Organic–Inorganic Nanohybrid Electrochemical Sensors from Multi-Walled Carbon Nanotubes Decorated with Zinc Oxide Nanoparticles and In-Situ Wrapped with Poly(2-methacryloyloxyethyl ferrocenecarboxylate) for Detection of the Content of Food Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of MWCNTs@ZnO Nanocomposites
2.2.2. Preparation of MWCNTs@ZnO/PMAEFc Nanohybrids
2.3. Preparation of Modified Electrode Sensors
2.4. Characterization and Measurements
2.5. Real Samples Analysis
3. Results and Discussion
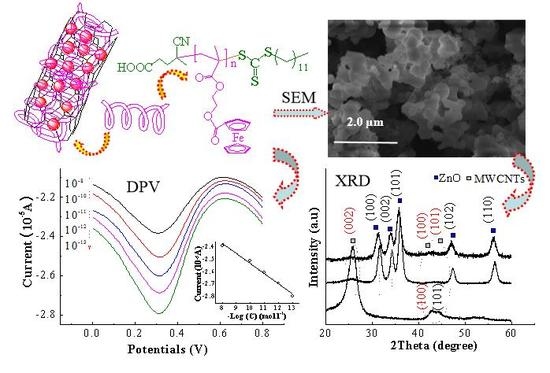
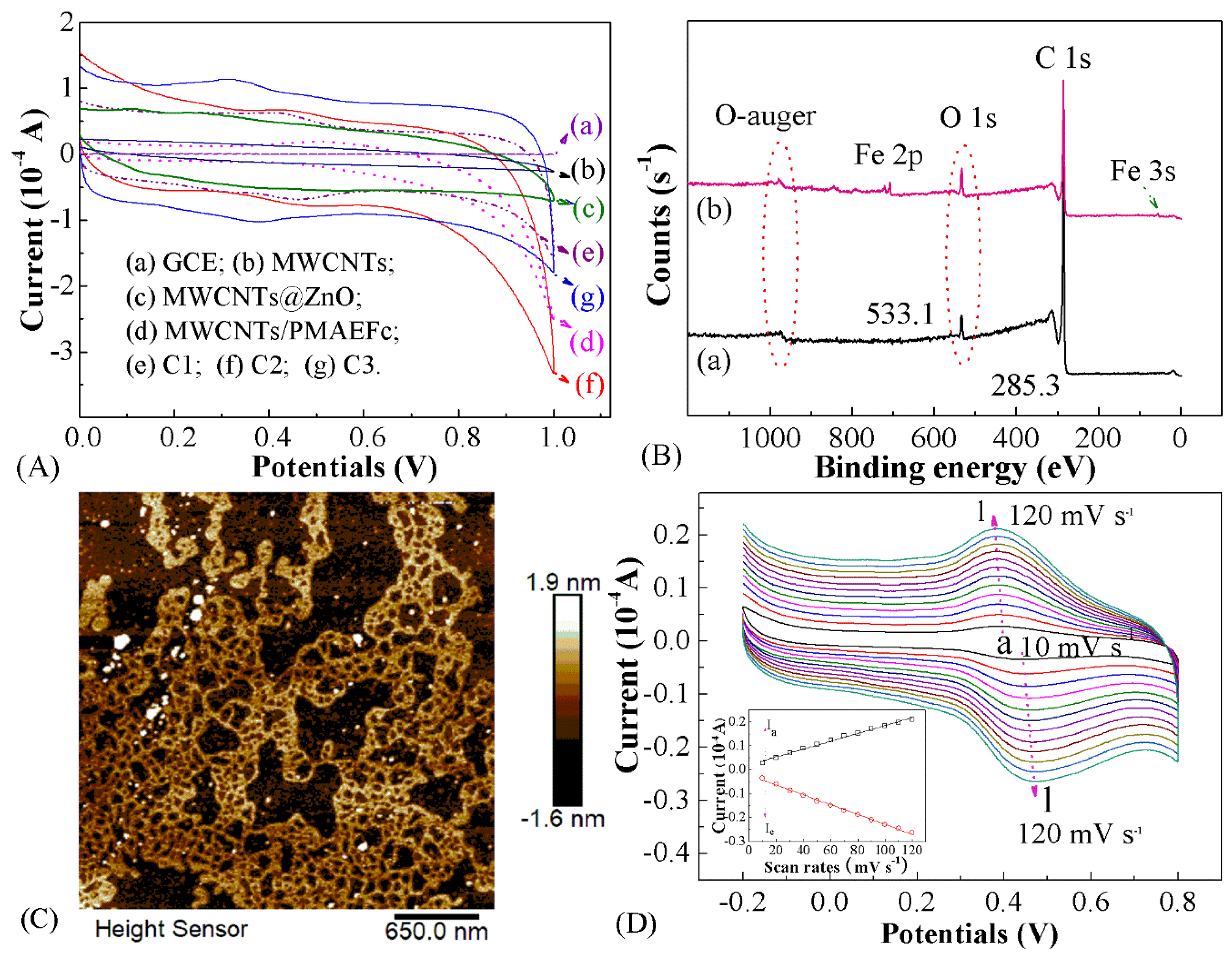
3.1. Structural Characterization
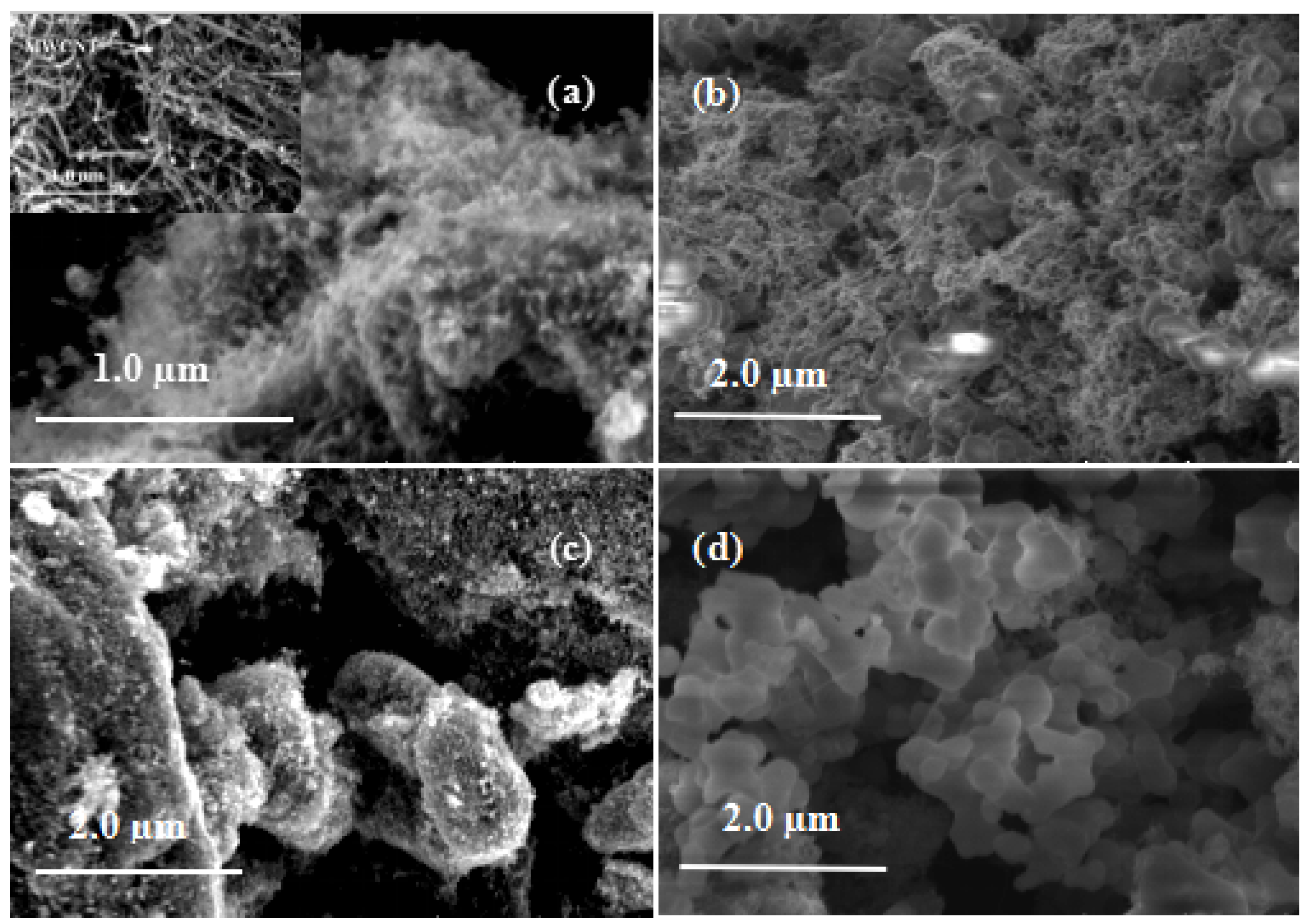
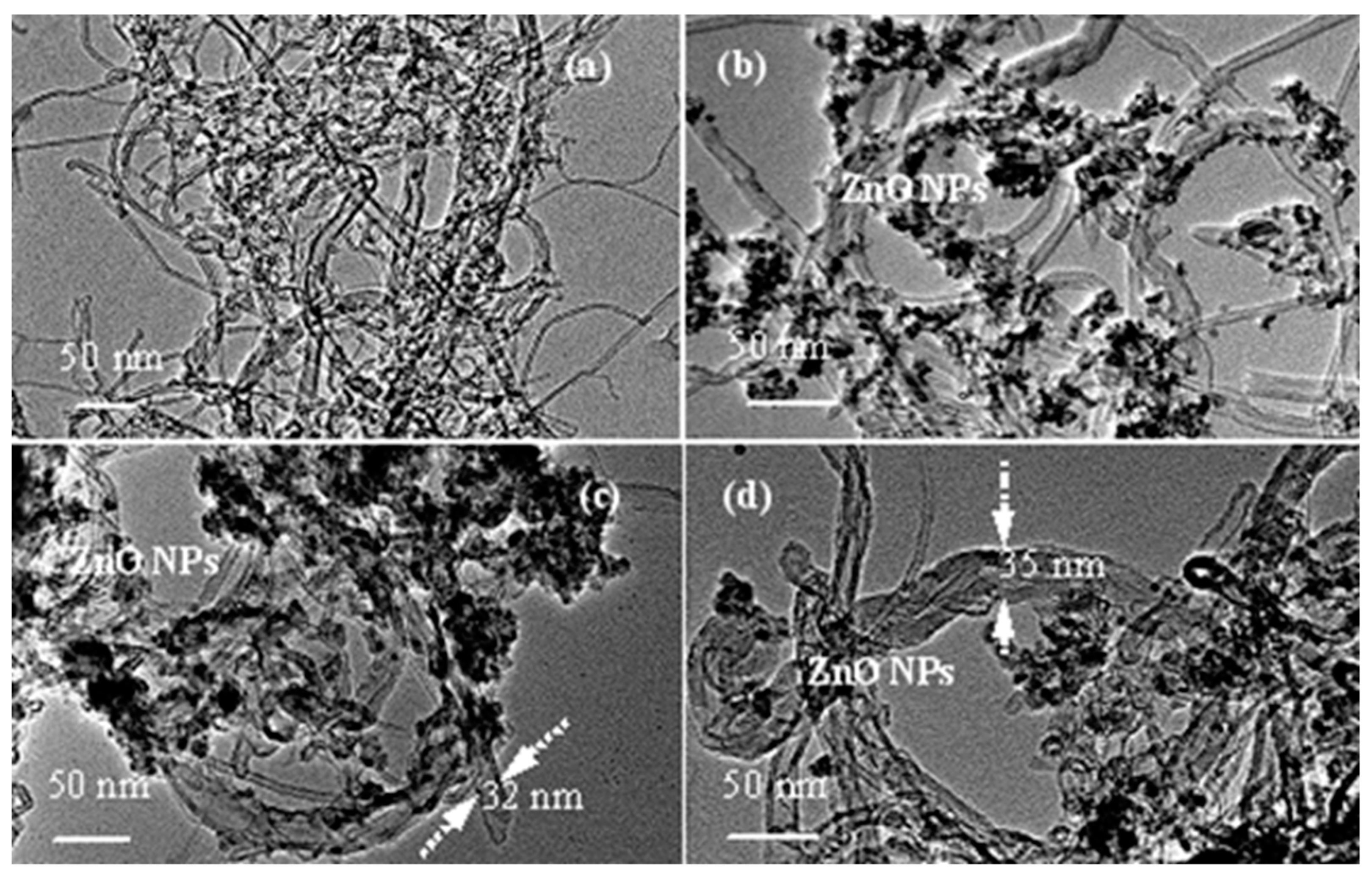
3.2. Morphologies of MWCNTs@ZnO/PMAEFc Nanohybrids
3.3. Electrochemical Characteristics
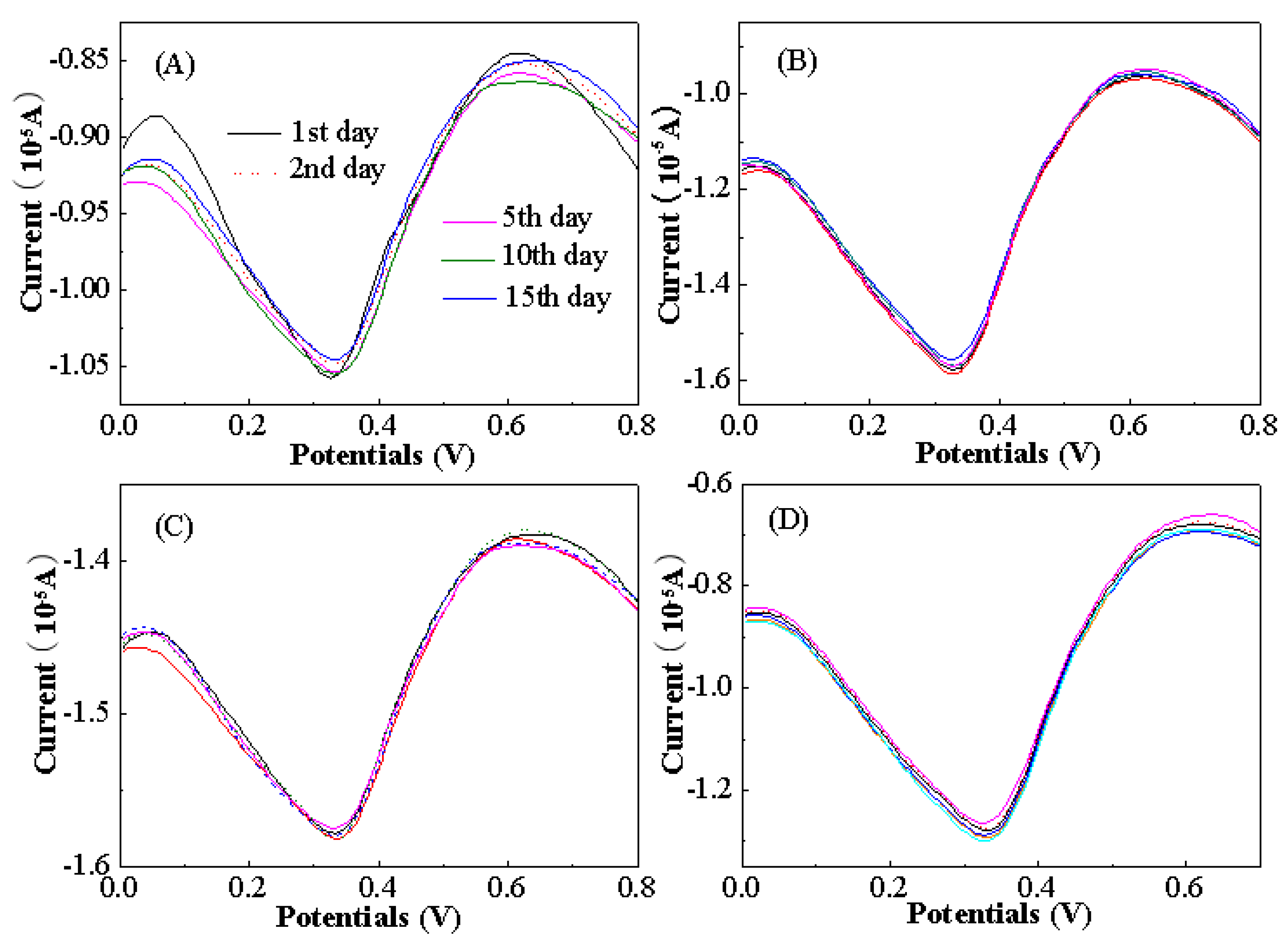
3.4. Applications of the Modified Electrochemical Sensors
3.5. Quantitative Detection Analysis of Aspartame
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rencuzogullari, E.; Tuylu, B.A.; Topaktas, M.; Ila, H.B.; Kayraldiz, A.; Arslan, M. Genotoxicity of Aspartame. Drug Chem. Toxicol. 2004, 27, 257–268. [Google Scholar] [CrossRef]
- Lin, H.; Oturan, N.H.; Wu, J.; Sharma, V.K.; Zhang, H. Removal of Artificial Sweetener Aspartame from Aqueous Media by Electrochemical Advanced Oxidation Processes. Chemosphere 2017, 167, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, A.; Tarannum, N. Water-compatible Aspartame-imprinted Polymer Grafted on Silica Surface for Selective Recognition in Aqueous Solution. Anal. Bioanal. Chem. 2013, 405, 4245–4252. [Google Scholar] [CrossRef]
- Stenberg, M.; Marko-Varga, G.; Öste, R. Racemization of Amino Acids During Classical and Microwave Oven Hydrolysis–application to Aspartame and A Maillard Reaction System. Food Chem. 2001, 74, 217–224. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Chen, S. A Facile HPLC Method for Optical Purity and Quantitative Measurements of Phenylalanine from the Hydrolyzed Aspartame Under Different pH and Temperature after its Derivatization with A Fluorescent Reagent. Amino Acids 2007, 33, 123–128. [Google Scholar]
- Cheng, C.; Wu, S.C. Analysis of the Enzymatic Racemization of D-aspartic Acid to L-aspartic Acid by the On-line Coupling of a Solid-phase Extraction Column and A Ligand-exchange High-performance Liquid Chromatography Column. J. Chromatogr. A 2000, 896, 299–310. [Google Scholar] [CrossRef]
- Stojkovic, M.; Mai, T.D.; Hauser, P.C. Determination of Artificial Sweeteners by Capillary Electrophoresis with Contactless Conductivity Detection Optimized by Hydrodynamic Pumping. AnalChim. Acta 2013, 787, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, A.B.; Fracassi da Silva, J.A.; de Jesus, D.P. Simultaneous Determination of Aspartame, Cyclamate, Saccharin and Acesulfame-K in Soft Drinks and Tabletop Sweetener Formulations by Capillary Electrophoresis with Capacitively Coupled Contactless Conductivity Detection. Food Chem. 2011, 124, 1714–1717. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, S.C. Simultaneous Analysis of Aspartame and its Hydrolysis Products of Coca–Cola Zero by On-line Post Column Derivation Fluorescence Detection and Ultraviolet Detection Coupled Two-dimensional High-performance Liquid Chromatography. J. Chromatogr. A 2011, 1218, 2976–2983. [Google Scholar] [CrossRef]
- Odac, D.; Timur, S.; Telefoncu, A. Carboxyl Esterase-alcohol Oxidase Based Biosensor for the Aspartame Determination. Food Chem. 2004, 84, 493–496. [Google Scholar] [CrossRef]
- Choi du, H.; Kim, N.A.; Nam, T.S.; Lee, S.; Jeong, S.H. Evaluation of Taste-Masking Effects of Pharmaceutical Sweeteners with an Electronic Tongue System. Drug. Dev. Ind. Pharm. 2014, 40, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Migon, D.; Gębicki, J.; Kamysz, W. Critical Review of Electronic Nose and Tongue Instruments Prospects in Pharmaceutical Analysis. Anal. Chim. Acta 2019, 1077, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Elrouby, M. Electrochemical Applications of Carbon Nanotube. J. Nanotechnol. Adv. Mat. 2013, 1, 23–38. [Google Scholar]
- Babaei, A.; Taheri, A.R. Nafion/Ni(OH)2 Nanoparticles-carbon Nanotube Composite Modified Glassy Carbon Electrode as a Sensor for Simultaneous Determination of Dopamine and Serotonin in the Presence of Ascorbic Acid. Sens. Actuator B 2013, 176, 543–551. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Zhai, X.; Yang, X.; Zhao, H.; Wang, J.; Dong, A.; Wang, Z. Application of β-cyclodextrin–reduced grapheme oxide nanosheets for enhanced electrochemical sensing of the nitenpyram residue in real samples. New J. Chem. 2017, 41, 2169–2177. [Google Scholar] [CrossRef]
- Gao, F.; Fan, T.; Ou, S.; Wu, J.; Zhang, X.; Luo, J.; Li, N.; Yao, Y.; Mou, Y.; Liao, X.; et al. Highly Ffficient Electrochemical Sensing Platform for Sensitive Detection DNA Methylation, and Methyltransferase Activity Based on Ag NPs Decorated Carbon Nanocubes. Biosens. Bioelectron. 2018, 99, 201–208. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Li, J.; Kuang, X.; Fan, Y.; Wei, G.; Su, Z. Electrostatic Assembly of Peptide Nanofiber−Biomimetic Silver Nanowires onto Graphene for Electrochemical Sensors. Acs Macro Lett. 2014, 3, 529–533. [Google Scholar] [CrossRef]
- Wang, N.; Lin, M.; Dai, H.; Ma, H. Functionalized Gold Nanoparticles/Reduced Graphene Oxide Nanocomposites for Ultrasensitive Electrochemical Sensing of Mercury Ions Based on Thymine–Mercury–Thymine structure. Biosens. Bioelectron. 2016, 79, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Bussy, C.; Boucetta, H.A.; Kostarelos, K. Safety Considerations for Graphene: Lessons Learnt from Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 692–701. [Google Scholar] [CrossRef]
- Bathinapatla, A.; Kanchi, S.; Singh, P.; Sabela, M.I.; Bisetty, K. Fabrication of Copper Nanoparticles Decorated Multiwalled Carbon Nanotubes as a High Performance Electrochemical Sensor for the Detection of Neotame. Biosens. Bioelectron. 2015, 67, 200–207. [Google Scholar] [CrossRef]
- Shobha Jeykumari, D.R.; Raman, K.; Narayanan, S. Nanobiocomposite Electrochemical Biosensor Utilizing Synergic Action of Neutral Red Functionalized Carbon Nanotubes. Micro Nano Lett. 2012, 4, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Xu, J.; Liu, J.; Wang, X.; Chen, B. Disease-Related Detection with Electrochemical Biosensors: A Review. Sensors 2017, 17, 2375. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A Review on Graphene-Based Nanocomposites for Electrochemical and Fluorescent Biosensors. Rsc Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Hajnorouzi, A.; Afzalzadeh, R.; Ghanati, F. Ultrasonic Irradiation Effects on Electrochemical Synthesis of ZnO Nanostructures. Ultrason. Sonochem. 2014, 21, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, A. Nanoparticle Catalysts. J. Phys. D. Appl. Phys. 2009, 42, 233001. [Google Scholar] [CrossRef]
- Fang, X.; Liu, J.; Wang, J.; Zhao, H.; Ren, H.; Lia, Z. Dual Signal Amplification Strategy of Au Nanopaticles/ZnO Nanorods Hybridized Reduced Graphene Nanosheet and Multienzyme Functionalized Au@ZnO Composites for Ultrasensitive Electrochemical Detection of Tumor Biomarker. Biosens. Bioelectron. 2017, 97, 218–225. [Google Scholar] [CrossRef]
- Chazalviel, J.N.; Allongue, P. On the Origin of the Efficient Nanoparticle Mediated Electron Transfer Across A Self-assembled Monolayer. J. Am. Chem. Soc. 2011, 133, 762–764. [Google Scholar] [CrossRef]
- Balgobind, K.; Kanchi, S.; Sharma, D.; Bisetty, K.; Sabela, M.I. Hybrid of ZnONPs/MWCNTs for Electrochemical Detection of Aspartame in Food and Beverage Samples. J. Electroanal. Chem. 2016, 774, 51–57. [Google Scholar] [CrossRef]
- Rabti, A.; Raouafi, N.; Merkoçi, A. Bio (Sensing) Devices Based on Ferroceneefunctionalized Graphene and Carbon Nanotubes. Carbon 2016, 108, 481–514. [Google Scholar] [CrossRef]
- Fatoni, A.; Numnuam, A.; Kanatharana, P.; Limbut, W.; Thammakhet, C.; Thavarungkul, P. A Highly Stable Oxygen-independent Glucose Biosensor Based on A Chitosan-albumin Cryogel Incorporated with Carbon Nanotubes and Ferrocene. Sens. Actuators B 2013, 185, 725–734. [Google Scholar] [CrossRef]
- Qiu, J.D.; Xiong, M.; Liang, R.P.; Peng, H.P.; Liu, F. Synthesis and Characterization of Ferrocene Modified Fe3O4@Au Magnetic Nanoparticles and its Application. Biosens. Bioelectron. 2009, 24, 2649–2653. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Dong, W.F.; Hempenius, M.A.; Möhwald, H.; Vancso, G.J. Redox-controlled Molecular Permeability of Composite-wall Microcapsules. Nat. Mater. 2006, 5, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Xu, L.; Yan, D.X.; Li, Z.M. Conductive Polymer Composites with Segregated Structures. Prog. Polym. Sci. 2014, 39, 1908–1933. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ahanjan, K.; Taghavi, M.; Ghaemy, M. A Novel Voltammetric Sensor Employing Zinc Oxide Nanoparticles and A New Ferrocene-derivative Modified Carbon Paste Electrode for Determination of Captopril in Drug Samples. Anal. Methods 2016, 8, 1780–1788. [Google Scholar] [CrossRef]
- Sonker, R.K.; Singh, M.; Kumar, U.; Yadav, B.C. MWCNT Doped ZnO Nanocomposite Thin Film as LPG Sensing. J. Inorg. Organomet. Polym. 2016, 26, 1434–1440. [Google Scholar] [CrossRef]
- Sarkara, D.; Ghosha, C.K.; Chattopadhyay, K.K. Carbon Doped ZnO Thin Film: Unusual Nonlinear Variation in Band Gap and Electrical Characteristic. Appl. Surf. Sci. 2017, 418, 252–257. [Google Scholar] [CrossRef]
- Sonker, R.K.; Sharma, A.; Shahabuddin, M.; Tomar, M.; Gupta, M. Low Temperature Sensing of NO2 Gas Using SnO2-ZnO Nanocomposite Sensor. Adv. Mater. Lett. 2013, 4, 196–201. [Google Scholar] [CrossRef]
- Scheibe, B.; Borowiak-Palen, E.; Kalenczuk, R.J. Oxidation and Reduction of Multiwalled Carbon Nanotubes-preparation and Characterization. Mater. Charact. 2010, 61, 185–191. [Google Scholar] [CrossRef]
- Nithya, A.; Jothivenkatachalam, K. Chitosan Assisted Synthesis of ZnO Nanoparticles: An Efficient Solar Light Driven Photocatalyst and Evaluation of Antibacterial Activity. J. Mater. Sci. Mater. Electron. 2015, 26, 10207–10216. [Google Scholar] [CrossRef]
- Alimohammady, M.; Jahangiria, M.; Kianib, F.; Tahermansouri, H. A New Modified MWCNTs with 3-aminopyrazole as A Nanoadsorbent for Cd (II) Removal from Aqueous Solutions. J. Environ. Chem. Eng. 2017, 5, 3405–3417. [Google Scholar] [CrossRef]
- Teng, Y.Q.; Zhang, X.A.; Fu, Y.; Liu, H.J.; Wang, Z.C.; Jin, L.T.; Zhang, W. Optimized Ferrocene-functionalized ZnO Nanorods for Signal Amplification in Electrochemical Immunoassay of Escherichia Coli. Biosens. Bioelectron. 2011, 26, 4661–4666. [Google Scholar] [CrossRef]
- Xiao, F.J.; Luo, Y.J. Ferrocene Containing Hyperbranched Polyester: Structure and Catalytic Performance for Thermal Decomposition of Ammonium Perchlorate. Adv. Mater. Res. 2011, 197, 1225–1230. [Google Scholar] [CrossRef]
- Feng, Q.; Tang, D.; Lv, H.; Zhang, W.; Li, W. Surface-initiated ATRP to Modify ZnO Nanoparticles with Poly(N-isopropylacrylamide): Temperature-controlled Switching of Photocatalysis. J. Alloy Compd. 2017, 69, 185–194. [Google Scholar] [CrossRef]
- Vanaja, A.; Rao, K.S. Effect of Co Doping on Structural and Optical Properties of Zinc Oxide Nanoparticles Synthesized by Sol-sel Method. Adv. Naonoparticles 2015, 5, 83–89. [Google Scholar] [CrossRef]
- Jiang, B.B.; Han, C.P.; Li, B.; He, Y.J.; Lin, Z.Q. In-situ Crafting of ZnFe2O4 Nanoparticles Impregnated within Continuous Carbon Network as Advanced Anode Materials. Acs Nano 2016, 23, 2728–2735. [Google Scholar] [CrossRef]
- Liao, N.; Lia, Y.W.; Jin, S.L.; Sang, S.B.; Liu, G.F. Reduced Brittleness of Multi-walled Carbon Nanotubes (MWCNTs) Containing Al2O3-C Refractories with Boron Carbide. Mater. Sci. Eng. A 2017, 698, 80–87. [Google Scholar] [CrossRef]
- Zhang, C.H.; Wang, G.F.; Ji, Y.L.; Liu, M. Enhancement in Analytical Hydrazine Based on Gold Nanoparticles Deposited on ZnO-MWCNTs Films. Sens. Actuators B 2010, 150, 247–253. [Google Scholar] [CrossRef]
- Zou, Y.L.; Qi, Z.G.; Jiang, W.J.; Duan, J.L.; Ma, Z.S. MWCNTs Enhanced ZnO Nanoparticles as Anode for Lithium Ion Batteries. Mater. Lett. 2017, 199, 57–60. [Google Scholar] [CrossRef]
- Ren, Y.; Mao, X.; Hatton, T.A. An Asymmetric Electrochemical System with Complementary Tunability in Hydrophobicity for Selective Separations of Organics. Acs Cent. Sci. 2019, 58, 1396–1406. [Google Scholar] [CrossRef]
- Saleem, M.; Yu, H.; Wang, L.; Abdin, Z.; Khalid, H.; Akram, M.; Abbasi, N.M.; Huang, J. Review on Synthesis of Ferrocene-based Redox Polymers and Derivatives and Their Application in Glucose Sensing. Anal. Chim. Acta 2015, 876, 9–25. [Google Scholar] [CrossRef]
- Niu, X.; Mo, Z.; Yang, X.; Shuai, C.; Liu, N.; Guo, R. Graphene-ferrocene Functionalized Cyclodextrin Composite with High Electrochemical Recognition Capability for Phenylalanine Enantiomers. Bioelectrochemistry 2019, 128, 74–82. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.J. Effect of Silver Doped MWCNTs on the Electrical Properties of Conductive MWCNTs/PMMA Thin Films. Syn. Met. 2010, 160, 123–126. [Google Scholar] [CrossRef]
- Wang, M.W. Alignment of Multiwall Carbon Nanotubes in Polymer Composites by Dielectrophoresis. Jpn. Soc. Appl. Phys. 2009, 48, 035002. [Google Scholar] [CrossRef]
- Senel, M.; Cevik, E.; Abasiyanik, M.F. Amperometric Hydrogen Peroxide Biosensor Based on Covalent Immobilization of Horseradish Peroxidase on Ferrocene Containing Polymeric Mediator. Sens. Actuators B 2010, 145, 444–450. [Google Scholar] [CrossRef]
- Beitollahi, H.; Ivari, S.G.; Torkzadeh-Mahani, M. Voltammetric Determination of 6-thioguanine and Folic Acid Using A Carbon Paste Electrode Modified with ZnO-CuO Nanoplates and Modifier. Mater. Sci. Eng. C 2016, 69, 128–133. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Solgi, M. Sensitive Warfarin Sensor Based on Cobalt Oxide Nanoparticles Electrodeposited at Multi-walled Carbon Nanotubes Modified Glassy Carbon Electrode (CoxOyNPs/MWCNTs/GCE). Electrochim. Acta 2017, 246, 689–698. [Google Scholar] [CrossRef]
- Zare, H.R.; Namazian, M.; Coote, M.L. Experimental and Theoretical Studies of Electrochemical Characteristics of 3,4-dihydroxyphenylacetic Acid (DOPAC). Electrochim. Acta 2009, 54, 5353–5357. [Google Scholar] [CrossRef]
- Yu, C.M.; Ji, W.Y.; Wang, Y.D.; Bao, N.; Gu, H.Y. Graphene Oxide-modified Electrodes for Sensitive Determination of Diethylstilbestrol. Nanotechnology 2013, 24, 115502–115510. [Google Scholar] [CrossRef]
- Kirgöz, Ü.A.; Odaci, D.; Timur, S.; Merkoçi, A.; Alegret, S.; Beşün, N.; Telefoncu, A. A Biosensor Based on Graphite Epoxy Composite Electrode for Aspartame and Ethanol Detection. Anal. Chim. Acta 2006, 570, 165–169. [Google Scholar] [CrossRef]
- Radulescu, M.C.; Bucur, B.; Bucur, M.P.; Radu, G.L. Bienzymatic Biosensor for Rapid Detection of Aspartame by Flow Injection Analysis. Sensors 2014, 14, 1028–1038. [Google Scholar] [CrossRef] [Green Version]
- Tiu, B.D.N.; Pernites, R.B.; Tiu, S.B.; Advincula, R.C. Detection of Aspartame via Microsphere-Patterned and Molecularly Imprinted Polymer Arrays. Colloid. Surf. A 2016, 495, 149–158. [Google Scholar] [CrossRef]
- De Carvalho, R.C.; Netto, A.D.P.; Marques, F.F.d.C. Simultaneous Determination of Strontium Ranelate and Aspartame in Pharmaceutical Formulation for the Treatment of Postmenopausal Osteoporosis by Capillary Zone Electrophoresis. Microchem. J. 2014, 117, 214–219. [Google Scholar] [CrossRef]
- Medeiros, R.A.; de Carvalho, A.E.; Rocha-Filho, R.C.; Filho, O.F. Simultaneous Square-wave Voltammetric Determination of Aspartame and Cyclamate Using A Boron-doped Diamond Electrode. Talanta 2008, 76, 685–689. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. FTIR Determination of Aspartame and Acesulfame-K in Tabletop Sweeteners. J. Agric. Food Chem. 2004, 52, 7798–7803. [Google Scholar] [CrossRef]
- Herzog, G.; Kam, V.; Berduque, A.; Arrigan, D.W.M. Detection of food additives by voltammetry at the liquid-liquid interface. J. Agric. Food Chem. 2008, 56, 4304–4310. [Google Scholar] [CrossRef]
- Kokotou, M.G.; Thomaidis, N.S. Determination of eight artificial sweeteners in wastewater by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal. Methods 2013, 5, 3825–3833. [Google Scholar] [CrossRef]
- Fernandes, V.N.O.; Fernandes, L.B.; Vasconcellos, J.P.; Jager, A.V.; Tonin, F.G.; de Oliveira, M.A.L. Simultaneous analysis of aspartame, cyclamate, saccharin and acesulfame-K by CZE under UV detection. Anal. Methods 2013, 5, 1524–1532. [Google Scholar] [CrossRef]
- Deroco, P.B.; Medeiros, R.A.; Rocha-Filho, R.C.; Fatibello-Filho, O. Simultaneous voltammetric determination of aspartame and acesulfame-K in food products using an anodically pretreated boron-doped diamond electrode. Anal. Methods 2015, 7, 2135–2140. [Google Scholar] [CrossRef]



| Electrode/Method | LOD | RSD | Real Samples | Aspartame Content | Citation |
|---|---|---|---|---|---|
| Bienzymatic (AOX and CaE) biosensor/FIA | 0.2 μM | 2.7% | Pepsi Max no sugar | 129 ± 5.2 mg L−1 | [60] |
| Pepsi Light | 152 ± 7.0 mg L−1 | ||||
| Coca-Cola | 58 ± 2.2~82 ± 3.1 mg L−1 | ||||
| Erdomed 175 and 225 | 466 ± 19~7512 ± 288 mg L−1 | ||||
| Polyterthiophene film sensor/SMIT | 31 μM | NR | NR * | NR | [61] |
| ZnONPs/MWCNTs/GCE/DPV | 3.68 μM | NR | Coke Zero, Huletts and Sprite Zero | 40.36, 27.92 and 44.30 μM | [28] |
| Capillary zone electrophoresis (CZE) | 0.3 μg mL−1 | <2.6% | Protos | 5 μg ml−1 | [62] |
| Boron-doped diamond (BDD) electrode/SWV | 3.5 × 10−7 M | 1.3% | Powder juice drink | 6.8 mg 100 mL−1 | [63] |
| Carbonated guaraná drink | 12.0 mg 100 mL−1 | ||||
| FTIR | 0.10% | 0.17% | NR | NR | [64] |
| DPV | 30 μM | NR | A tabletop sweetener | 20.8 ± 0.2 mg g−1 | [65] |
| Sugar-free cola A | 502 ± 26 mg L−1 | ||||
| Sugar-free cola B | 1083 ± 176 mg L−1 | ||||
| Sugar-free lemonade A, B, and C | 466 ± 125~1175 ± 124 mg L−1 | ||||
| A sugar-free apple juice | 1496 ± 217 mg L−1 | ||||
| HILIC | 1.6 μg L−1 | 10% | Wastewater | 0 | [66] |
| CZE-UV | 6.8 mg L−1 | <4.0% | Lemon tea sachet | NR | [67] |
| Anodically pretreated boron-doped diamond electrode/DPV | 1.60 μM | ≤2.9% | Carbonated drinks | 22 ± 2~35 ± 1 mg 100 mL−1 | [68] |
| Powder juice drink 1 | 44 ± 4 mg 100 mL−1 | ||||
| Tabletop sweetener 1 | 7.0 ± 0.3 mg 100 mL−1 | ||||
| MWCNTs@ZnO/PMAEFcGCE/DPV | 1.35 × 10−9 M | 1.7% | Coke Zero | 35.36 ± 0.01 μM (1.05 ± 0.00 mg 100 mL−1) | This work |
| Sprite Zero | 40.20 ± 0.03 μM (1.18 ± 0.01 mg 100 mL−1) | ||||
| Mango juice | 0.95 ± 0.02 mM (27.90 ± 0.53 mg 100 mL−1) | ||||
| Kiwi fruit juice | 1.33 ± 0.01mM (39.41 ± 0.35 mg 100 mL−1) | ||||
| Strawberry juice | 0.88 ± 0.02 mM (25.79 ± 0.67 mg 100 mL−1) | ||||
| Lipton lipya tea sachet | 1.53 ± 0.04 mM (0.45 ± 0.01 mg 100 mL−1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.-W.; Cui, Z.-M.; Liu, Z.-Q.; Xu, F.; Chen, Y.-S.; Luo, Y.-L. Organic–Inorganic Nanohybrid Electrochemical Sensors from Multi-Walled Carbon Nanotubes Decorated with Zinc Oxide Nanoparticles and In-Situ Wrapped with Poly(2-methacryloyloxyethyl ferrocenecarboxylate) for Detection of the Content of Food Additives. Nanomaterials 2019, 9, 1388. https://doi.org/10.3390/nano9101388
Xu J-W, Cui Z-M, Liu Z-Q, Xu F, Chen Y-S, Luo Y-L. Organic–Inorganic Nanohybrid Electrochemical Sensors from Multi-Walled Carbon Nanotubes Decorated with Zinc Oxide Nanoparticles and In-Situ Wrapped with Poly(2-methacryloyloxyethyl ferrocenecarboxylate) for Detection of the Content of Food Additives. Nanomaterials. 2019; 9(10):1388. https://doi.org/10.3390/nano9101388
Chicago/Turabian StyleXu, Jing-Wen, Zhuo-Miao Cui, Zhan-Qing Liu, Feng Xu, Ya-Shao Chen, and Yan-Ling Luo. 2019. "Organic–Inorganic Nanohybrid Electrochemical Sensors from Multi-Walled Carbon Nanotubes Decorated with Zinc Oxide Nanoparticles and In-Situ Wrapped with Poly(2-methacryloyloxyethyl ferrocenecarboxylate) for Detection of the Content of Food Additives" Nanomaterials 9, no. 10: 1388. https://doi.org/10.3390/nano9101388