First-Principles Study of Thermo-Physical Properties of Pu-Containing Gd2Zr2O7
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
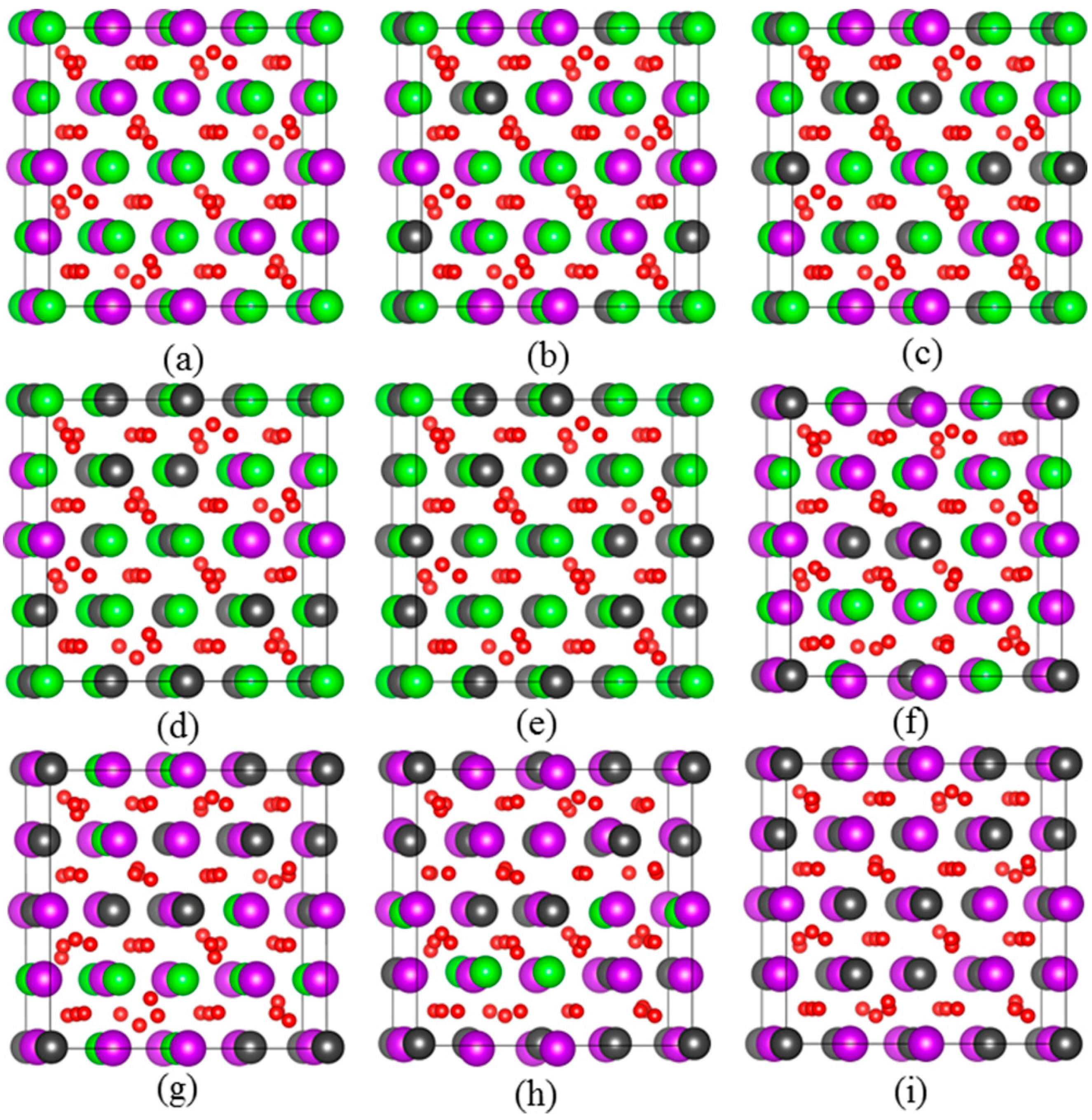
3.1. Structural Stability of Pu Incorporation into Gd2Zr2O7
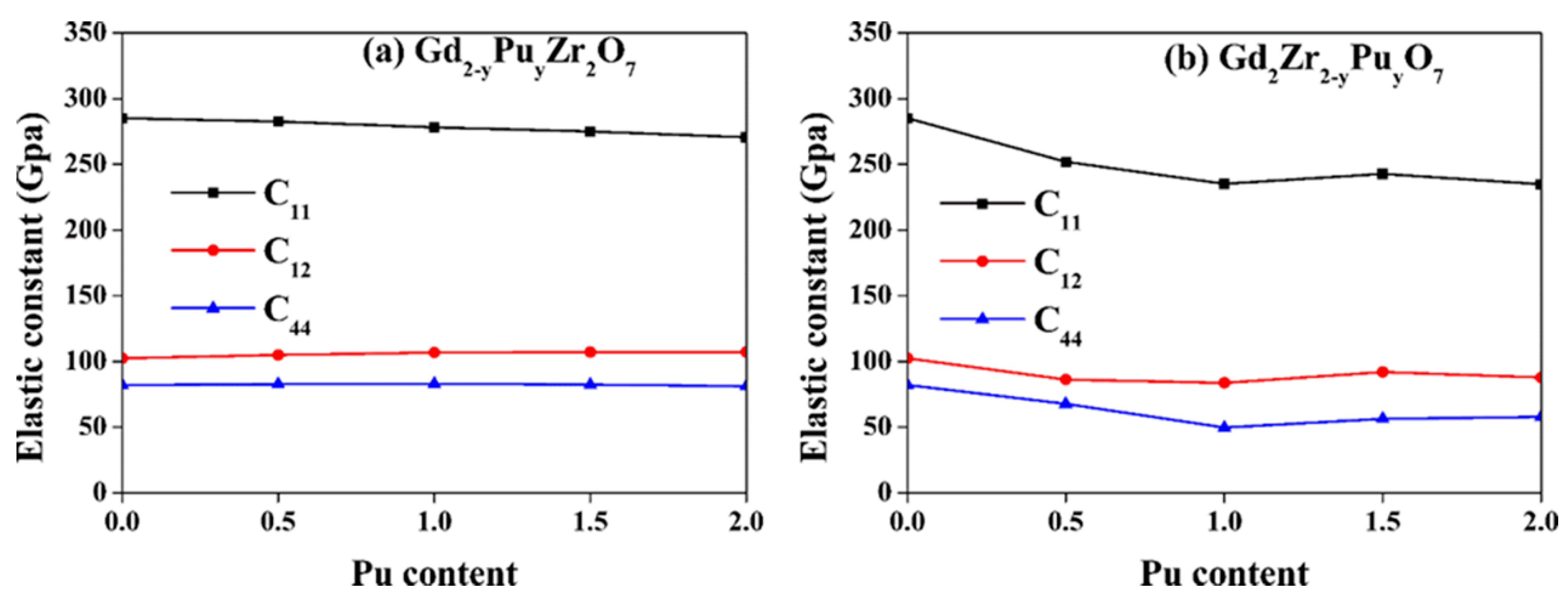
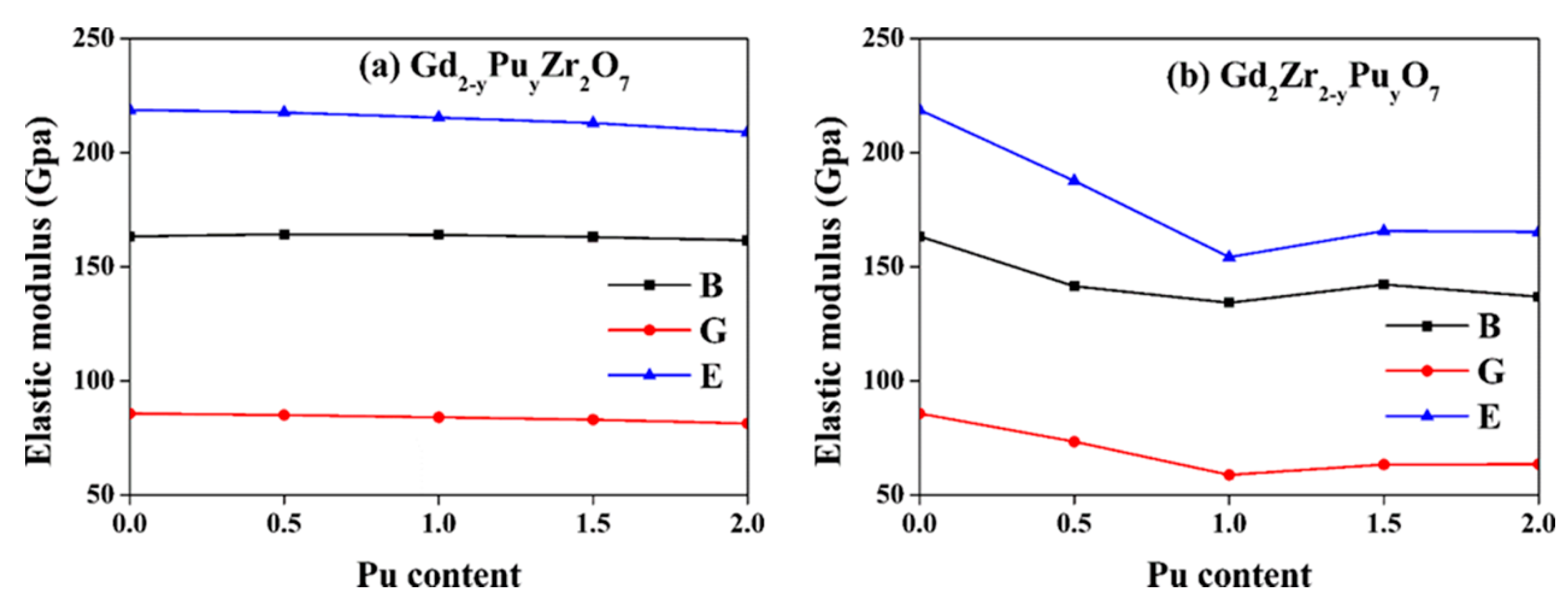
3.2. Elastic Constants and Elastic Moduli of Gd2−yPuyZr2O7 and Gd2Zr2−yPuyO7
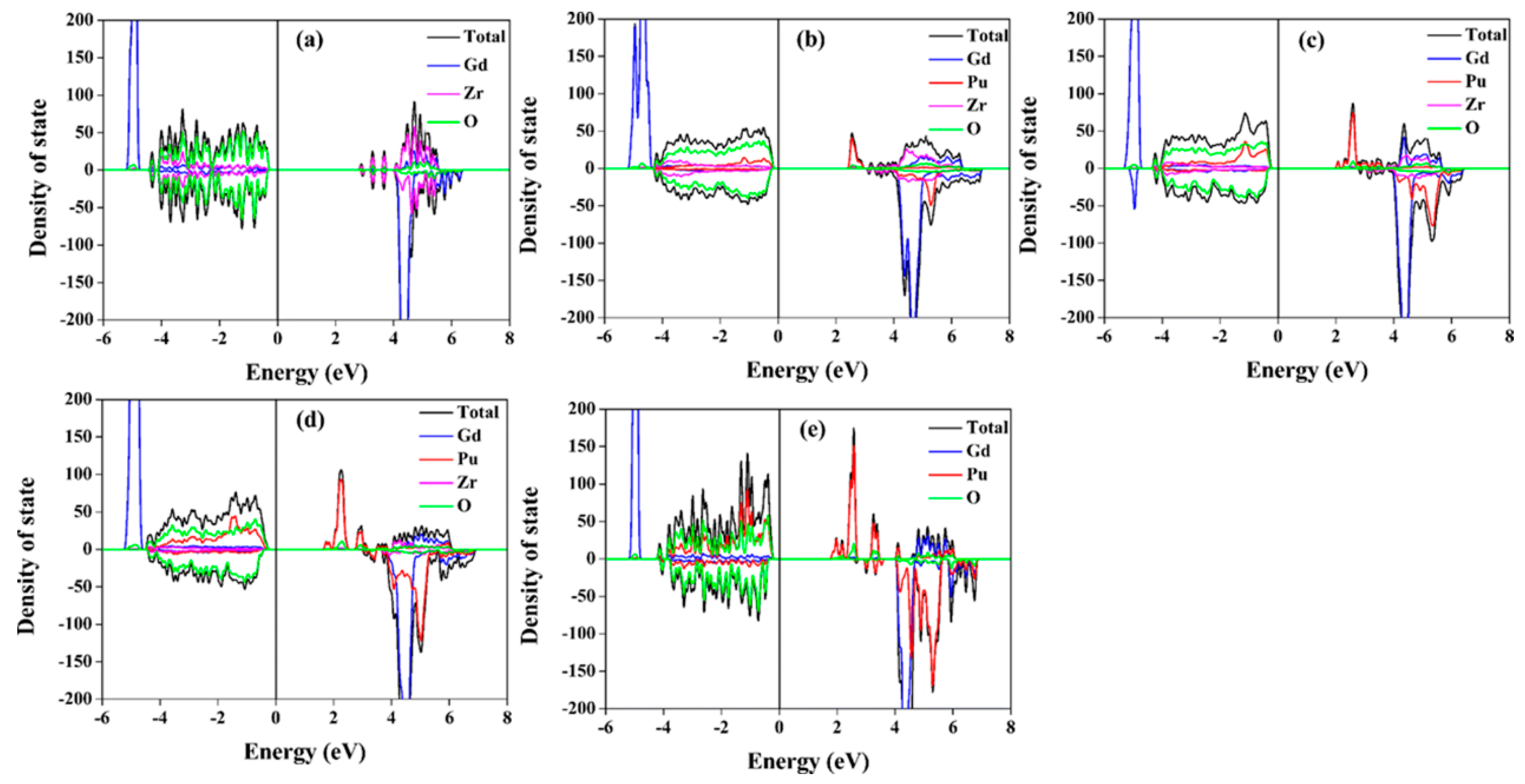
3.3. Ductility, Elastic Anisotropy, Debye Temperature and Electronic Structures of Pu-Doped Gd2Zr2O7
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ewing, R.C. Nuclear waste forms for actinides. Proc. Natl. Acad. Sci. USA 1999, 96, 3432–3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.Y.; Jiang, M.; Zhao, F.A.; Liu, Z.J.; Zu, X.T. Thermal and mechanical stability, electronic structure and energetic properties of Pu-containing pyrochlores: La2−yPuyZr2O7 and La2Zr2−yPuyO7 (0 ≤ y ≤ 2). J. Nucl. Mater. 2015, 466, 162–171. [Google Scholar] [CrossRef]
- Ewing, R.C.; Weber, W.J.; Lian, J. Nuclear waste disposal—Pyrochlore (A2B2O7): Nuclear waste form for the immobilization of plutonium and “minor” actinides. J. Appl. Phys. 2004, 95, 5949–5971. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 6th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1986; pp. 106–107. [Google Scholar]
- Chroneos, A.; Rushton, M.J.D.; Jiang, C.; Tsoukalas, L.H. Nuclear wasteform materials: Atomistic simulation case studies. J. Nucl. Mater. 2013, 441, 29–39. [Google Scholar] [CrossRef]
- Wang, S.X.; Begg, B.D.; Wang, L.M.; Ewing, R.C.; Weber, W.J.; Kutty, K.V.G. Radiation stability of gadolinium zirconate: A waste form for plutonium disposition. J. Mater. Res. 1999, 14, 4470–4473. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Minervini, L.; Grimes, R.W.; Valdez, J.A.; Ishimaru, M.; Li, F.; McClellan, K.J.; Hartmann, T. Radiation tolerance of complex oxides. Science 2000, 289, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J. Plutonium immobilization and radiation effects. Science 2000, 289, 2051–2052. [Google Scholar] [CrossRef]
- Devanathan, R.; Weber, W.J.; Gale, J.D. Radiation tolerance of ceramics—Insights from atomistic simulation of damage accumulation in pyrochlores. Energy Environ. Sci. 2010, 3, 1551–1559. [Google Scholar] [CrossRef]
- Patwe, S.J.; Tyagi, A.K. Solubility of Ce4+ and Sr2+ in the pyrochlore lattice of Gd2Zr2O7 for simulation of Pu and alkaline earth metal. Ceram. Int. 2006, 32, 545–548. [Google Scholar] [CrossRef]
- Mandal, B.P.; Tyagi, A.K. Pyrochlores: Potential multifunctional materials. BARC Newslett. 2010, 313, 6–13. [Google Scholar]
- Zhao, P.Z.; Li, L.Y.; Xu, S.M.; Zhang, Q. Synthesis and structural transformations of (Gd1−xCex)2Zr2O7+x: An analogue for Pu immobilization. Acta Phys. Chim. Sin. 2013, 29, 1168–1172. [Google Scholar]
- Reid, D.P.; Stennett, M.C.; Hyatt, N.C. The fluorite related modulated structures of the Gd2(Zr2−xCex)O7 solid solution: An analogue for Pu disposition. J. Solid State Chem. 2012, 191, 2–9. [Google Scholar] [CrossRef]
- Patwe, S.J.; Ambekar, B.R.; Tyagi, A.K. Synthesis, characterization and lattice thermal expansion of some compounds in the system Gd2CexZr2−xO7. J. Alloys Compd. 2005, 389, 243–246. [Google Scholar] [CrossRef]
- Zhao, F.A.; Xiao, H.Y.; Jiang, M.; Liu, Z.J.; Zu, X.T. A DFT+U study of Pu immobilization in Gd2Zr2O7. J. Nucl. Mater. 2015, 467, 937–948. [Google Scholar] [CrossRef]
- Williford, R.E. Computer simulation of Pu3+ and Pu4+ substitutions in gadolinium zirconate. J. Nucl. Mater. 2001, 299, 140–147. [Google Scholar] [CrossRef]
- Cleave, A.; Grimes, R.W.; Sickafus, K. Plutonium and uranium accommodation in pyrochlore oxides. Philos. Mag. 2005, 85, 967–980. [Google Scholar] [CrossRef]
- Wang, X.J.; Xiao, H.Y.; Zu, X.T.; Weber, W.J. Study of cerium solubility in Gd2Zr2O7 by DFT + U calculations. J. Nucl. Mater. 2011, 419, 105–111. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Xiao, P. Role and determining factor of substitutional defects on thermal conductivity: A study of La2(Zr1−xBx)2O7 (B = Hf, Ce, 0 ≤ x ≤ 0.5) pyrochlore solid solutions. Acta Mater. 2014, 68, 106–115. [Google Scholar] [CrossRef]
- Shimamura, K.; Arima, T.; Idemitsu, K.; Inagaki, Y. Thermophysical properties of rare-earth-stabilized zirconia and zirconate pyrochlores as surrogates for actinide-doped zirconia. Int. J. Thermophys. 2007, 28, 1074–1084. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmiiller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Cohen, R.E. More accurate generalized gradient approximation for solids. Phys. Rev. B 2006, 73, 235116. [Google Scholar] [CrossRef]
- Constantin, L.A.; Terentjevs, A.; Della Sala, F.; Fabiano, E. Gradient-dependent upper bound for the exchange-correlation energy and application to density functional theory. Phys. Rev. B 2015, 91, 041120(R). [Google Scholar] [CrossRef]
- Constantin, L.A.; Terentjevs, A.; Della Sala, F.; Cortona, P.; Fabiano, E. Semiclassical atom theory applied to solid-state physics. Phys. Rev. B 2016, 93, 045126. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Wei, S.H.; Ferreira, L.G.; Bernard, J.E.; Zunger, A. Electronic properties of random alloys: Special quasirandom structures. Phys. Rev. B 1990, 42, 9622–9649. [Google Scholar] [CrossRef]
- Zunger, A.; Wei, S.; Ferreira, L.G.; Bernard, J.E. Special quasirandom structures. Phys. Rev. Lett. 1990, 65, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Wolverton, C.; Sofo, J.; Chen, L.Q.; Liu, Z.K. First-principles study of binary bcc alloys using special quasirandom structures. Phys. Rev. B 2004, 69, 214202. [Google Scholar] [CrossRef]
- Jiang, C.; Stanek, C.R.; Sickafus, K.E.; Uberuaga, B.P. First-principles prediction of disordering tendencies in pyrochlore oxides. Phys. Rev. B 2009, 79, 104203. [Google Scholar] [CrossRef]
- McCleskey, T.M.; Bauer, E.; Jia, Q.; Burrell, A.K.; Scott, B.L.; Conradson, S.D.; Mueller, A.; Roy, L.; Wen, X.; Scuseria, G.E.; et al. Optical band gap of NpO2 and PuO2 from optical absorbance of epitaxial films. J. Appl. Phys. 2013, 113. [Google Scholar] [CrossRef]
- Chikalla, T.D.; McNeilly, C.E.; Skavdahl, R.E. The plutonium-oxygen system. J. Nucl. Mater. 1964, 12, 131–141. [Google Scholar] [CrossRef]
- Jomard, G.; Amadon, B.; Bottin, F.; Torrent, M. Structural, thermodynamic, and electronic properties of plutonium oxides from first principles. Phys. Rev. B 2008, 78, 075125. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, P.; Zhao, X.-G. First-principles local density approximation plus U and generalized gradient approximation plus U study of plutonium oxides. J. Chem. Phys. 2008, 128, 084705. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-D.; Martin, R.L.; Henderson, T.M.; Scuseria, G.E. Density Functional Theory Studies of the Electronic Structure of Solid State Actinide Oxides. Chem. Rev. 2013, 113, 1063–1096. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.P.; Banerji, A.; Sathe, V.; Deb, S.K.; Tyagi, A.K. Order–disorder transition in Nd2−yGdyZr2O7 pyrochlore solid solution: An X-ray diffraction and Raman spectroscopic study. J. Solid State Chem. 2007, 180, 2643–2648. [Google Scholar] [CrossRef]
- Yamazaki, S.; Yamashita, T.; Matsui, T.; Nagasaki, T. Thermal expansion and solubility limits of plutonium-doped lanthanum zirconates. J. Nucl. Mater. 2001, 294, 183–187. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Cystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Lian, J.; Zu, X.T.; Kutty, K.V.G.; Chen, J.; Wang, L.M.; Ewing, R.C. Ion-irradiation-induced amorphization of La2Zr2O7 pyrochlore. Phys. Rev. B 2002, 66, 054108. [Google Scholar] [CrossRef]
- Ravindran, P.; Fast, L.; Korzhavyi, P.A.; Johansson, B.; Wills, J.; Eriksson, O. Density functional theory for calculation of elastic properties of orthorhombic crystals: Application to TiSi2. J. Appl. Phys. 1998, 84, 4891–4904. [Google Scholar] [CrossRef]
- Lan, G.; Ouyang, B.; Song, J. The role of low-lying optical phonons in lattice thermal conductance of rare-earth pyrochlores: A first-principle study. Acta Mater. 2015, 91, 304–317. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wang, B.T.; Zhao, X. Ground-state properties and high-pressure behavior of plutonium dioxide: Density functional theory calculations. Phys. Rev. B 2010, 82, 144110. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, M.P.; de Vries, K.J.; Burggraaf, A.J. Oxygen ion and mixed conductivity in compounds with the fluorite and pyrochlore structure. Solid State Ion. 1983, 9, 913–919. [Google Scholar] [CrossRef]
- Wu, J.; Wei, X.; Padtur, N.P.; Klemens, P.G.; Gell, M.; Garcia, E.; Miranzo, P.; Osendi, M.I. Low-thermal-conductivity rare-earth zirconates for potential thermal-barrier-coating applications. J. Am. Ceram. Soc. 2002, 85, 3031–3035. [Google Scholar] [CrossRef]
- Zhao, F.A.; Xiao, H.Y.; Bai, X.M.; Liu, Z.J.; Zu, X.T. Effects of Nd doping on the mechanical properties and electronic structures of Gd2Zr2O7: A first-principles-based study. J. Mater. Sci. 2018, 53, 16423–16438. [Google Scholar] [CrossRef]
- Chung, D.H. Elastic moduli of single crystal and polycrystalline MgO. Philos. Mag. 1963, 8, 833–841. [Google Scholar] [CrossRef]
- Voigt, W. Ueber die Beziehung zwischen den beiden Elasticitätsconstanten isotroper Körper. Annalen der Physik 1889, 274, 573–587. [Google Scholar] [CrossRef]
- Reuss, A. Account of the liquid limit of mixed crystals on the basis of the plasticity condition for single crystal. Z. Angew. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.Y.; Li, F.Z.; Zhou, Y.C. Theoretical elastic stiffness, structural stability and thermal conductivity of La2T2O7 (T = Ge, Ti, Sn, Zr, Hf) pyrochlore. Acta Mater. 2010, 58, 4369–4377. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.B.; Zhao, F.A.; Jiang, M.; Xiao, H.Y.; Liu, Z.J.; Zu, X.T. Impact of isovalent and aliovalent substitution on the mechanical and thermal properties of Gd2Zr2O7. Sci. Rep. 2017, 7, 6399. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 2009, 45, 823–843. [Google Scholar] [CrossRef]
- Fine, M.E.; Brown, L.D.; Marcus, H.L. Elastic constants versus melting temperature in metals. Scr. Metall. 1984, 18, 951–956. [Google Scholar] [CrossRef]
- Ledbetter, H.; Migliori, A. A general elastic-anisotropy measure. J. Appl. Phys. 2006, 100, 063516. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal elastic anisotropy index. Phys. Rev. Lett. 2008, 101, 055504. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xiao, B.; Wan, C.L.; Qu, Z.X.; Huang, Z.C.; Chen, J.C.; Zhou, R.; Pan, W. Electronic structure, mechanical properties and thermal conductivity of Ln2Zr2O7 (Ln = La, Pr, Nd, Sm, Eu and Gd) pyrochlore. Acta Mater. 2011, 59, 1742–1760. [Google Scholar] [CrossRef]
- Zhao, F.A.; Xiao, H.Y.; Liu, Z.J.; Li, S.; Zu, X.T. A DFT study of mechanical properties, thermal conductivity and electronic structures of Th-doped Gd2Zr2O7. Acta Mater. 2016, 121, 299–309. [Google Scholar] [CrossRef]



| a0 | xO48f | Eg (eV) | d<Gd-O48f> | d<Gd-O8b> | d<Pu-O48f> | d<Pu-O8b> | d<Zr-O48f> | |
|---|---|---|---|---|---|---|---|---|
| y = 0 | 10.666 | 0.339 | 2.86 | 2.553 | 2.309 | - | - | 2.109 |
| Exp. | 10.540 [38] | 0.345 [41] | 2.483 [38] | |||||
| Cal. | 10.66 [18] | 0.339 [18] | 2.548 [18] | 2.307 [18] | 2.110 [18] | |||
| y = 0.5 | 10.703 | 0.337 | 2.33 | 2.561 | 2.302 | 2.582 | 2.369 | 2.111 |
| y = 1.0 | 10.736 | 0.337 | 2.27 | 2.578 | 2.284 | 2.591 | 2.366 | 2.114 |
| y = 1.5 | 10.768 | 0.336 | 2.33 | 2.574 | 2.286 | 2.605 | 2.349 | 2.116 |
| y = 2.0 | 10.802 | 0.335 | 2.37 | - | - | 2.615 | 2.339 | 2.117 |
| Exp. | 10.70 [39] |
| a0 | xO48f | Eg (eV) | d<Gd-O48f> | d<Gd-O8b> | d<Pu-O48f> | d<Zr-O48f> | |
|---|---|---|---|---|---|---|---|
| y = 0 | 10.666 | 0.339 | 2.86 | 2.553 | 2.309 | - | 2.109 |
| Exp. | 10.540 [38] | 0.345 [41] | 2.483 [38] | ||||
| Cal. | 10.66 [18] | 0.339 [18] | 2.548 [18] | 2.307 [18] | 2.110 [18] | ||
| y = 0.5 | 10.750 | 0.342 | 2.33 | 2.513 | 2.338 | 2.296 | 2.110 |
| y = 1.0 | 10.836 | 0.344 | 1.99 | 2.555 | 2.347 | 2.228 | 2.114 |
| y = 1.5 | 10.909 | 0.350 | 1.68 | 2.508 | 2.364 | 2.273 | 2.121 |
| y = 2.0 | 11.003 | 0.350 | 1.75 | 2.552 | 2.382 | 2.234 | - |
| C11 | C12 | C44 | B | G | E | ||
|---|---|---|---|---|---|---|---|
| Gd2Zr2O7 | 285.1 | 102.5 | 82.1 | 163.4 | 85.7 | 218.8 | |
| Cal [43] | 289 | 103 | 85 | 165 | 88 | 224 | |
| Exp [45,46] | 153 | 80 | 205 | ||||
| Exp [20] | 174 | 92 | 236 | ||||
| Gd1.5Pu0.5Zr2O7 | 282.6 | 105.1 | 82.7 | 164.3 | 85.1 | 217.6 | |
| Gd1.0Pu1.0Zr2O7 | 278.1 | 106.9 | 83.1 | 164.0 | 84.1 | 215.4 | |
| Gd0.5Pu1.5Zr2O7 | 274.9 | 107.2 | 82.5 | 163.1 | 83.0 | 213.0 | |
| Pu2Zr2O7 | 270.6 | 107.3 | 81.2 | 161.7 | 81.4 | 209.1 | |
| Cal [2] | 306 | 131.8 | 90.2 | ||||
| Gd2Zr1.5Pu0.5O7 | 251.9 | 86.3 | 67.7 | 141.5 | 73.4 | 187.7 | |
| Gd2Zr1.0Pu1.0O7 | 235.2 | 83.8 | 49.8 | 134.3 | 58.9 | 154.2 | |
| Gd2Zr0.5Pu1.5O7 | 242.8 | 92.0 | 56.5 | 142.3 | 63.4 | 165.7 | |
| Gd2Pu2O7 | 234.8 | 87.9 | 57.8 | 136.9 | 63.6 | 165.3 |
| Gd | Pu | Zr | O48f | O8b | |
|---|---|---|---|---|---|
| Gd2Zr2O7 | 2.16 | - | 2.26 | −1.25 | −1.37 |
| Gd1.5Pu0.5Zr2O7 | 2.15 | 2.10 | 2.27 | −1.25 | −1.35 |
| Gd1.0Pu1.0Zr2O7 | 2.13 | 2.11 | 2.27 | −1.24 | −1.35 |
| Gd0.5Pu1.5Zr2O7 | 2.15 | 2.09 | 2.28 | −1.24 | −1.33 |
| Pu2Zr2O7 | - | 2.08 | 2.27 | −1.23 | −1.32 |
| Gd2Zr1.5Pu0.5O7 | 2.14 | 2.36 | 2.26 | −1.25 | −1.37 |
| Gd2Zr1.0Pu1.0O7 | 2.15 | 2.31 | 2.27 | −1.25 | −1.37 |
| Gd2Zr0.5Pu1.5O7 | 2.15 | 2.34 | 2.24 | −1.25 | −1.36 |
| Gd2Pu2O7 | 2.16 | 2.30 | - | −1.26 | −1.38 |
| Gd2Zr2O7 | 1.907 | 0.01355 | 4666.0 | 580.2 | 0.277 | |
| Exp. [45,46] | 1.913 | 0.276 | ||||
| Exp. [20] | 1.891 | 513.3 | 0.274 | |||
| Cal. [53] | 2.004 | 0.00420 | 4833.5 | 612.9 | 0.286 | |
| Cal. [43] | 0.273 | |||||
| Gd1.5Pu0.5Zr2O7 | 1.931 | 0.00598 | 4533.7 | 560.7 | 0.279 | |
| Gd1.0Pu1.0Zr2O7 | 1.950 | 0.00105 | 4367.8 | 540.2 | 0.281 | |
| Gd0.5Pu1.5Zr2O7 | 1.964 | 0.00032 | 4247.4 | 522.5 | 0.282 | |
| Pu2Zr2O7 | 1.987 | 0.00004 | 4106.4 | 503.8 | 0.285 | |
| Gd2Zr1.5Pu0.5O7 | 1.928 | 0.04881 | 4124.6 | 508.7 | 0.279 | |
| Gd2Zr1.0Pu1.0O7 | 2.278 | 0.21353 | 3591.7 | 439.5 | 0.309 | |
| Gd2Zr0.5Pu1.5O7 | 2.243 | 0.10062 | 3590.2 | 435.9 | 0.306 | |
| Gd2Pu2O7 | 2.151 | 0.06923 | 3471.3 | 418.3 | 0.299 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhao, F.; Xiao, H.; Zhang, H.; Gong, H.; Zhang, S.; Liu, Z.; Zu, X. First-Principles Study of Thermo-Physical Properties of Pu-Containing Gd2Zr2O7. Nanomaterials 2019, 9, 196. https://doi.org/10.3390/nano9020196
Li P, Zhao F, Xiao H, Zhang H, Gong H, Zhang S, Liu Z, Zu X. First-Principles Study of Thermo-Physical Properties of Pu-Containing Gd2Zr2O7. Nanomaterials. 2019; 9(2):196. https://doi.org/10.3390/nano9020196
Chicago/Turabian StyleLi, Pengcheng, Fengai Zhao, Haiyan Xiao, Haibin Zhang, Hengfeng Gong, Sa Zhang, Zijiang Liu, and Xiaotao Zu. 2019. "First-Principles Study of Thermo-Physical Properties of Pu-Containing Gd2Zr2O7" Nanomaterials 9, no. 2: 196. https://doi.org/10.3390/nano9020196





