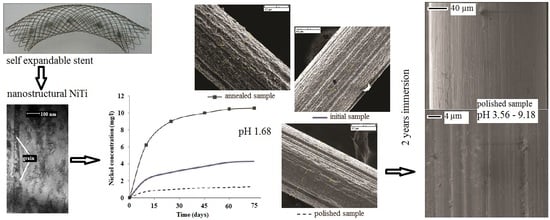
Ion Release and Surface Characterization of Nanostructured Nitinol during Long-Term Testing
Abstract
:1. Introduction
2. Materials and Methods
3. Results
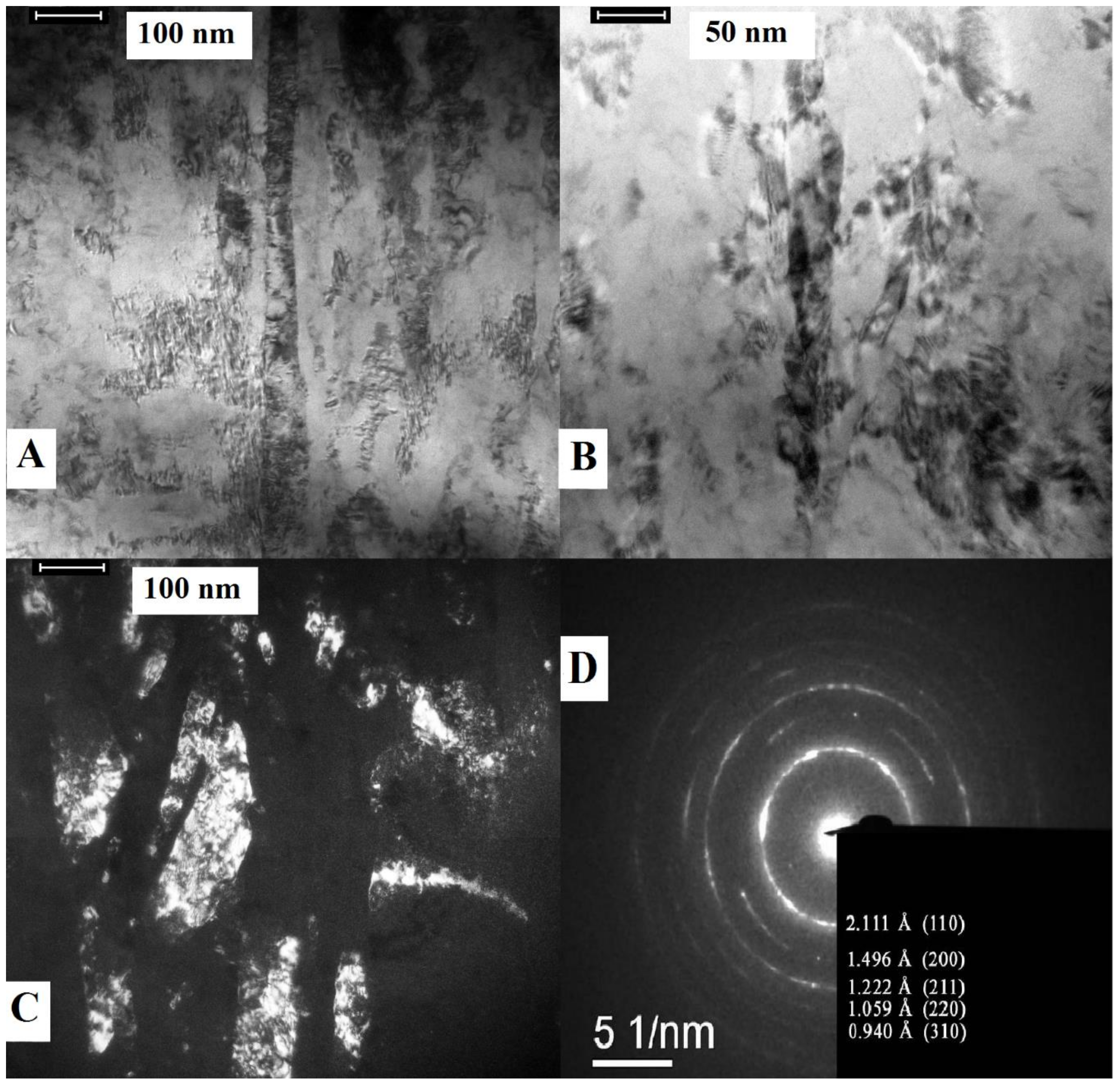
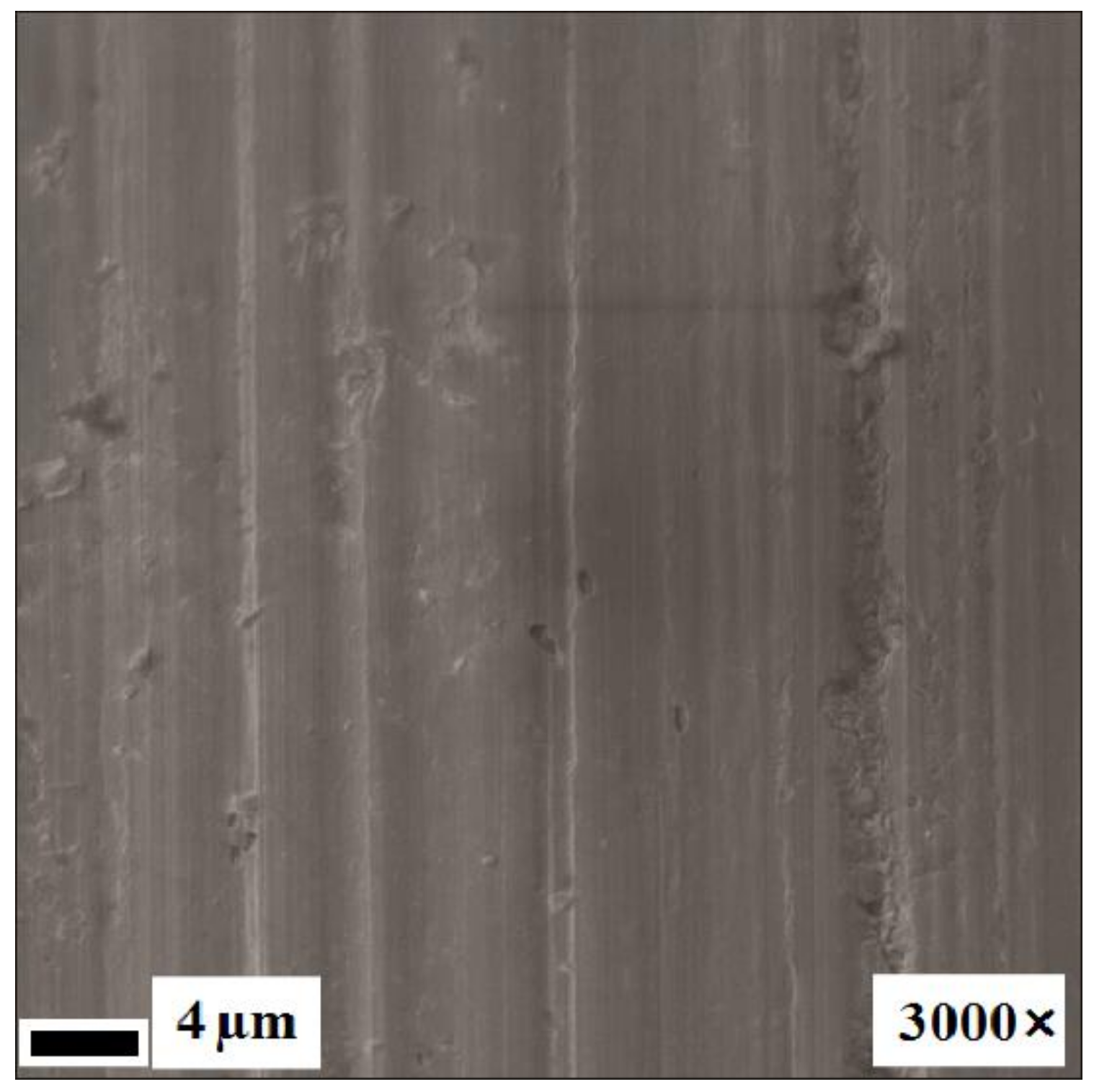
3.1. Structure Analysis Before Corrosion
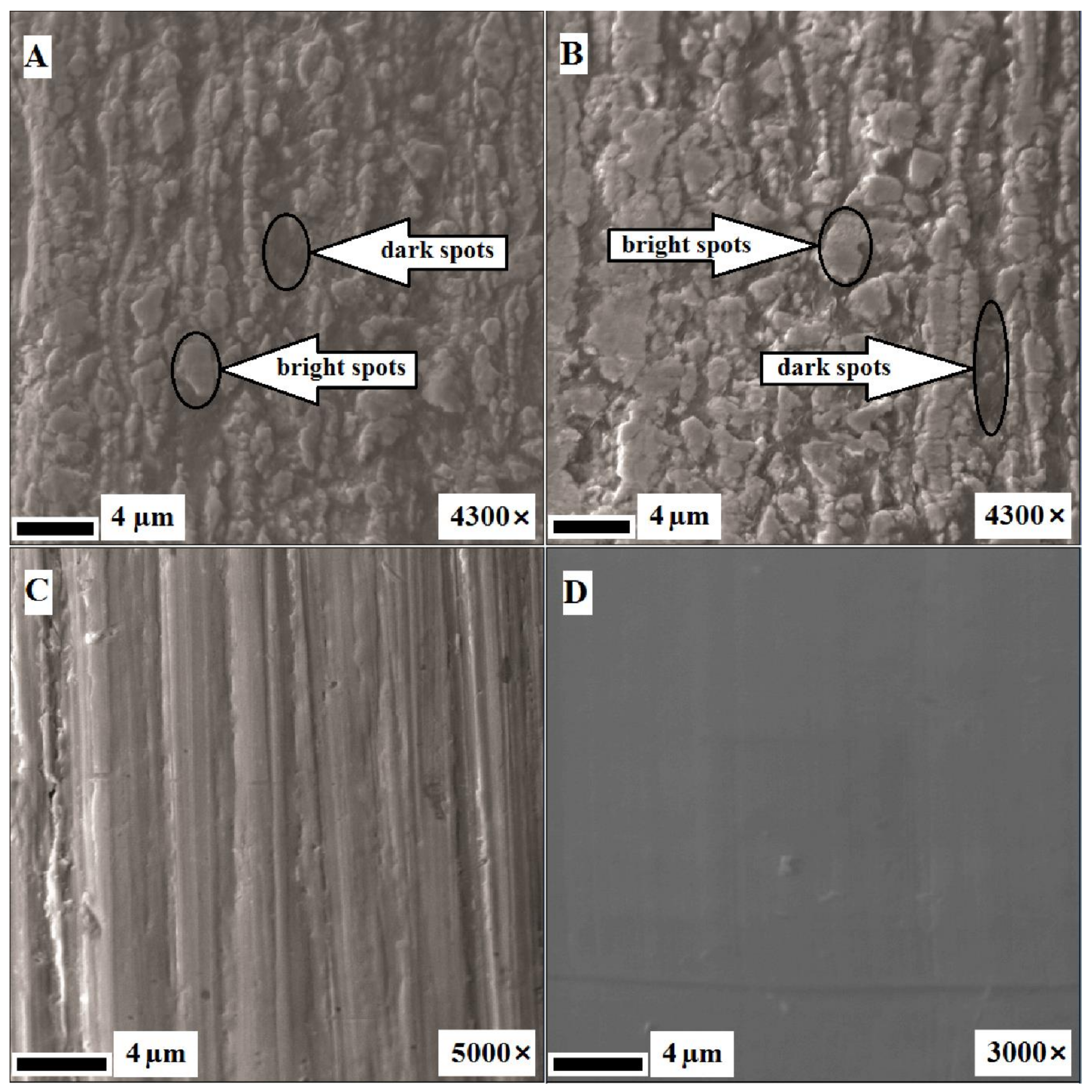
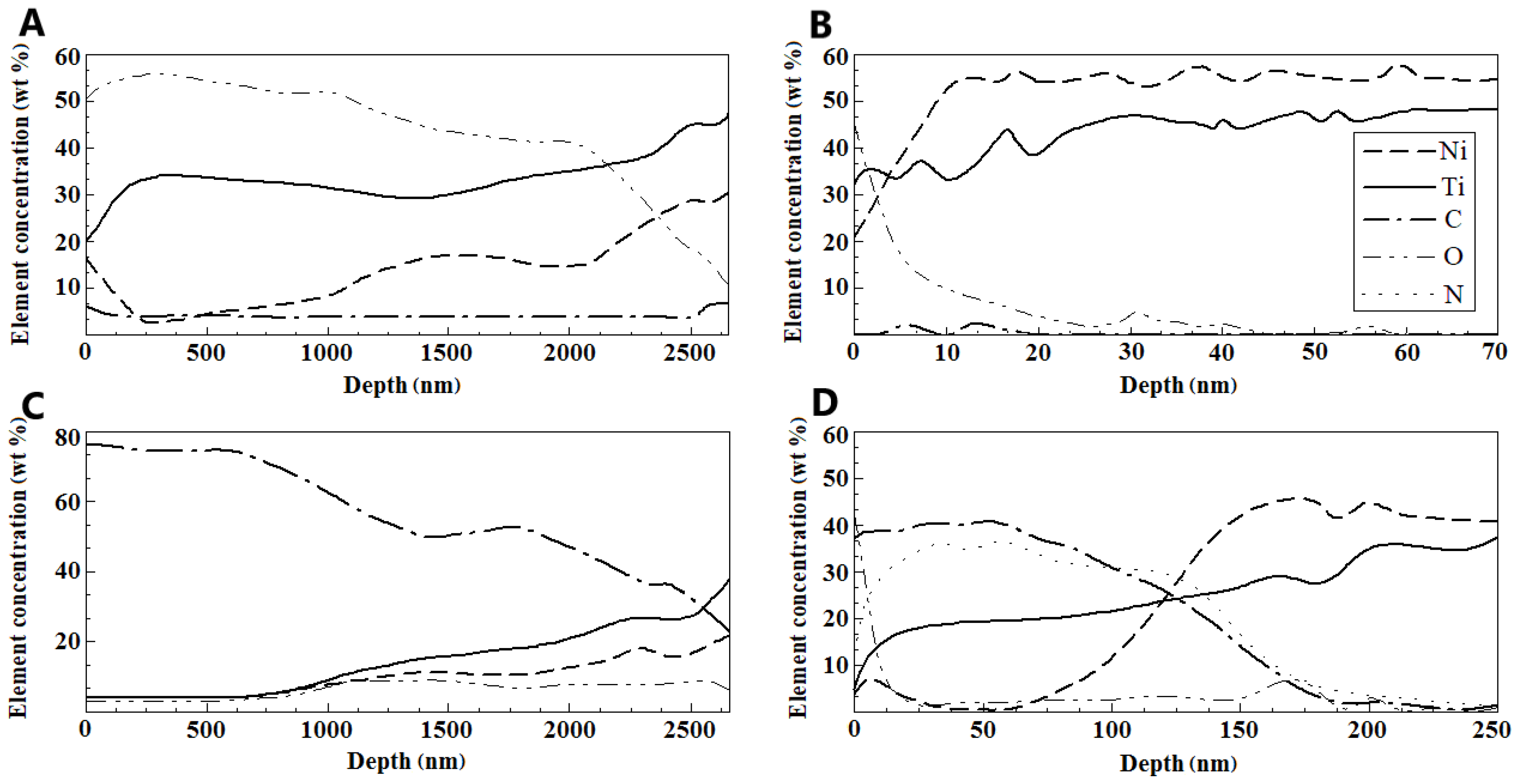
3.2. Surface Analysis After Corrosion
3.3. Metallic Ion Release in Solutions
4. Discussion
4.1. Structure Analysis Before and After Corrosion
4.2. Metallic Ion Release in Solutions
4.3. Effect of Nanostructure and Treatment on Corrosion Behavior
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gunther, V.E.; Hodorenko, V.N.; Yasenchuk, Y.F.; Chekalkin, T.L. Nikelid Titana, Meditsynsky Material Novogo Pokoleniya [Titanium Nickelide, Medical Material of New Generation]; MITs Publishing House: Tomsk, Russia, 2006. [Google Scholar]
- Petrini, L.; Migliavacca, F. Biomedical applications of shape memory alloys. J. Metall. 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Pelton, A.R.; Huang, G.H.; Moine, P.; Sinclair, R. Effects of thermal cycling on microstructure and properties in nitinol. Mater. Sci. Eng. 2012, 532, 130–138. [Google Scholar] [CrossRef]
- Duerig, T.W.; Melton, K.N.; Wayman, C.M.; Stockel, D. Engineering Aspects of Shape–Memory Alloys; Butterworth Heinemann Ltd.: Oxford, UK, 1990. [Google Scholar]
- Stoeckel, D. Nitinol medical devices and implants. Minim. Invasive Ther. Allied Technol. 2000, 9, 81–88. [Google Scholar] [CrossRef]
- Shabalovskaya, S. On the nature of the biocompatibility and medical applications of NiTi shape memory and superelastic alloys. Bio Med. Mater. Eng. 1996, 6, 267–289. [Google Scholar]
- Zhang, Y.; Zhang, Z.; Xie, Y.; Wang, S.; Qiu, Q.; Zhou, Y.; Zeng, G. Toxicity of nickel ions and comprehensive analysis of nickel ion-associated gene expression profiles in THP-1 cells. Mol. Med. Rep. 2015, 12, 3273–3278. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Lin, S.J.; Chen, Y.L.; Su, Y.Y.; Lai, S.T.; Wu, G.J.; Kwok, C.F.; Chung, K.H. The cytotoxicity of corrosion products of nitinol stent wire on cultured smooth muscle cells. J. Biomed. Mater. Res. 2000, 52, 395–403. [Google Scholar] [CrossRef]
- Bour, H.; Nicolas, J.F.; Garrigue, J.L.; Demiden, A.; Schmitt, D. Establishment of nickel-specific T cell lines from patients with allergic contact dermatitis: Comparison of different protocols. Clin. Immunol. Immunopathol. 1994, 73, 142–145. [Google Scholar] [CrossRef]
- Wataha, J.; O’Dell, N.; Singh, B.; Ghazi, M.; Whitford, G.; Lockwood, P. Relating nickel-induced tissue inflammation to Ni release in vivo. J. Biomed. Mater. Res. 2001, 58, 537–544. [Google Scholar] [CrossRef]
- Uo, M.; Watari, F.; Yokoyama, A.; Matsuno, H.; Kawasaki, T. Dissolution of nickel and tissue response observed by X-ray scanning analytical microscopy. Biomaterials 1999, 20, 747–755. [Google Scholar] [CrossRef]
- Lu, X.; Bao, X.; Huang, Y.; Qu, Y.; Lu, H.; Lu, Z. Mechanisms of cytotoxicity of nickel ions based on gene expression profiles. Biomaterials 2009, 30, 141–148. [Google Scholar] [CrossRef]
- Trépanier, C.; Pelton, A.R. Effect of strain on the corrosion resistance of nitinol and stainless steel in simulated physiological environment. In Proceedings of the SMST: The International Conference on Shape Memory and Superelastic Technologies, Monterey, CA, USA, 5–8 May 2003; pp. 393–398. [Google Scholar]
- Huang, H.H. Surface characterizations and corrosion resistance of nickel–titanium orthodontic archwires in artificial saliva of various degrees of acidity. J. Biomed. Mater. Res. 2005, 74, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Es-Souni, M.; Fischer-Brandies, H. On the properties of two binary NiTi shape memory alloys. Effects of surface finish on the corrosion behaviour and in vitro biocompatibility. Biomaterials 2002, 23, 2887–2894. [Google Scholar] [CrossRef]
- Trepanier, C.; Tabizian, M.; Yahia, L.H.; Bilodeau, L.; Piron, D.L. Effect of modification of oxide layer on NiTi stent corrosion resistance. J. Biomed. Mater. Res. 1998, 43, 433–440. [Google Scholar] [CrossRef]
- Sun, E.X.; Fine, S.; Nowak, W.B. Electrochemical behavior of nitinol alloy in Ringer’s solution. J. Mater. Sci. Mater. Med. 2002, 13, 959–964. [Google Scholar] [CrossRef]
- Trepanier, C.; Tabrizian, M.; Yahia, L.H.; Bilodeau, L.; Piron, D.L. Improvement of the corrosion resistance of NiTi stents by surface treatments. Mater. Res. Soc. Symp. Proc. 1997, 459, 363–368. [Google Scholar] [CrossRef]
- Trepanier, C.; Fino, J.; Zhu, L.; Pelton, A.R. Corrosion resistance of oxidized Nitinol. In Proceedings of the SMST: The International Conference on Shape Memory and Superelastic Technologies, Monterey, CA, USA, 5–8 May 2003; pp. 267–276. [Google Scholar]
- Huang, H.H. Variation in corrosion resistance of nickel-titanium wires from different manufacturers. Angle Orthod. 2005, 75, 661–665. [Google Scholar]
- Figueira, N.; Silva, T.M.; Carmezim, M.J.; Fernandes, J.C.S. Corrosion behaviour of NiTi alloy. Electrochim. Acta 2009, 54, 921–926. [Google Scholar] [CrossRef]
- Rocher, P.; El Medawar, L.; Hornez, J.C.; Traisnel, M.; Breme, J.; Hildebrand, H.F. Biocorrosion and cytocompatibility assessment of NiTi shape memory alloys. Scr. Mater. 2004, 50, 255–260. [Google Scholar] [CrossRef]
- Huang, H.H.; Chiu, Y.H.; Lee, T.H.; Wu, S.C.; Yang, H.W.; Su, K.H.; Hsu, C.C. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials 2003, 24, 3585–3592. [Google Scholar] [CrossRef]
- Hu, T.; Chu, C.; Xin, Y.; Wu, S.; Yeung, K.W.K.; Chu, P.K. Corrosion products and mechanism on NiTi shape memory alloy in physiological environment. J. Mater. Res. 2010, 25, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Shabalovskaya, S.A.; He Tian Anderegg, J.W.; Schryvers, D.U.; Carroll, W.U.; Van Humbeeck, J. The influence of surface oxides on the distribution and release of nickel from nitinol wires. Biomaterials 2009, 30, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Cisse, O.; Savagodo, O.; Wu, M.; Yahia, L. Effect of surface treatment of NiTi alloy on its corrosion behavior in Hank’s solution. J. Biomed. Mater. Res. 2002, 61, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Trepanier, C.; Venugopalan, R.; Messer, R.; Zimmerman, J.; Pelton, A.R. Effect of passivation treatments on nickel release from nitinol. In Proceedings of the 6th World Biomaterials Congress, Kamuela, HI, USA, 15–20 May 2000. [Google Scholar]
- Chu, C.L.; Guo, C.; Sheng, X.B.; Dong, Y.S.; Lin, P.H.; Yeung, K.W.K.; Chu, P.K. Microstructure, nickel suppression and mechanical characteristics of electropolished and photoelectrocatalytically oxidized biomedical nickel titanium shape memory alloy. Acta Biomater. 2009, 5, 2238–2245. [Google Scholar] [CrossRef]
- Michiardi, A.; Aparicio, C.; Planell, J.A.; Gil, F.J. New oxidation treatment of NiTi shape memory alloys to obtain Ni-free surfaces and to improve biocompatibility. J. Biomed. Mater. Res. 2006, 77, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Wu, S.L.; Chan, Y.L.; Chu Paul, K.; Chung, C.Y.; Chu, C.L.; Yeung, K.W.K.; Lu, W.W.; Cheung, K.M.C.; Luk, K.D.K. Surface characteristics, biocompatibility, and mechanical properties of nickel-titanium plasma-implanted with nitrogen at different implantation voltages. J Biomed. Mater. Res. 2007, 82, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Kanemura, T.; Yokoyama, K.; Sakai, J. Effects of acid type on corrosion and fracture behavior of Ni–Ti superelastic alloy under sustained tensile load in physiological saline solution containing hydrogen peroxide. Corros. Sci. 2008, 50, 2785–2795. [Google Scholar] [CrossRef]
- Ryhanen, J.; Niemi, E.; Serlo, W.; Niemela, E.; Sandvik, P.; Pernu, H.; Salo, T. Biocompatibility of nickel–titanium shape memory metal and its corrosion behavior in human cell cultures. J. Biomed. Mater. Res. 1997, 35, 451–457. [Google Scholar] [CrossRef]
- Tomić, S.; Rudolf, R.; Brunčko, M.; Anžel, I.; Savić, V.; Čolić, M. Response of monocyte-derived dendritic cells to rapidly solidified nickel-titanium ribbons with shape memory properties. Eur. Cells Mater. 2012, 23, 58–81. [Google Scholar] [CrossRef]
- Clarke, B.; Kingshott, P.; Hou, X.; Rochev, Y.; Gorelov, A.; Carroll, W. Effect of nitinol wire surface properties on albumin adsorption. Acta Biomater. 2007, 3, 103–111. [Google Scholar] [CrossRef]
- Bishara, S.E.; Barrett, R.D.; Selim, M.I. Biodegradation of orthodontic appliances. Part II: Changes in the blood level of nickel. Am. J. Orthod. Dentofac. 1993, 103, 115–119. [Google Scholar] [CrossRef]
- Assad, M.; Lemieux, N.; Rivard, C.H.; Yahia, L.H. Comparative in vitro biocompatibility of nickel-titanium, pure nickel, pure titanium, and stainless steel: Genotoxicity and atomic absorption evaluation. Bio Med. Mater. Eng. 1999, 9, 1–12. [Google Scholar]
- Wever, D.J.; Veldhuizen, A.G.; Sanders, M.M.; Schakenraad, J.M.; van Horn, J.R. Cytotoxic, allergic and genotoxic activity of a nickel-titanium alloy. Biomaterials 1997, 18, 1115–1120. [Google Scholar] [CrossRef]
- Prymak, O.; Bogdanski, D.; Köller, M.; Esenwein, S.A.; Muhr, G.; Beckmann, F.; Donath, T.; Assad, M.; Epple, M. Morphological characterization and in vitro biocompatibility of a porous nickel–titanium alloy. Biomaterials 2005, 26, 5801–5807. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, S.; Yeung, K.W.K.; Chan, Y.L.; Hu, T.; Xu, Z.; Liu, X.; Chung, J.C.Y.; Cheung, K.M.C.; Chu, P.K. Relationship between osseointegration and superelastic biomechanics in porous NiTi scaffolds. Biomaterials 2011, 32, 330–338. [Google Scholar] [CrossRef]
- Kapanen, A.; Ryhanen, J.; Danilov, A.; Tuukkanen, J. Effect of nickel-titanium shape memory metal alloy on bone formation. Biomaterials 2001, 22, 2475–2480. [Google Scholar] [CrossRef]
- Berger-Gorbet, M.; Broxup, B.; Rivard, C.; Yahia, L.H. Biocompatibility testing of NiTi screws using immunohistochemistry on sections containing metallic implants. J. Biomed. Mater. Res. 1996, 32, 243–248. [Google Scholar] [CrossRef]
- Zhu, L.; Trepanier, C.; Fino, J.; Pelton, A.R. Oxidation of nitinol and its effect on corrosion resistance. In Proceedings of the ASM Materials & Processes for Medical Device Conference, Anaheim, CA, USA, 8–10 September 2003; pp. 156–161. [Google Scholar]
- Firstov, G.S.; Vitchev, R.G.; Kumar, H.; Blanpain, B.; Van Humbeeck, J. Surface oxidation of NiTi shape memory alloy. Biomaterials 2002, 23, 4863–4871. [Google Scholar] [CrossRef]
- Barison, S.; Cattarin, S.; Daolio, S.; Musiani, M.; Tuissi, A. Characterisation of surface oxidation of nickel–titanium alloy by ion-beam and electrochemical techniques. Electrochim. Acta 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Simka, W.; Baron, A.; Szewczenko, J.; Marciniak, J. Electrochemical behavior of Ni-Ti alloy after surface modification. J. Achiev. Mater. Manuf. Eng. 2006, 18, 111–114. [Google Scholar]
- Bakhshi, R.; Darbyshire, A.; Evans, J.E.; You, Z.; Lu, J.; Seifalian, A.M. Polymeric coating of surface modified nitinol stent with POSS-nanocomposite polymer. Colloids Surf. B Biointerfaces 2011, 86, 93–105. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A.; Anderegg, J.W. Surface spectroscopic characterization of TiNi nearly equiatomic shape memory alloys for implants. J. Vac. Sci. Technol. 1995, 13, 2624–2632. [Google Scholar] [CrossRef]
- Ryhanen, J.; Kallioinen, M.; Tuukkanen, J.; Lehenkari, P.; Junila, J.; Niemela, K.E.; Sandvik, P.; Serlo, W. Bone modelling and cell-material interface response induced by nickel-titanium shape memory alloy after periosteal implantation. Biomaterials 1999, 20, 1309–1317. [Google Scholar] [CrossRef]
- Trepanier, C.; Leung, T.K.; Tabrizian, M.; Yahia, H.; Bienvenu, J.G.; Tanguay, J.F.; Piron, D.L.; Bilodeau, L. Preliminary investigation of the effects of surface treatments on biological response to shape memory NiTi stents. J. Biomed. Mater. Res. 1999, 48, 165–171. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A.; Rondelli, G.; Anderegg, J.; Xiong, J.P.; Wu, M. Comparative corrosion performance of black oxide, sandblasted, and fine-drawn nitinol wires in potentiodynamic and potentiostatic tests: Effects of chemical etching and electropolishing. J. Biomed. Mater. Res. 2004, 69, 223–231. [Google Scholar] [CrossRef]
- Sevostyanov, M.A.; Fedotov, A.Y.; Kolmakov, A.G.; Zabolotnyi, V.T.; Barinov, S.M.; Goncharenko, B.A.; Komlev, V.S.; Baikin, A.S.; Sergienko, K.V.; Teterina, A.Y.; et al. Mechanical properties of nanostructured nitinol/chitosan composite material. Inorg. Mater. Appl. Res. 2014, 5, 344–346. [Google Scholar] [CrossRef]
- Flamini, D.O.; Saugo, M.; Saidman, S.B. Electrodeposition of polypyrrole on Nitinol alloy in the presence of inhibitor ions for corrosion protection. Corros. Sci. 2014, 81, 36–44. [Google Scholar] [CrossRef]
- Maho, A.; Delhalle, J.; Mekhalif, Z. Study of the formation process and the characteristics of tantalum layers electrodeposited on nitinol plates in the 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide ionic liquid. Electrochim. Acta 2013, 89, 346–358. [Google Scholar] [CrossRef]
- Petrović, Z.; Katić, J.; Metikoš-Huković, M.; Dadafarin, H.; Omanovic, S. Modification of a nitinol surface by phosphonate self-assembled monolayers. J. Electrochem. Soc. Phys. Anal. Electrochem. 2011, 158, F159–F165. [Google Scholar] [CrossRef]
- Armitage, D.A.; Parker, T.L.; Grant, D.M. Biocompatibility and hemocompatibility of surface-modified NiTi alloys. J. Biomed. Mater. Res. 2003, 66, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, A.; Yokoyama, K.; Sakai, J. Evaluation of hydrogen absorption behaviour during acid etching for surface modification of commercial pure Ti, Ti–6Al–4V and Ni–Ti superelastic alloys. Corros. Sci. 2010, 52, 1130–1138. [Google Scholar] [CrossRef]
- Shi, P.; Cheng, F.T.; Man, H.C. Improvement in corrosion resistance of NiTi by anodization in acetic acid. Mater. Lett. 2007, 61, 2385–2388. [Google Scholar] [CrossRef]
- Devillers, S.; Barthélémy, B.; Fery, I.; Delhalle, J.; Mekhalif, Z. Functionalization of nitinol surface toward a versatile platform for post-grafting chemical reactions. Electrochim. Acta 2011, 56, 8129–8137. [Google Scholar] [CrossRef]
- Chu, C.L.; Wang, R.M.; Yin, L.H.; Pu, Y.P.; Lin, P.H.; Dong, Y.S.; Chung, C.Y.; Yeung, K.W.K.; Chu, P.K. Effects of anodic oxidation in H2SO4 electrolyte on the biocompatibility of NiTi shape memory alloy. Mater. Lett. 2008, 62, 3512–3514. [Google Scholar] [CrossRef]
- Wong, M.H.; Cheng, F.T.; Man, H.C. Laser oxidation of NiTi for improving corrosion resistance in Hanks’ solution. Mater. Lett. 2007, 61, 3391–3394. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Li, P.; Bilek, M.M.M.; Boxman, R.L.; McKenzie, D.R.; Chu, P.K. Evaluation of corrosion resistance and cytocompatibility of graded metal carbon film on Ti and NiTi prepared by hybrid cathodic arc/glow discharge plasma-assisted chemical vapor deposition. Corros. Sci. 2015, 97, 126–138. [Google Scholar] [CrossRef]
- Hassel, A.W.; Neelakantan, L.; Zelenkevych, A.; Ruh, A.; Spiegel, M. Selective de-alloying of NiTi by oxochloridation. Corros. Sci. 2008, 50, 1368–1375. [Google Scholar] [CrossRef]
- Maho, A.; Kanoufi, F.; Combellas, C.; Delhalle, J.; Mekhalif, Z. Electrochemical investigation of nitinol/tantalum hybrid surfaces modified by alkylphosphonic self-assembled monolayers. Electrochim. Acta 2014, 116, 78–88. [Google Scholar] [CrossRef]
- Nasakina, E.O.; Baikin, A.S.; Sevost’yanov, M.A.; Kolmakov, A.G.; Zabolotnyi, V.T.; Solntsev, K.A. Properties of nanostructured titanium nickelide and composite based on it. Theor. Found. Chem. Eng. 2014, 48, 477–486. [Google Scholar] [CrossRef]
- Yan, X.; Yang, D.; Liu, X. Electrochemical behavior of YAG laser-welded NiTi shape memory alloy. Trans. Nonferrous Met. Soc. China 2006, 16, 572–576. [Google Scholar] [CrossRef]
- Plant, S.D.; Grant, D.M.; Leach, L. Surface modification of NiTi alloy and human platelet activation under static and flow conditions. Mater. Lett. 2007, 61, 2864–2867. [Google Scholar] [CrossRef]
- Wong, M.H.; Cheng, F.T.; Man, H.C. In situ hydrothermal synthesis of oxide film on NiTi for improving corrosion resistance in Hanks’ solution. Scr. Mater. 2007, 56, 205–208. [Google Scholar] [CrossRef]
- Katić, J.; Metikoš-Huković, M.; Škapin, S.D.; Petravić, M.; Varašanec, M. The potential-assisted deposition as valuable tool for producing functional apatite coatings on metallic materials. Electrochim. Acta 2014, 127, 173–179. [Google Scholar] [CrossRef]
- Hu, T.; Chu, C.L.; Yin, L.H.; YPPu Dong, Y.S.; Guo, C.; Sheng, X.B.; Chung, J.C.Y.; Chu, P.K. In vitro biocompatibility of titanium-nickel alloy with titanium oxide film by H2O2 oxidation. Trans. Nonferrous Met. Soc. China 2007, 17, 553–557. [Google Scholar] [CrossRef]
- Chu, C.L.; Hu, T.; Wu, S.L.; Dong, Y.S.; Yin, L.H.; Pu, Y.P.; Lin, P.H.; Chung, C.Y.; Yeung, K.W.K.; Chu, P.K. Surface structure and properties of biomedical NiTi shape memory alloy after Fenton’s oxidation. Acta Biomater. 2007, 3, 795–806. [Google Scholar] [CrossRef]
- Simka, W.; Kaczmarek, M.; Baron-Wiecheć, A.; Nawrat, G.; Marciniak, J.; Żak, J. Electropolishing and passivation of NiTi shape memory alloy. Electrochim. Acta 2010, 55, 2437–2441. [Google Scholar] [CrossRef]
- Thierry, B.; Tabrizian, M.; Savadogo, O.; Yahia, L.H. Effects of sterilization processes on NiTi alloy: Surface characterization. J. Biomed. Mater. Res. 2000, 49, 88–98. [Google Scholar] [CrossRef]
- Armitage, D.A.; Grant, D.M. Characterisation of surface-modified nickel titanium alloys. Mater. Sci. Eng. 2003, 349, 89–97. [Google Scholar] [CrossRef]
- Nasakina, E.O.; Baikin, A.S.; Sergienko, K.V.; Sevost’yanov, M.A.; Kolmakov, A.G.; Goncharenko, B.A.; Zabolotnyi, V.T.; Fadeev, R.S.; Fadeeva, I.S.; Gudkov, S.V.; et al. Biocompatibility of nanostructured nitinol with titanium or tantalum surface composite layers formed by magnetron sputtering. Dokl. Chem. 2015, 461, 86–88. [Google Scholar] [CrossRef]
- Plant, S.D.; Grant, D.M.; Leach, L. Behaviour of human endothelial cells on surface modified NiTi alloy. Biomaterials 2005, 26, 5359–5367. [Google Scholar] [CrossRef]
- Jacques, A.; Barthélémy, B.; Delhalle, J.; Mekhalif, Z. Nitinol modified by in situ generated diazonium from its nitro precursor for the SI-ATRP of 2-hydroxyethyl methacrylate. J. Electrochem. Soc. 2015, 162, G94–G102. [Google Scholar] [CrossRef]
- Jacques, A.; Devillers, S.; Arrotin, B.; Delhalle, J.; Mekhalif, Z. Polyelectrolyte multilayers deposition on nitinol modified by in situ generated diazonium in gentle conditions. J. Electrochem. Soc. 2014, 161, G55–G62. [Google Scholar] [CrossRef]
- Tan, L.; Dodd, R.A.; Crone, W.C. Corrosion and wear-corrosion behavior of NiTi modified by plasma source ion implantation. Biomaterials 2003, 24, 3931–3939. [Google Scholar] [CrossRef]
- Nasakina, E.O.; Sevostyanov, M.A.; Golberg, M.A.; Dyomin, K.Y.; Baikin, A.S.; Goncharenko, B.A.; Cherkasov, V.A.; Kolmakov, A.G.; Zabolotnyy, V.T. LongTerm Corrosion Tests of Nanostructural Nitinol of (55.91 wt % Ni, 44.03 wt % Ti) Composition under Static Conditions: Composition and Structure before and after Corrosion. Inorg. Mater. Appl. Res. 2015, 6, 53–58. [Google Scholar] [CrossRef]
- Eliades, T.; Eliades, G.; Athanasiou, A.E.; Bradley, T.G. Surface characterization of retrieved NiTi orthodontic arcwires. Eur. J. Orthod. 2000, 22, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, R.; Trepanier, C. Assessing the corrosion behaviour of Nitinol for minimally-invasive device design. Minim. Invasive Ther. Allied Technol. 2000, 9, 67–74. [Google Scholar] [CrossRef]
- Venugopalan, R.; Trepanier, C.; Pelton, A.R.; Lucas, L.C. Comparative electrochemical behavior of NiTi and 316L stainless steel. In Proceedings of the 25th Annual Meeting, Providence, RI, USA, 10–13 May 1999. [Google Scholar]
- Trépanier, C.; Gong, X.Y.; Ditter, T.; Pelton, A.; Neely, Y.; Grishaber, R. Effect of wear and crevice on the corrosion resistance of overlapped stents. In Proceedings of the SMST: The International Conference on Shape Memory and Superelastic Technologies, Pacific Grove, CA, USA, 7–11 May 2006; pp. 265–276. [Google Scholar]
- Zhuk, N.P. Kurs Korrozii i Zashhity Metallov [The Course of Corrosion and Protection of Metals]; Metallurgy: Moscow, Russia, 1976. [Google Scholar]
- Ulig, G.G.; Revi, G.U. Korrozija i Bor’ba s nej. Vvedenie v Korrozionnuju Nauku i Tehniku [Corrosion and Its Prevention]; Chemistry: Leningrad, Russia, 1989. [Google Scholar]
- Li, X.; Wang, J.; Han, E.-H.; Ke, W. Influence of fluoride and chloride on corrosion behavior of NiTi orthodontic wires. Acta Biomater. 2007, 3, 807–815. [Google Scholar] [CrossRef]
- Shcherbakov, A.I.; Kasatkina, I.V.; Dorofeeva, V.N.; Zalavutdinov, R.K. Ustojchivost’ passivnogo sostojanija splava «pamjati» TiNi k pittingovoj korrozii [Passive condition stability of ‘memory’ alloy TiNi to pitting]. Korroziya: Materialy i Zashchita [Corros. Mater. Prot.] 2007, 12, 6–9. [Google Scholar]
- Trépanier, C.; Pelton, A.R. Effect of temperature and pH on the corrosion resistance of Nitinol. In Proceedings of the ASM Materials and Processes for Medical Devices Conference, Boston, MA, USA, 14–16 November 2005; pp. 392–397. [Google Scholar]
- Trépanier, C.; Pelton, A.R. Effect of temperature and pH on the corrosion resistance of passivated nitinol and stainless steel. In Proceedings of the International Conference on Shape Memory and Superelastic Technologies, Baden-Baden, Germany, 3–7 October 2004; pp. 361–366. [Google Scholar]
- Tian, H.; Schryvers, D.; Shabalovskaya, S.; Van Humbeeck, J. Microstructure of surface and subsurface layers of a Ni-Ti shape memory microwire. Microsc. Microanal. 2009, 15, 62–70. [Google Scholar] [CrossRef]
- Mishnaevsky, L., Jr.; Levashov, E.; Valiev, R.Z.; Segurado, J.; Sabirov, I.; Enikeev, N.; Prokoshkin, S.; Solov’yov, A.V.; Korotitskiy, A.; Gutmanas, E.; et al. Nanostructured titanium-based materials for medical implants: Modeling and development. Mater. Sci. Eng. 2014, 81, 1–19. [Google Scholar] [CrossRef]
- Nolan, M.; Tofail, S.A.M. Density functional theory simulation of titanium migration and reaction with oxygen in the early stages of oxidation of equiatomic NiTi alloy. Biomaterials 2010, 31, 3439–3448. [Google Scholar] [CrossRef]
- Shcherbakov, A.I.; Kasatkina, I.V.; Zalavutdinov, R.K. Ustojchivost’ splava «pamjati» TiNi k selektivnoj korrozii [Resistance of ‘memory’ alloy TiNi to selective corrosion]. Korroziya: Materialy i zashchita [Corros. Mater. Prot.] 2007, 12, 14–16. [Google Scholar]
- Amirhanova, N.A.; Valiev, R.Z.; Adasheva, S.L.; Prokof’ev, E.A. Issledovanie korrozionnyh i jelektrohimicheskih svojstv splavov na osnove nikelida titana v krupnozernistom i ul’tramelkozernistom sostojanijah [Investigation of corrosion and electrochemical properties of alloys based on nickel titanium in coarse and ultrafine states]. J. Ufa State Aviat. Tech. Univ. 2006, 7, 143–146. [Google Scholar]
- Korotin, D.M.; Bartkowski, S.; Kurmaev, E.Z.; Cholakh, S.O.; Müller, M.; Neumann, M.; Gunderov, D.; Valiev, R.Z.; Cholakh, S.O. Arsenic contamination of coarse-grained and nanostructured nitinol surfaces induced by chemical treatment in hydrofluoric acid. J. Biomed. Mater. Res. 2012, 100, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.Y.; Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Valiev, R.Z. Enhanced in vitro biocompatibility of ultrafine-grained titanium with hierarchical porous surface. Appl. Surf. Sci. 2011, 257, 5634–5640. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Ruan, L.; Valiev, R.Z. Enhanced corrosion resistance and cellular behavior of ultrafine-grained biomedical NiTi alloy with a novel SrO–SiO2–TiO2 sol–gel coating. Appl. Surf. Sci. 2011, 257, 5913–5918. [Google Scholar] [CrossRef]
- Zhang, X.N. Biomechanical and biocorrosion properties of nanostructured titanium. Adv. Mater. Res. 2007, 29–30, 51–54. [Google Scholar] [CrossRef]
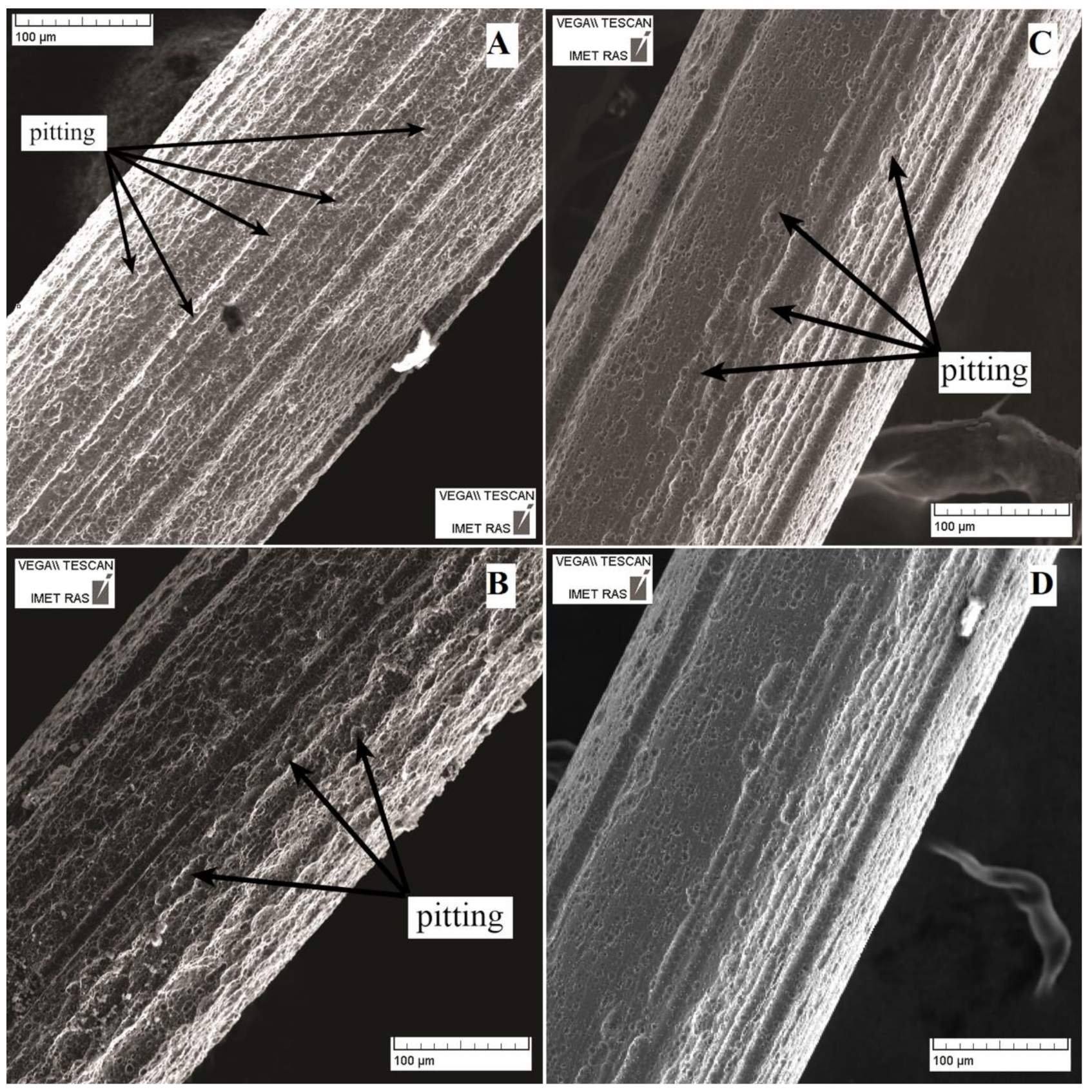
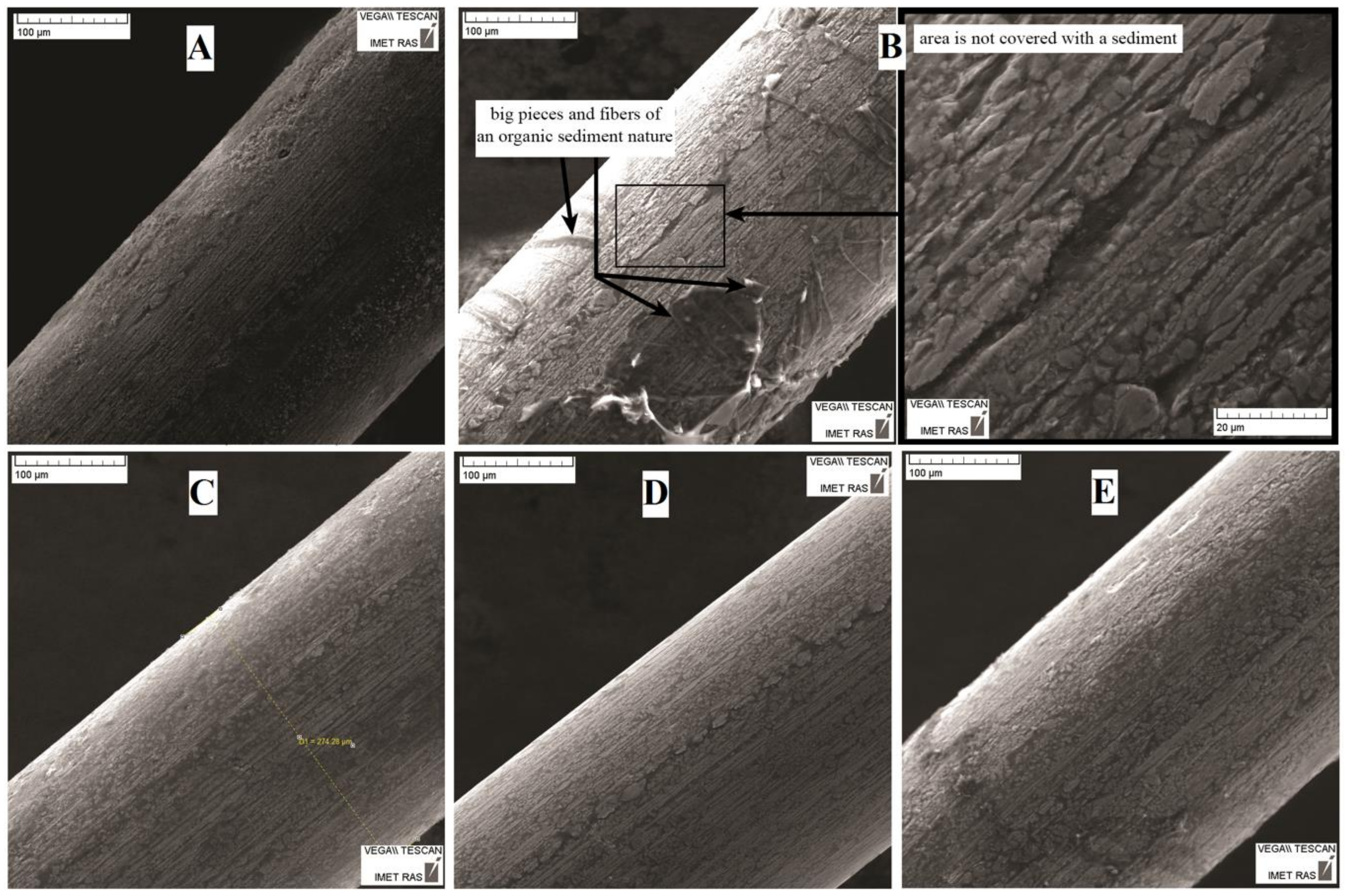
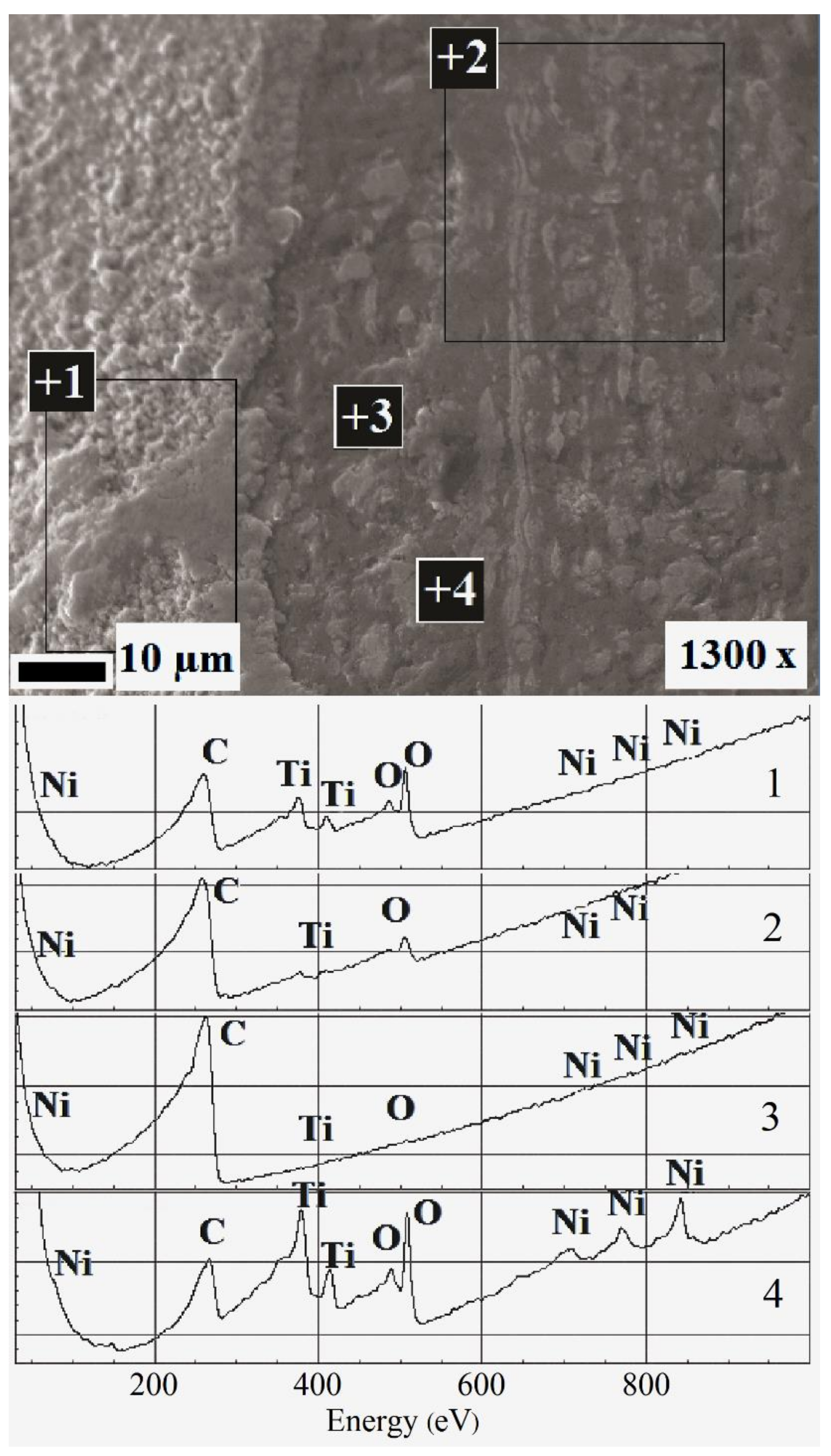

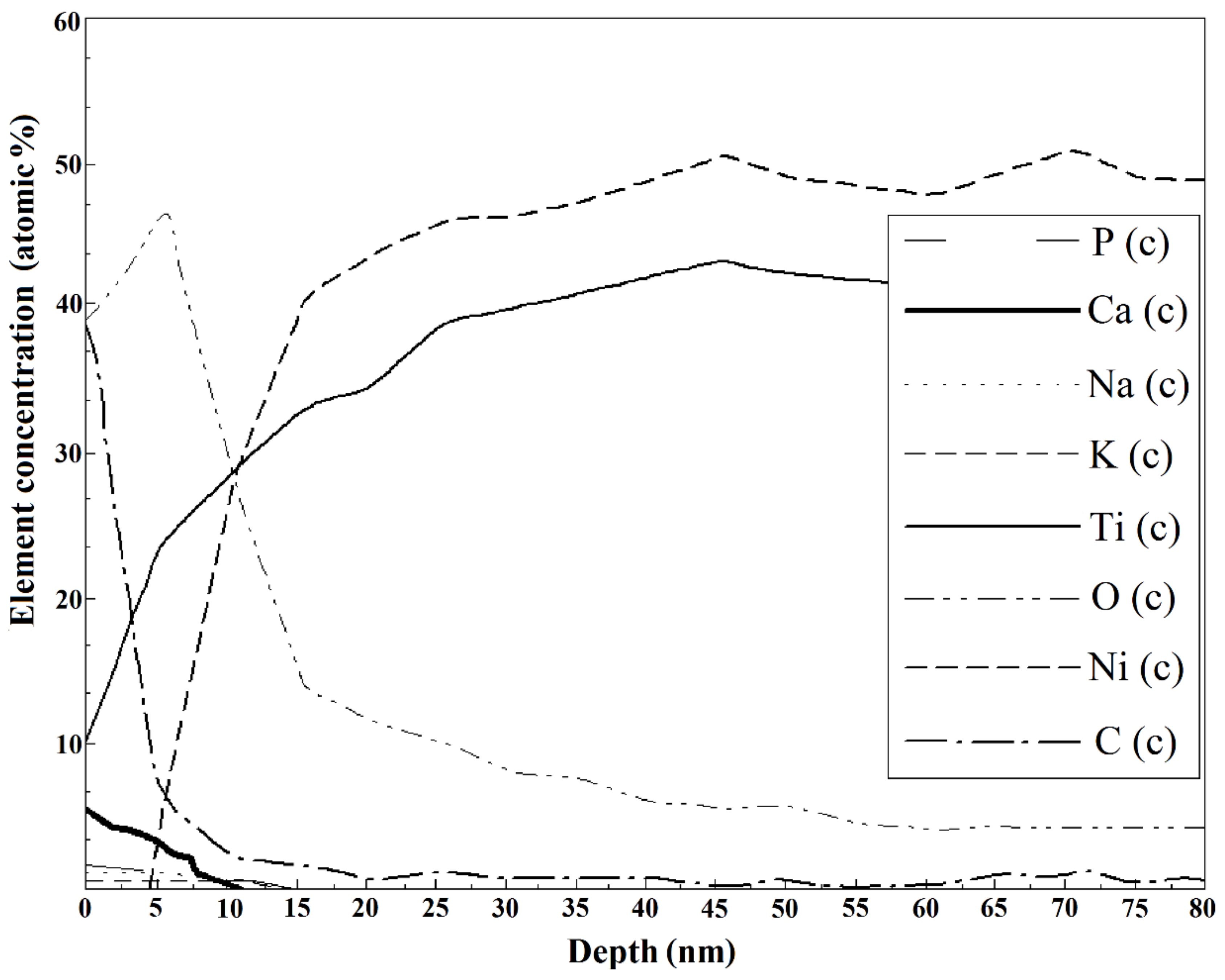

| Solution Number | pH | Composition |
|---|---|---|
| 1 | 1.68 | Potassium tetraoxalate KH3C4O8·2H2O, 0.05 M |
| 2 | 3.56 | Acid potassium tartrate C4H5O6K, 0.025 M |
| 3 | 4.01 | Acid potassium phthalate C8H5O4K, 0.05 M |
| 4 | 6.31 | Sodium chloride NaCl, 0.9 wt % |
| 5 | 9.18 | Acid sodium tetraborate Na2B4O7·10H2O, 0.05 M |
| 6 | 7.36 | Artificial plasma: NaCl (92.3 mM), NaHCO3 (26.3 mM), K2HPO4 (0.9 mM), KCl (2.7 mM), NaH2PO4 (0.22 mM), CaCl2 (2.5 mM), MgSO4·7H2O (0.82 mM), Na2SO4 (1.48 mM), d-glucose C6H12O6 (5.55 mM) [16,17,20,23,26,27,29,30,46,49,78] |
| 7 | 1.56 | HCl (aq.), 0.0275 M |
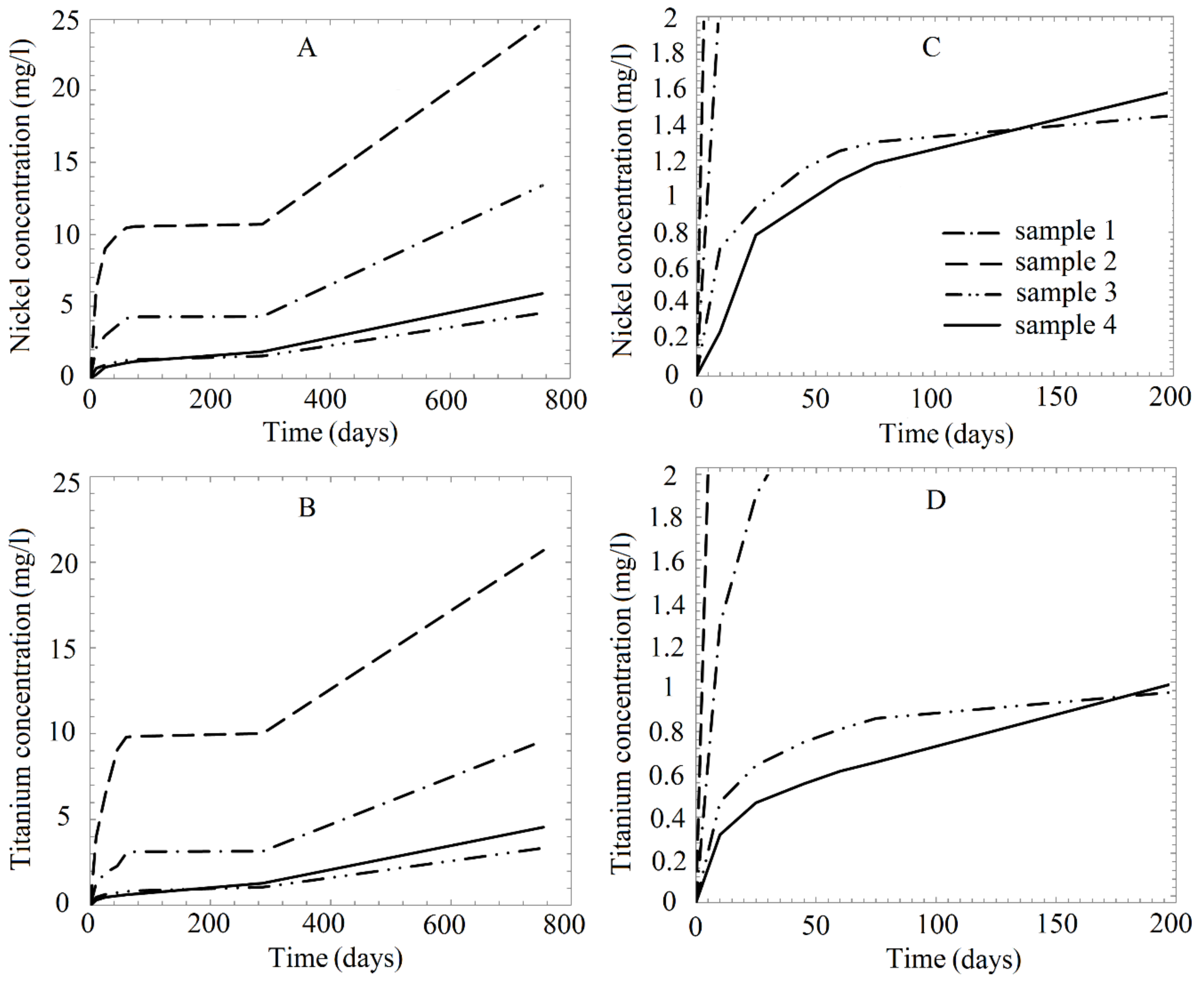
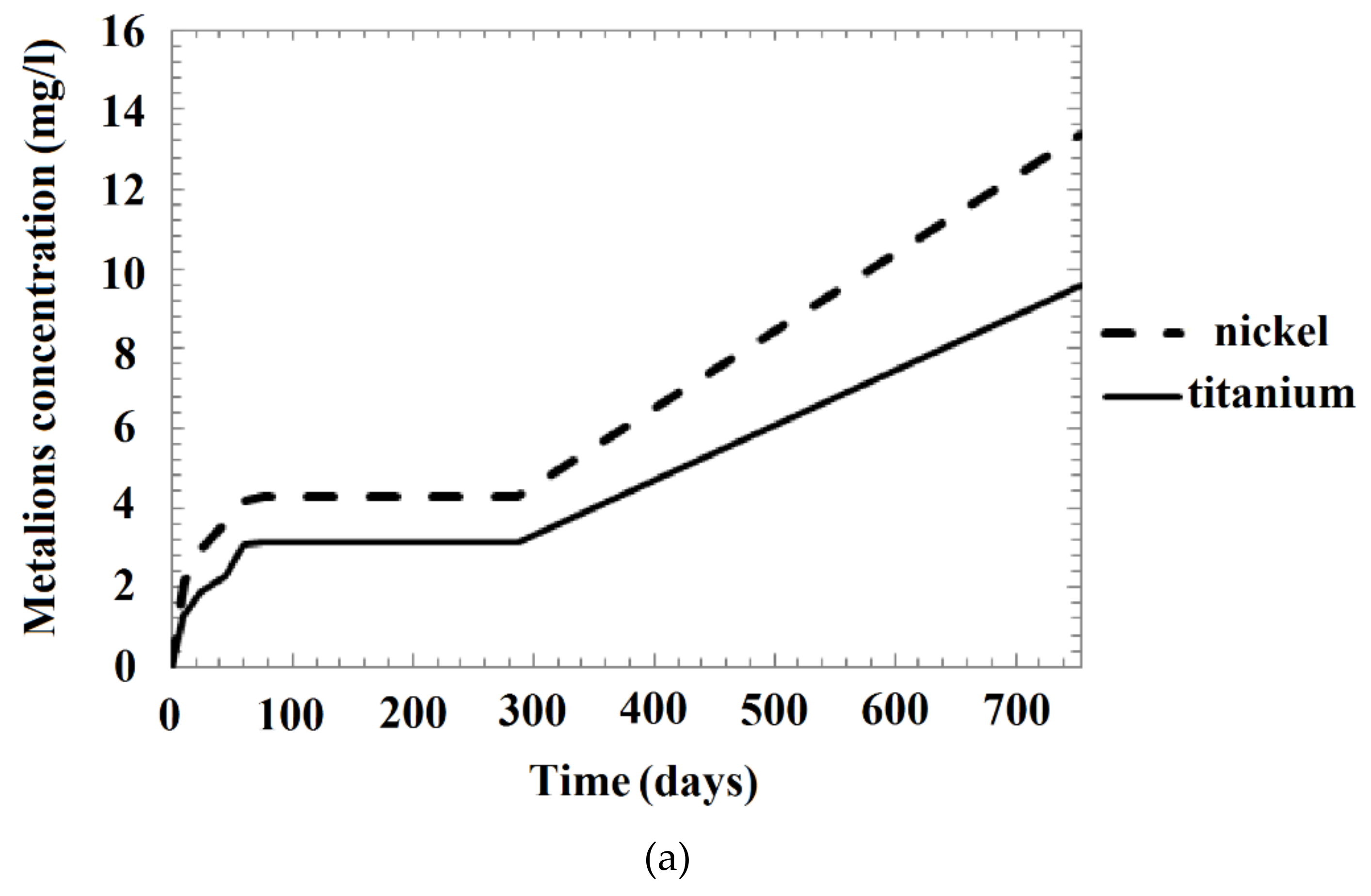
| Solution Number | Time (Days) | Ion Concentration in Solution (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| As-Received Sample (1) | Annealed Sample (2) | Mechanically Treated Sample (3) | Annealed Mechanically Treated Sample (4) | ||||||
| Ti | Ni | Ti | Ni | Ti | Ni | Ti | Ni | ||
| 1 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.000 | 0.000 | 0.000 | 0.000 |
| 10 | 1.30 | 2.13 | 4.00 | 6.20 | 0.470 | 0.710 | 0.317 | 0.245 | |
| 25 | 1.90 | 2.97 | 6.50 | 9.00 | 0.640 | 0.940 | 0.467 | 0.784 | |
| 45 | 2.30 | 3.67 | 9.08 | 9.97 | 0.750 | 1.150 | 0.556 | 0.958 | |
| 60 | 3.05 | 4.16 | 9.81 | 10.44 | 0.810 | 1.250 | 0.614 | 1.087 | |
| 75 | 3.14 | 4.26 | 9.86 | 10.55 | 0.860 | 1.300 | 0.656 | 1.182 | |
| 287 | 3.15 | 4.29 | 10.01 | 10.70 | 1.070 | 1.550 | 1.284 | 1.860 | |
| 754 | 9.60 | 13.39 | 20.70 | 24.60 | 3.340 | 4.550 | 4.559 | 5.898 | |
| 2 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | - | 0.05 | - | 0.10 | - | 0.03 | - | 0.010 | |
| 25 | - | 0.18 | - | 0.40 | - | 0.07 | - | 0.058 | |
| 45 | - | 0.38 | 0.08 | 0.61 | - | 0.11 | - | 0.092 | |
| 60 | 0.04 | 0.44 | 0.09 | 0.67 | - | 0.13 | - | 0.113 | |
| 75 | 0.06 | 0.47 | 0.09 | 0.87 | - | 0.14 | - | 0.127 | |
| 287 | 0.12 | 1.05 | 0.16 | 1.59 | - | 0.19 | - | 0.228 | |
| 754 | 0.32 | 1.34 | 0.48 | 2.19 | 0.09 | 0.32 | 0.11 | 0.415 | |
| 3 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | - | 0.03 | - | 0.04 | - | 0.01 | - | 0.003 | |
| 25 | - | 0.06 | - | 0.07 | - | 0.02 | - | 0.017 | |
| 45 | - | 0.07 | - | 0.14 | - | 0.02 | - | 0.017 | |
| 60 | - | 0.09 | - | 0.14 | - | 0.03 | - | 0.026 | |
| 75 | - | 0.10 | - | 0.18 | - | 0.03 | - | 0.027 | |
| 287 | - | 0.41 | - | 0.57 | - | 0.10 | - | 0.120 | |
| 754 | 0.01 | 0.50 | 0.01 | 0.63 | - | 0.14 | - | 0.181 | |
| 4 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | - | 0.50 | 0.01 | 0.88 | - | 0.04 | - | 0.014 | |
| 25 | - | 0.76 | 0.05 | 1.33 | - | 0.12 | - | 0.100 | |
| 45 | 0.01 | 0.94 | 0.09 | 1.55 | - | 0.15 | - | 0.125 | |
| 60 | 0.03 | 0.95 | 0.10 | 1.56 | - | 0.17 | - | 0.148 | |
| 75 | 0.04 | 0.97 | 0.12 | 1.59 | - | 0.19 | - | 0.173 | |
| 287 | 0.08 | 1.20 | 0.17 | 2.08 | - | 0.24 | - | 0.288 | |
| 764 | 0.12 | 1.28 | 0.19 | 2.17 | - | 0.39 | - | 0.506 | |
| 7 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | 0.515 | 1.94 | no | no | no | no | no | no | |
| Diameter Before Immersion (µm) | Diameter After Immersion (µm) | Sample | pH |
|---|---|---|---|
| 280.00 | 253.74 | As-received | 1.68 |
| 280.00 | 275.59 | As-received | 3.56 |
| 280.00 | 277.14 | As-received | 4.01 |
| 280.00 | 275.16 | As-received | 6.31 |
| 280.00 | 280.00 | As-received | 9.18 |
| 280.00 | 252.46 | Annealed | 1.68 |
| 270.00 | 257.00 | Mechanically treated | 1.68 |
| 280.00 | 270.03 | As-received | 1.5 (HCl) |
| Solution pH | 1.68 | 3.56 | 4.01 | 6.31/7.36 | 9.18 |
|---|---|---|---|---|---|
| Oxide layer thickness (nm) | 8–13 | 13–17 | 15–20 | 23–28 | ~10 |
| Nickel surface concentration (atomic %) | 8 | 5 | 3 | 0 | 3 |
| Depth of nickel etching (nm) | 10 | 10 | 10 | 20 | 10 |
| Maximum oxygen surface concentration (atomic %) | ~60–70 atomic.% at a depth of 2.5–7 nm | ||||
| Solution | Time (Days) | Ni Concentration in Solution (mg/L) | Reference | ||
|---|---|---|---|---|---|
| As-Received Sample (Commercial) | Mechanically Treated and/or Electropolished Sample | ||||
| 0.9% NaCl | 15 | 0.005–1.000 | [25] | ||
| 30 | 0.010–1.480 | ||||
| 60 | 0.020–1.620 | ||||
| 120 | 0.025–1.760 | ||||
| 180 | 0.030–1.800 | ||||
| Hank’s physiological solution (pH 7.4) | 1 | 0.200 | 0.200 | [26] | |
| 2 | 0.300 | 0.220 | |||
| 6 | 0.350–0.400 | 0.250 | |||
| 9 | 0.500–0.850 | 0.250 | |||
| 14 | 0.550–0.900 | 0.250 | |||
| 18 | 0.600–1.050 | 0.250 | |||
| 1 | 1.300 | [27] | |||
| Simulated body fluid (SBF) (pH 7.4) | 14 | 0.195 | [28] | ||
| 35 | 0.205 | ||||
| 70 | 0.500 | ||||
| 30 | 0.290 | [29] | |||
| 35 | 0.950 | [30] | |||
| (cell-free) Culture medium (pH 7.4) | 2 | 0.152 | [32] | ||
| 2 | 118.930 | [33] | |||
| 3 | 0.081 | [22] | |||
| 6 | 0.176 | ||||
| Artificial saliva | pH 2.5 | 1 | 0.255–1.275 | [23] | |
| 3 | 1.530–4.080 | ||||
| 7 | 2.040–5.610 | ||||
| 14 | 4.080–10.200 | ||||
| 28 | 4.080–15.300 | ||||
| pH 3.75 | 1 | 0.128–0.893 | |||
| 3 | 0.255–1.020 | ||||
| 7 | 0.255–1.275 | ||||
| 14 | 0.255–1.785 | ||||
| 28 | 0.383–2.550 | ||||
| pH 6.25 | 1 | 0.038–0.191 | |||
| 3 | 0.153–0.561 | ||||
| 7 | 0.153–0.714 | ||||
| 14 | 0.153–0.714 | ||||
| 28 | 0.153–0.765 | ||||
| 0.9% NaCl | 2 | 0.013 | [24] | ||
| 5 | 0.020 | ||||
| 8 | 0.050 | ||||
| 12 | 0.065 | ||||
| 30 | 0.120 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasakina, E.O.; Sudarchikova, M.A.; Sergienko, K.V.; Konushkin, S.V.; Sevost’yanov, M.A. Ion Release and Surface Characterization of Nanostructured Nitinol during Long-Term Testing. Nanomaterials 2019, 9, 1569. https://doi.org/10.3390/nano9111569
Nasakina EO, Sudarchikova MA, Sergienko KV, Konushkin SV, Sevost’yanov MA. Ion Release and Surface Characterization of Nanostructured Nitinol during Long-Term Testing. Nanomaterials. 2019; 9(11):1569. https://doi.org/10.3390/nano9111569
Chicago/Turabian StyleNasakina, Elena O., Maria A. Sudarchikova, Konstantin V. Sergienko, Sergey V. Konushkin, and Mikhail A. Sevost’yanov. 2019. "Ion Release and Surface Characterization of Nanostructured Nitinol during Long-Term Testing" Nanomaterials 9, no. 11: 1569. https://doi.org/10.3390/nano9111569