Preparation and Characterization of Biomimetic Hydroxyapatite Nanocrystals by Using Partially Hydrolyzed Keratin as Template Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HA Nanocrystals
2.3. Characterization of HA Nanocrystals
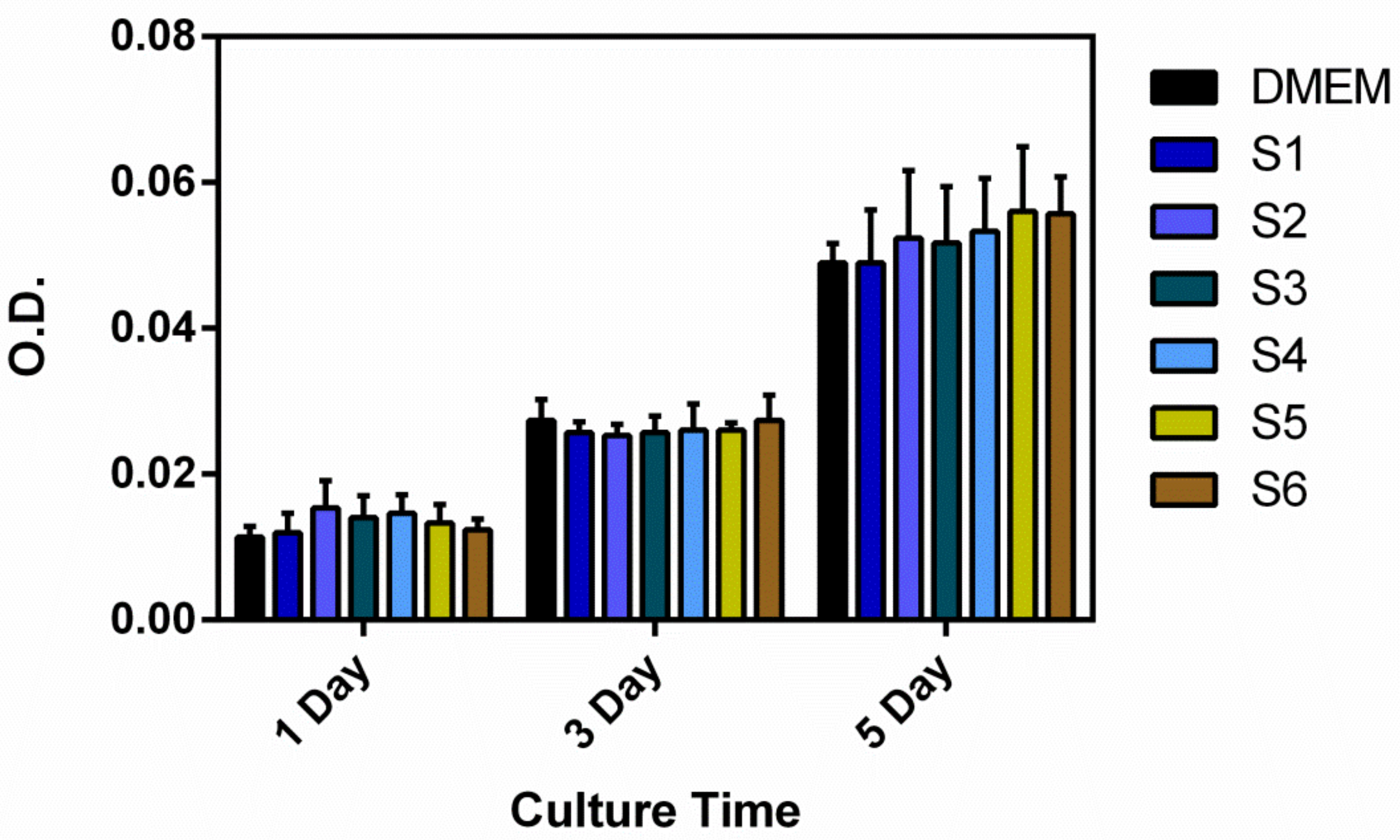
2.4. In Vitro Cytocompatibility
2.5. Statistical Analysis
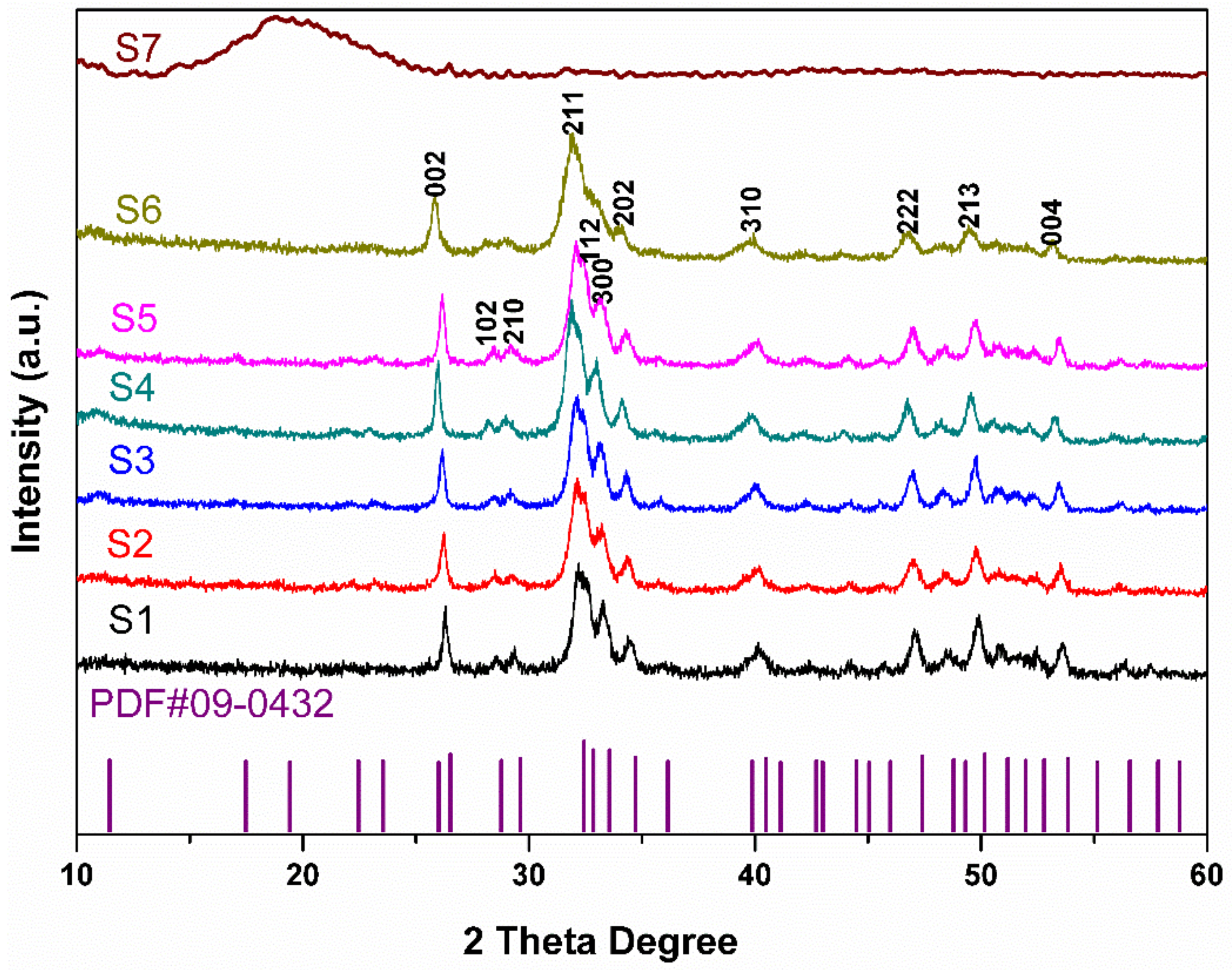
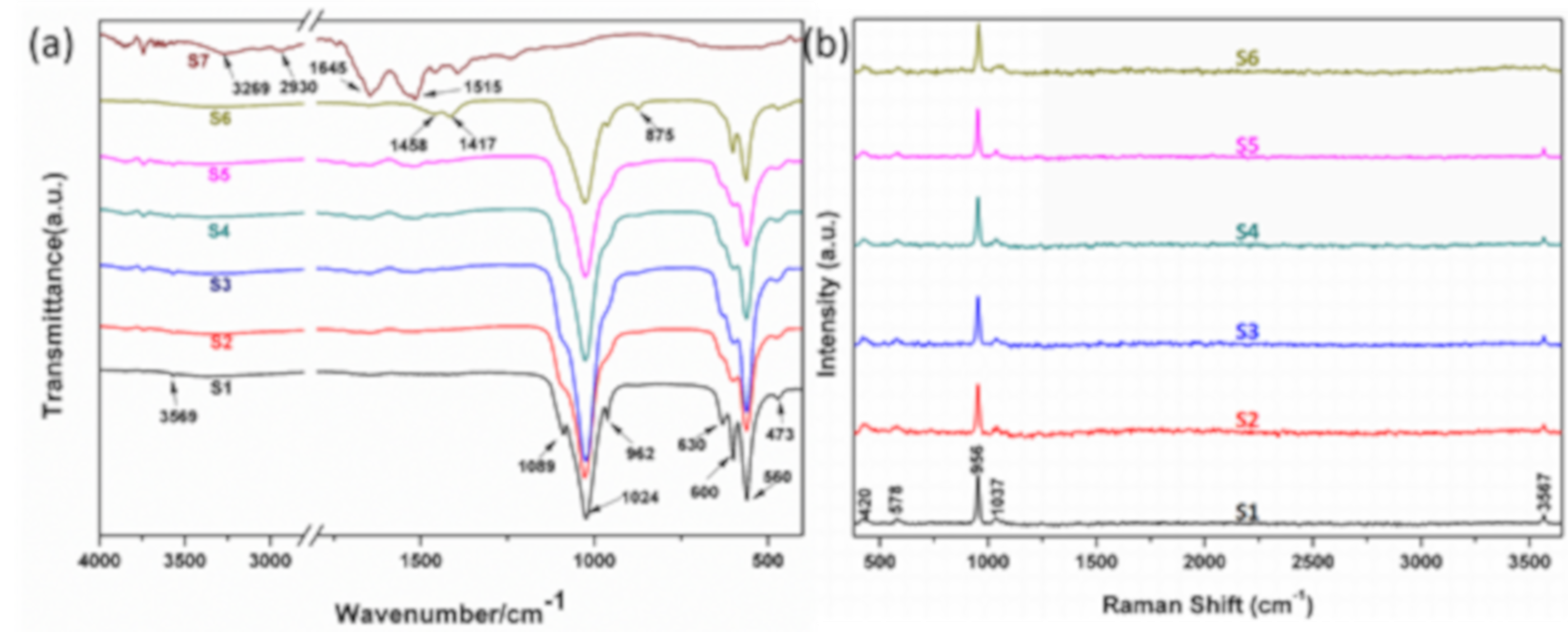
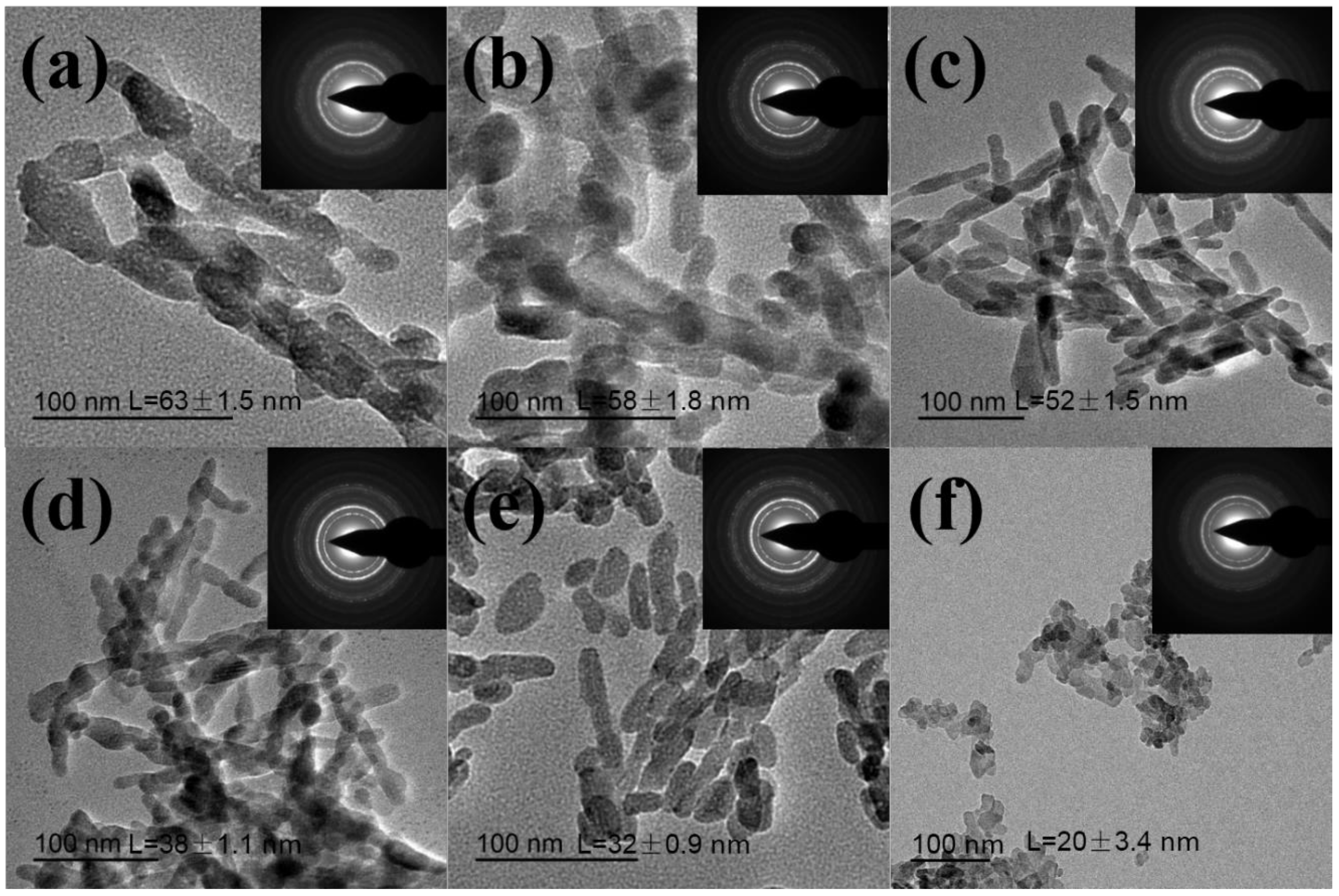
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cölfen, H. Biomineralization: A crystal-clear view. Nat. Mater. 2010, 9, 960–961. [Google Scholar] [CrossRef] [PubMed]
- Finnemore, A.; Cunha, P.; Shean, T.; Vignolini, S.; Guldin, S.; Oyen, M.; Steiner, U. Biomimetic layer-by-layer assembly of artificial nacre. Nat. Commun. 2012, 3, 966. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.; Ramachandrarao, P. Morphological changes in biomimetically synthesized hydroxyapatite and silver nanoparticles for medical applications. J. Mater. Sci. 2009, 44, 2264–2270. [Google Scholar] [CrossRef]
- Wang, Y.; Azaïs, T.; Robin, M.; Vallée, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.-M.; Nassif, N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Sun, Y.; Chen, X.; Zhu, P.; Wei, S. Biomimetic synthesis and biocompatibility evaluation of carbonated apatites template-mediated by heparin. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2905–2913. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Chen, J.; Zhao, B.; Zhu, P. Synthesis of biocompatible hydroxyapatite using chitosan oligosaccharide as a template. Materials 2015, 8, 8097–8105. [Google Scholar] [CrossRef]
- Campinho, M.A.; Silva, N.; Sweeney, G.E.; Power, D.M. Molecular, cellular and histological changes in skin from a larval to an adult phenotype during bony fish metamorphosis. Cell Tissue Res. 2007, 327, 267–284. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, A.; Kumar, A.; Kee, C.G.; Kamyab, H.; Saufi, S.M. An efficient conversion of waste feather keratin into ecofriendly bioplastic film. Clean Technol. Environ. Policy 2018, 20, 2157–2167. [Google Scholar] [CrossRef]
- Vasilevich, F.I.; Bobyleva, O.V.; Sapozhnikova, A.I.; Gordiyenko, I.M.; Gorbacheva, M.V. Products recycling waste fur production: New capabilities to use. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 1590. [Google Scholar]
- Okazaki, M.; Yoshimura, K.; Fujiwara, H.; Suzuki, Y.; Harii, K. Induction of hard keratin expression in non-nail-matrical keratinocytes by nail-matrical fibroblasts through epithelial-mesenchymal interactions. Plast. Reconstr. Surg. 2003, 111, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.; Madhan, B.; Shanmugam, G. Extraction and characterization of keratin from bovine hoof: A potential material for biomedical applications. Springerplus 2014, 3, 596. [Google Scholar] [CrossRef] [PubMed]
- Leichner, C.; Steinbring, C.; Baus, R.A.; Baecker, D.; Gust, R.; Bernkop-Schnurch, A. Reactive keratin derivatives: A promising strategy for covalent binding to hair. J. Colloid Interface Sci. 2019, 534, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Xing, Y.; Yu, W.D.; Liu, H.L. Wool keratin and silk sericin composite films reinforced by molecular network reconstruction. J. Mater. Sci. 2018, 53, 5418–5428. [Google Scholar] [CrossRef]
- Bigi, A.; Burghammer, M.; Falconi, R.; Koch, M.H.J.; Panzavolta, S.; Riekel, C. Twisted plywood pattern of collagen fibrils in teleost scales: An x-ray diffraction investigation. J. Struct. Biol. 2001, 136, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Anton, M.; Santé-Lhoutellier, V. The “sisters” α-helices of collagen, elastin and keratin recovered from animal by-products: Functionality, bioactivity and trends of application. Trends Food Sci. Technol. 2016, 51, 65–75. [Google Scholar] [CrossRef]
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Microstructure, mechanical, and biomimetic properties of fish scales from Pagrus major. J. Struct. Biol. 2003, 142, 327–333. [Google Scholar] [CrossRef]
- Mondal, B.; Mondal, S.; Mondal, A.; Mandal, N. Fish scale derived hydroxyapatite scaffold for bone tissue engineering. Mater. Charact. 2016, 121, 112–124. [Google Scholar] [CrossRef]
- Pon-On, W.; Suntornsaratoon, P.; Charoenphandhu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. Synthesis and investigations of mineral ions-loaded apatite from fish scale and PLA/chitosan composite for bone scaffolds. Mater. Lett. 2018, 221, 143–146. [Google Scholar] [CrossRef]
- Chen, S.; Ikoma, T.; Ogawa, N.; Migita, S.; Kobayashi, H.; Hanagata, N. In vitro formation and thermal transition of novel hybrid fibrils from type I fish scale collagen and type I porcine collagen. Sci. Technol. Adv. Mater. 2010, 11, 035001. [Google Scholar] [CrossRef]
- Takagi, Y.; Ura, K. Teleost fish scales: A unique biological model for the fabrication of materials for corneal stroma regeneration. J. Nanosci. Nanotechnol. 2007, 7, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.S.; Shin, T.J. Supramolecular assembly of collagen fibrils into collagen fiber in fish scales of red seabream, Pagrus major. J. Struct. Biol. 2009, 168, 332–336. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, H.; Geng, X.; Wang, J.; Xiao, J.; Zhu, P. Effect of spray distance on microstructure and tribological performance of suspension plasma-sprayed hydroxyapatite–titania composite coatings. J. Thermal Spray Technol. 2016, 25, 1255–1263. [Google Scholar] [CrossRef]
- Granito, R.N.; Muniz Renno, A.C.; Yamamura, H.; de Almeida, M.C.; Menin Ruiz, P.L.; Ribeiro, D.A. Hydroxyapatite from fish for bone tissue engineering: A promising approach. Int. J. Mol. Cell. Med. 2018, 7, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Balas, F.; Pérez-Pariente, J.; Vallet-Regí, M. In vitro bioactivity of silicon-substituted hydroxyapatites. J. Biomed. Mater. Res. Part A 2010, 66, 364–375. [Google Scholar]
- Wang, J.; Xue, C.; Zhu, P. Hydrothermal synthesis and structure characterization of flower-like self assembly of silicon-doped hydroxyapatite. Mater. Lett. 2017, 196, 400–402. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; TenHuisen, K.S. Preparation of magnesium-substituted hydroxyapatite powders by the mechanochemical–hydrothermal method. Biomaterials 2004, 25, 4647–4657. [Google Scholar] [CrossRef]
- Silva, C.C.; Graça, M.P.F.; Valente, M.A.; Góes, J.C.; Sombra, A.S.B. Microwave preparation, structure and electrical properties of calcium–sodium–phosphate biosystem. J. Non-Cryst. Solids 2006, 352, 3512–3517. [Google Scholar] [CrossRef]
- Choi, J.; Panthi, G.; Liu, Y.; Kim, J.; Chae, S.-H.; Lee, C.; Park, M.; Kim, H.-Y. Keratin/poly (vinyl alcohol) blended nanofibers with high optical transmittance. Polymer 2015, 58, 146–152. [Google Scholar] [CrossRef]
- Xue, C.; Chen, Y.; Huang, Y.; Zhu, P. Hydrothermal synthesis and biocompatibility study of highly crystalline carbonated hydroxyapatite nanorods. Nanoscale Res. Lett. 2015, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.-X.; Lv, Y.; Niu, Y.-R.; Zhao, X.-H.; Cao, D.-S.; Tang, J.; Sun, X.-C.; Chen, K.-Z. Physicochemical and biological properties of bovine-derived porous hydroxyapatite/collagen composite and its hydroxyapatite powders. Ceram. Int. 2017, 43, 16792–16798. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, U.; Pattanayak, D.K.; Vijayalakshmi, U. Snail shell derived natural hydroxyapatite: Effects on NIH-3T3 cells for orthopedic applications. Adv. Manuf. Process. 2016, 31, 206–216. [Google Scholar] [CrossRef]
- Yamini, D.; Devanand, V.G.; Kumar, J.; Ramakrishnan, V. Raman scattering studies on PEG functionalized hydroxyapatite nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Shi, J.; Peng, B.; Chen, L. The effect of alginate addition on the structure and morphology of hydroxyapatite/gelatin nanocomposites. Compos. Sci. Technol. 2006, 66, 1532–1538. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, H.; Yin, J.; Yang, B.; Zhang, Y.; Li, J.; Yao, F. Hydroxyapatite crystal formation in the presence of polysaccharide. Cryst. Growth Des. 2016, 16, 1247–1255. [Google Scholar] [CrossRef]
- Geng, Z.; Yuan, Q.; Zhuo, X.; Li, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Bao, H.; Li, X. Synthesis, characterization, and biological evaluation of nanostructured hydroxyapatite with different dimensions. Nanomaterials 2017, 7, 38. [Google Scholar] [CrossRef]
- Okada, M.; Furuzono, T. Hydroxylapatite nanoparticles: Fabrication methods and medical applications. Sci. Technol. Adv. Mater. 2012, 13, 5233–5240. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. Natural enamel wear—A physiological source of hydroxylapatite nanoparticles for biofilm management and tooth repair? Med. Hypotheses 2010, 74, 670–672. [Google Scholar] [CrossRef]
- Chaudhry, A.A.; Haque, S.; Kellici, S.; Boldrin, P.; Rehman, I.; Khalid, F.A.; Darr, J.A. Instant nano-hydroxyapatite: A continuous and rapid hydrothermal synthesis. Chem. Commun. 2006, 21, 2286–2288. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Pallela, R.; Bhatnagar, I.; Kim, S.K. Chitosan-amylopectin/hydroxyapatite and chitosan-chondroitin sulphate/hydroxyapatite composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2012, 51, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.; Bernhardt, A.; Heinemann, S.; Stachel, I.; Meyer, M.; Gelinsky, M. Biomimetically mineralized salmon collagen scaffolds for application in bone tissue engineering. Biomacromolecules 2012, 13, 1059–1066. [Google Scholar] [CrossRef] [PubMed]

| Sample | Ca(NO3)2·4H2O | (NH4)2HPO4 | Partially Hydrolyzed Keratin |
|---|---|---|---|
| S1 | 9.54 g | 3.20 g | — |
| S2 | 9.54 g | 3.20 g | 0.02 g |
| S3 | 9.54 g | 3.20 g | 0.1 g |
| S4 | 9.54 g | 3.20 g | 0.2 g |
| S5 | 9.54 g | 3.20 g | 0.6 g |
| S6 | — | — | — |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Zhao, K.; Lin, L.; Wang, J.; Liu, Y.; Zhu, P. Preparation and Characterization of Biomimetic Hydroxyapatite Nanocrystals by Using Partially Hydrolyzed Keratin as Template Agent. Nanomaterials 2019, 9, 241. https://doi.org/10.3390/nano9020241
Gao C, Zhao K, Lin L, Wang J, Liu Y, Zhu P. Preparation and Characterization of Biomimetic Hydroxyapatite Nanocrystals by Using Partially Hydrolyzed Keratin as Template Agent. Nanomaterials. 2019; 9(2):241. https://doi.org/10.3390/nano9020241
Chicago/Turabian StyleGao, Chunxia, Ke Zhao, Liwei Lin, Jinyu Wang, Yang Liu, and Peizhi Zhu. 2019. "Preparation and Characterization of Biomimetic Hydroxyapatite Nanocrystals by Using Partially Hydrolyzed Keratin as Template Agent" Nanomaterials 9, no. 2: 241. https://doi.org/10.3390/nano9020241




