials. 2019; 9(2):259. https://doi.org/10.3390/nano9020259
Chicago/Turabian StyleAaen, Ragnhild, Fredrik Wernersson Brodin, Sébastien Simon, Ellinor Bævre Heggset, and Kristin Syverud. 2019. "Oil-in-Water Emulsions Stabilized by Cellulose Nanofibrils—The Effects of Ionic Strength and pH" Nanomaterials 9, no. 2: 259. https://doi.org/10.3390/nano9020259





