Neurotoxicity of Nanomaterials: An Up-to-Date Overview
Abstract
:1. Introduction
2. Toxicity Assessment of Nanomaterials
2.1. Nanomaterial Characterization
2.2. In Vitro Studies
2.3. In Vivo Studies
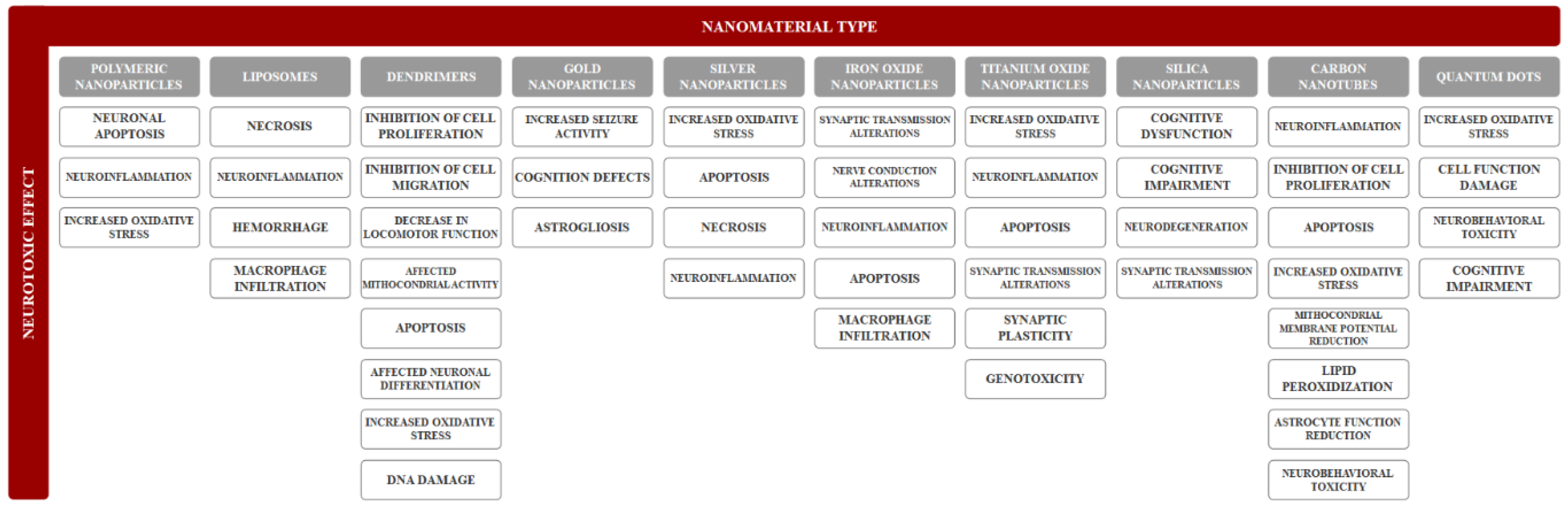
3. Neurotoxicity of Nanomaterials
3.1. Organic Nanomaterials
3.1.1. Polymeric Nanoparticles
3.1.2. Liposomes
3.1.3. Dendrimers
3.2. Inorganic Nanomaterials
3.2.1. Inorganic Nanoparticles
3.2.2. Carbon Nanotubes
3.2.3. Quantum Dots
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kargozar, S.; Mozafari, M. Nanotechnology and nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Faisal, N.; Kumar, K. Polymer and metal nanocomposites in biomedical applications. Biointerface Res. Appl. Chem. 2017, 7, 2286–2294. [Google Scholar]
- Ramsden, J.J. Chapter 1—What is nanotechnology? In Applied Nanotechnology, 3rd ed.; Ramsden, J.J., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 3–13. [Google Scholar]
- Husain, Q. Nanosupport bound lipases their stability and applications. Biointerface Res. Appl. Chem. 2017, 7, 2194–2216. [Google Scholar]
- Kaphle, A.; Navya, P.N.; Umapathi, A.; Daima, H.K. Nanomaterials for agriculture, food and environment: Applications, toxicity and regulation. Environ. Chem. Lett. 2018, 16, 43–58. [Google Scholar] [CrossRef]
- Silva, G.A. Neuroscience nanotechnology: Progress, opportunities and challenges. Nat. Rev. Neurosci. 2006, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Pathakoti, K.; Manubolu, M.; Hwang, H.-M. Chapter 48—Nanotechnology applications for environmental industry. In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 894–907. [Google Scholar]
- Ayodele, A.T.; Valizadeh, A.; Adabi, M.; Esnaashari, S.S.; Madani, F.; Khosravani, M.; Adabi, M. Ultrasound nanobubbles and their applications as theranostic agents in cancer therapy: A review. Biointerface Res. Appl. Chem. 2017, 7, 2253–2262. [Google Scholar]
- Higa, A.M.; Mambrini, G.P.; Hausen, M.; Strixino, F.T.; Leite, F.L. Ag-nanoparticle-based nano-immunosensor for anti-glutathione s-transferase detection. Biointerface Res. Appl. Chem. 2016, 6, 1053–1058. [Google Scholar]
- Melo, A.; Amadeu, M.S.; Lancellotti, M.; Hollanda, L.M.d.; Machado, D. The role of nanomaterials in cosmetics: National and international legislative aspects. Química Nova 2015, 38, 599–603. [Google Scholar] [CrossRef]
- Boverhof, D.R.; Bramante, C.M.; Butala, J.H.; Clancy, S.F.; Lafranconi, M.; West, J.; Gordon, S.C. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. RTP 2015, 73, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Sudha, P.N.; Sangeetha, K.; Vijayalakshmi, K.; Barhoum, A. Chapter 12—Nanomaterials history, classification, unique properties, production and market. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Barhoum, A., Makhlouf, A.S.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 341–384. [Google Scholar]
- Karak, N. Chapter 1—Fundamentals of nanomaterials and polymer nanocomposites. In Nanomaterials and Polymer Nanocomposites; Karak, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–45. [Google Scholar]
- Bolocan, A.; Mihaiescu, D.E.; Andronescu, E.; Voicu, G.; Grumezescu, A.M.; Ficai, A.; Vasile, B.Ş.; Bleotu, C.; Chifiriuc, M.C.; Pop, C.S. Biocompatible hydrodispersible magnetite nanoparticles used as antibiotic drug carriers. Roman. J. Morphol. Embryol. 2015, 56, 365–370. [Google Scholar]
- Balaure, P.C.; Popa, R.A.; Grumezescu, A.M.; Voicu, G.; Rădulescu, M.; Mogoantă, L.; Bălşeanu, T.A.; Mogoşanu, G.D.; Chifiriuc, M.C.; Bleotu, C.; et al. Biocompatible hybrid silica nanobiocomposites for the efficient delivery of anti-staphylococcal drugs. Int. J. Pharm. 2016, 510, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Fufă, M.O.M.; Mihaiescu, D.E.; Mogoantă, L.; Bălşeanu, T.A.; Mogoşanu, G.D.; Grumezescu, A.M.; Bolocan, A. In vivo biodistribution of cntss using a balb/c mouse experimental model. Roman. J. Morphol. Embryol. 2015, 56, 1481–1493. [Google Scholar]
- Qiu, C.; Bennet, K.E.; Tomshine, J.R.; Hara, S.; Ciubuc, J.D.; Schmidt, U.; Durrer, W.G.; McIntosh, M.B.; Eastman, M.; Manciu, F.S. Ultrasensitive detection of neurotransmitters by surface enhanced raman spectroscopy for biosensing applications. Biointerface Res. Appl. Chem. 2017, 7, 1921–1926. [Google Scholar]
- Saleh, T.A.; Gupta, V.K. Chapter 4—Synthesis, classification, and properties of nanomaterials. In Nanomaterial and Polymer Membranes; Saleh, T.A., Gupta, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 83–133. [Google Scholar]
- Gao, H.; Jiang, X. Chapter 1—The medical applications of nanomaterials in the central nervous system. In Neurotoxicity of Nanomaterials and Nanomedicine; Jiang, X., Gao, H., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–31. [Google Scholar]
- Siddiqi, K.S.; Husen, A.; Sohrab, S.S.; Yassin, M.O. Recent status of nanomaterial fabrication and their potential applications in neurological disease management. Nanoscale Res. Lett. 2018, 13, 231. [Google Scholar] [CrossRef]
- Veloz-Castillo, M.F.; West, R.M.; Cordero-Arreola, J.; Arias-Carrion, O.; Mendez-Rojas, M.A. Nanomaterials for neurology: State-of-the-art. CNS Neurol. Disord. Drug Targets 2016, 15, 1306–1324. [Google Scholar] [CrossRef]
- Omrani, M.M.; Ansari, M.; Kiaie, N. Therapeutic effect of stem cells and nano-biomaterials on alzheimer’s disease. Biointerface Res. Appl. Chem. 2016, 6, 1814–1820. [Google Scholar]
- Huang, L.; Hu, J.; Huang, S.; Wang, B.; Siaw-Debrah, F.; Nyanzu, M.; Zhang, Y.; Zhuge, Q. Nanomaterial applications for neurological diseases and central nervous system injury. Prog. Neurobiol. 2017, 157, 29–48. [Google Scholar] [CrossRef]
- Shafiee, M.R.M.; Kargar, M. Preparation of aryl sulfonamides using cuo nanoparticles prepared in extractive rosmarinus officinalis leaves media. Biointerface Res. Appl. Chem. 2016, 6, 1257–1262. [Google Scholar]
- Xiong, J.; Gao, H. Neurotoxicity of nanomaterials: Where are we and what can we do? EC Pharmacol. Toxicol. 2017, 4, 93. [Google Scholar]
- Liu, Y.; He, Q. Chapter 2—The route of nanomaterials entering brain. In Neurotoxicity of Nanomaterials and Nanomedicine; Jiang, X., Gao, H., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 33–57. [Google Scholar]
- Gao, H.; Jiang, X. Introduction and overview. In Neurotoxicity of Nanomaterials and Nanomedicine; Jiang, X., Gao, H., Eds.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Li, J.; Martin, F.L. Chapter 4—Current perspective on nanomaterial-induced adverse effects: Neurotoxicity as a case example. In Neurotoxicity of Nanomaterials and Nanomedicine; Jiang, X., Gao, H., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 75–98. [Google Scholar]
- Xiaoli, F.; Longquan, S. Chapter 20—Neurotoxicity of nanomaterials. In Emerging Nanotechnologies in Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 421–444. [Google Scholar]
- Bencsik, A.; Lestaevel, P.; Guseva Canu, I. Nano- and neurotoxicology: An emerging discipline. Prog. Neurobiol. 2018, 160, 45–63. [Google Scholar] [CrossRef]
- Karmakar, A.; Zhang, Q.; Zhang, Y. Neurotoxicity of nanoscale materials. J. Food Drug Anal. 2014, 22, 147–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shvedova, A.; Pietroiusti, A.; Kagan, V. Nanotoxicology ten years later: Lights and shadows. Toxicol. Appl. Pharmacol. 2016, 299, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Qin, Z.; Zeng, W.; Yang, T.; Cao, Y.; Mei, C.; Kuang, Y. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol. Rev. 2017, 6, 279. [Google Scholar] [CrossRef]
- Fadeel, B.; Fornara, A.; Toprak, M.S.; Bhattacharya, K. Keeping it real: The importance of material characterization in nanotoxicology. Biochem. Biophys. Res. Commun. 2015, 468, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K. Chapter 1—Introduction to nanoparticles and nanotoxicology. In Engineered Nanoparticles; Academic Press: Boston, MA, USA, 2016; pp. 1–18. [Google Scholar]
- Blough, E.R. Nanomedicine in Drug Delivery; Taylor & Francis Group: Abingdon, UK, 2017. [Google Scholar]
- Van der Merwe, D.; Pickrell, J.A. Chapter 18—Toxicity of nanomaterials. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 319–326. [Google Scholar]
- Dusinska, M.; Tulinska, J.; El Yamani, N.; Kuricova, M.; Liskova, A.; Rollerova, E.; Rundén-Pran, E.; Smolkova, B. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing? Food Chem. Toxicol. 2017, 109, 797–811. [Google Scholar] [CrossRef] [Green Version]
- Salame, P.H.; Pawade, V.B.; Bhanvase, B.A. Chapter 3—Characterization tools and techniques for nanomaterials. In Nanomaterials for Green Energy; Bhanvase, B.A., Pawade, V.B., Dhoble, S.J., Sonawane, S.H., Ashokkumar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 83–111. [Google Scholar]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Saifi, M.A.; Khurana, A.; Godugu, C. Chapter 17—Nanotoxicology: Toxicity and risk assessment of nanomaterials*equal contribution. In Nanomaterials in Chromatography; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 437–465. [Google Scholar]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.K.; Singh, D.; Dubey, K.; Maurya, R.; Mittal, S.; Pandey, A.K. Chapter 3—Models and methods for in vitro toxicity. In In Vitro Toxicology; Dhawan, A., Kwon, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 45–65. [Google Scholar]
- Mihailoff, G.A.; Haines, D.E. Chapter 2—The cell biology of neurons and glia. In Fundamental Neuroscience for Basic and Clinical Applications, 5th ed.; Haines, D.E., Mihailoff, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 15–33. [Google Scholar]
- Stadelmann, C.; Wegner, C.; Bruck, W. Inflammation, demyelination, and degeneration—Recent insights from ms pathology. Biochim. Biophys. Acta 2011, 1812, 275–282. [Google Scholar] [CrossRef]
- Figueroa-González, G.; Pérez-Plasencia, C. Strategies for the evaluation of DNA damage and repair mechanisms in cancer. Oncol. Lett. 2017, 13, 3982–3988. [Google Scholar] [CrossRef] [Green Version]
- Romar, G.A.; Kupper, T.S.; Divito, S.J. Research techniques made simple: Techniques to assess cell proliferation. J. Investig. Dermatol. 2016, 136, e1–e7. [Google Scholar] [CrossRef]
- Poduri, A.; Volpe, J.J. Chapter 5—Neuronal proliferation. In Volpe’s Neurology of the Newborn, 6th ed.; Volpe, J.J., Inder, T.E., Darras, B.T., de Vries, L.S., du Plessis, A.J., Neil, J.J., Perlman, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 100–119. [Google Scholar]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méry, B.; Guy, J.-B.; Vallard, A.; Espenel, S.; Ardail, D.; Rodriguez-Lafrasse, C.; Rancoule, C.; Magné, N. In vitro cell death determination for drug discovery: A landscape review of real issues. J. Cell Death 2017. [Google Scholar] [CrossRef]
- Blanco, A.; Blanco, G. Chapter 32—Apoptosis. In Medical Biochemistry; Blanco, A., Blanco, G., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 791–796. [Google Scholar]
- Davila, J.C.; Levin, S.; Radi, Z.A. Chapter 8.21—Cell injury and necrosis. In Comprehensive Toxicology, 3rd ed.; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2018; pp. 404–453. [Google Scholar]
- Mitov, M.I.; Patil, V.S.; Alstott, M.C.; Dziubla, T.; Butterfield, D.A. Chapter 6—In vitro cellular assays for oxidative stress and biomaterial response. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 145–186. [Google Scholar]
- Lovisolo, D.; Dionisi, M.; Ruffinatti, F.A.; Distasi, C. Nanoparticles and potential neurotoxicity: Focus on molecular mechanisms. AIMS Mol. Sci. 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Dusinska, M.; Rundén-Pran, E.; Schnekenburger, J.; Kanno, J. Chapter 3—Toxicity tests: In vitro and in vivo. In Adverse Effects of Engineered Nanomaterials, 2nd ed.; Fadeel, B., Pietroiusti, A., Shvedova, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 51–82. [Google Scholar]
- Al-Lamki, R.S.; Bradley, J.R.; Pober, J.S. Human organ culture: Updating the approach to bridge the gap from in vitro to in vivo in inflammation, cancer, and stem cell biology. Front. Med. 2017, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, J.; Ju, D. Chapter 15—Neurotoxicity concern about the brain targeting delivery systems. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 377–408. [Google Scholar]
- O’Brown, N.M.; J. Pfau, S.; Gu, C. Bridging barriers: A comparative look at the blood-brain barrier across organisms. Genes Dev. 2018, 32, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Luhach, K.; Kulkarni, G.T. Chapter 4—In vitro and in vivo models of bbb to evaluate brain targeting drug delivery. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 53–101. [Google Scholar]
- Sachana, M.; Hargreaves, A.J. Chapter 9—Toxicological testing: In vivo and in vitro models. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 145–161. [Google Scholar]
- Costa, L.G.; Pellacani, C.; Guizzetti, M. Chapter 14—In vitro and alternative approaches to developmental neurotoxicity. In Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 241–253. [Google Scholar]
- Teleanu, D.; Chircov, C.; Grumezescu, A.; Volceanov, A.; Teleanu, R. Impact of nanoparticles on brain health: An up to date overview. J. Clin. Med. 2018, 7, 490. [Google Scholar] [CrossRef]
- Maurizi, L.; Papa, A.-L.; Boudon, J.; Sudhakaran, S.; Pruvot, B.; Vandroux, D.; Chluba, J.; Lizard, G.; Millot, N. Toxicological risk assessment of emerging nanomaterials: Cytotoxicity, cellular uptake, effects on biogenesis and cell organelle activity, acute toxicity and biodistribution of oxide nanoparticle. In Unraveling the Safety Profile of Nanoscale Particles and Materials; Gomes, A.C., Sárria, M.P., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Singh, N.; Joshi, A.; Toor, A.P.; Verma, G. Chapter 27—Drug delivery: Advancements and challenges. In Nanostructures for Drug Delivery; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 865–886. [Google Scholar]
- Yuan, Z.-Y.; Hu, Y.-L.; Gao, J.-Q. Brain localization and neurotoxicity evaluation of polysorbate 80-modified chitosan nanoparticles in rats. PLoS ONE 2015, 10, e0134722. [Google Scholar] [CrossRef]
- Voigt, N.; Henrich-Noack, P.; Kockentiedt, S.; Hintz, W.; Tomas, J.; Sabel, B.A. Toxicity of polymeric nanoparticles in vivo and in vitro. J. Nanopart. Res. Interdiscip. Forum Nanoscale Sci. Technol. 2014, 16, 2379. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Bansod, S.; Kon, K. Chapter 9—Tackling the problem of tuberculosis by nanotechnology: Disease diagnosis and drug delivery. In Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases; Rai, M., Kon, K., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 133–149. [Google Scholar]
- Abbina, S.; Parambath, A. Chapter 14—pegylation and its alternatives: A summary. In Engineering of Biomaterials for Drug Delivery Systems; Parambath, A., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 363–376. [Google Scholar]
- Kulkarni, V.S.; Shaw, C. Chapter 4—Formulating creams, gels, lotions, and suspensions. In Essential Chemistry for Formulators of Semisolid and Liquid Dosages; Kulkarni, V.S., Shaw, C., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 29–41. [Google Scholar]
- Karandikar, S.; Mirani, A.; Waybhase, V.; Patravale, V.B.; Patankar, S. Chapter 10—Nanovaccines for oral delivery-formulation strategies and challenges. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 263–293. [Google Scholar]
- Li, S.; Johnson, J.; Peck, A.; Xie, Q. Near infrared fluorescent imaging of brain tumor with ir780 dye incorporated phospholipid nanoparticles. J. Transl. Med. 2017, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, R.N. Liposomal drug delivery to the central nervous system. In Liposomes; Catala, A., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Huo, T.; Barth, R.F.; Yang, W.; Nakkula, R.J.; Koynova, R.; Tenchov, B.; Chaudhury, A.R.; Agius, L.; Boulikas, T.; Elleaume, H.; et al. Preparation, biodistribution and neurotoxicity of liposomal cisplatin following convection enhanced delivery in normal and f98 glioma bearing rats. PLoS ONE 2012, 7, e48752. [Google Scholar] [CrossRef] [PubMed]
- Priya, L.B.; Baskaran, R.; Padma, V.V. Chapter 21—Phytonanoconjugates in oral medicine. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 639–668. [Google Scholar]
- Verma, G.; Rajagopalan, M.D.; Valluru, R.; Sridhar, K.A. Chapter 7—Nanoparticles: A novel approach to target tumors. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 113–129. [Google Scholar]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Chapter 5—Effect of polymer-based nanoparticles on the assay of antimicrobial drug delivery systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar]
- Acharya, G.; Mitra, A.K.; Cholkar, K. Chapter 10—Nanosystems for diagnostic imaging, biodetectors, and biosensors. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A.K., Cholkar, K., Mandal, A., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 217–248. [Google Scholar]
- Srinageshwar, B.; Peruzzaro, S.; Andrews, M.; Johnson, K.; Hietpas, A.; Clark, B.; McGuire, C.; Petersen, E.; Kippe, J.; Stewart, A.; et al. Pamam dendrimers cross the blood-brain barrier when administered through the carotid artery in c57bl/6j mice. Int. J. Mol. Sci. 2017, 18, 628. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.A.G.; Wu, Y.; Fischer, S.; Liu, W.; Weil, T.; Müllen, K. Controlling cellular uptake and toxicity of polyphenylene dendrimers by chemical functionalization. ChemBioChem 2017, 18, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Kurokawa, Y.; Zeng, Q.; Win-Shwe, T.T.; Nansai, H.; Zhang, Z.; Sone, H. Effects of polyamidoamine dendrimers on a 3-d neurosphere system using human neural progenitor cells. Toxicol. Sci. 2016, 152, 128–144. [Google Scholar] [CrossRef]
- Calienni, M.N.; Feas, D.A.; Igartua, D.E.; Chiaramoni, N.S.; Alonso, S.D.V.; Prieto, M.J. Nanotoxicological and teratogenic effects: A linkage between dendrimer surface charge and zebrafish developmental stages. Toxicol. Appl. Pharmacol. 2017, 337, 1–11. [Google Scholar] [CrossRef]
- Zeng, Y.; Kurokawa, Y.; Win-Shwe, T.-T.; Zeng, Q.; Hirano, S.; Zhang, Z.; Sone, H. Effects of pamam dendrimers with various surface functional groups and multiple generations on cytotoxicity and neuronal differentiation using human neural progenitor cells. J. Toxicol. Sci. 2016, 41, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Vasquez, P.; Cayuman, F.R.; Diaz, C.; Fuentealba, J.; Aguayo, L.G.; Yevenes, G.E.; Alderete, J.; Guzman, L. Prevention of synaptic alterations and neurotoxic effects of pamam dendrimers by surface functionalization. Nanomaterials 2017, 8, 7. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.; Liu, J.; Feng, X.; Zhou, T.; Shao, L. Is neurotoxicity of metallic nanoparticles the cascades of oxidative stress? Nanoscale Res. Lett. 2016, 11, 291. [Google Scholar] [CrossRef]
- Flora, S.J.S. Chapter 8—The applications, neurotoxicity, and related mechanism of gold nanoparticles. In Neurotoxicity of Nanomaterials and Nanomedicine; Jiang, X., Gao, H., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 179–203. [Google Scholar]
- Das, M.; Ghosh, M.; Gharami, K.; Das, S. Chapter twelve—Thyroid hormone and astrocyte differentiation. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 106, pp. 283–312. [Google Scholar]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Struzynska, L.; Skalska, J. Mechanisms underlying neurotoxicity of silver nanoparticles. Adv. Exp. Med. Biol. 2018, 1048, 227–250. [Google Scholar]
- Sun, C.; Yin, N.; Wen, R.; Liu, W.; Jia, Y.; Hu, L.; Zhou, Q.; Jiang, G. Silver nanoparticles induced neurotoxicity through oxidative stress in rat cerebral astrocytes is distinct from the effects of silver ions. NeuroToxicology 2016, 52, 210–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotin, G.; Piant, S.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Chapter 2—Iron oxide nanoparticles for biomedical applications: Synthesis, functionalization, and application. In Iron Oxide Nanoparticles for Biomedical Applications; Mahmoudi, M., Laurent, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 43–88. [Google Scholar]
- Magro, M.; Baratella, D.; Bonaiuto, E.; de A. Roger, J.; Vianello, F. New perspectives on biomedical applications of iron oxide nanoparticles. Curr. Med. Chem. 2018, 25, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdiglesias, V.; Fernández-Bertólez, N.; Kiliç, G.; Costa, C.; Costa, S.; Fraga, S.; Bessa, M.J.; Pásaro, E.; Teixeira, J.P.; Laffon, B. Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J. Trace Elem. Med. Biol. 2016, 38, 53–63. [Google Scholar] [CrossRef]
- Aijie, C.; Huimin, L.; Jia, L.; Lingling, O.; Limin, W.; Junrong, W.; Xuan, L.; Xue, H.; Longquan, S. Central neurotoxicity induced by the instillation of zno and tio2 nanoparticles through the taste nerve pathway. Nanomedicine 2017, 12, 2453–2470. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, Y.; Liu, J.; Feng, X.; Zhou, T.; Shao, L. Unraveling the neurotoxicity of titanium dioxide nanoparticles: Focusing on molecular mechanisms. Beilstein J. Nanotechnol. 2016, 7, 645–654. [Google Scholar] [CrossRef]
- You, R.; Ho, Y.-S.; Hung, C.H.-L.; Liu, Y.; Huang, C.-X.; Chan, H.-N.; Ho, S.-L.; Lui, S.-Y.; Li, H.-W.; Chang, R.C.-C. Silica nanoparticles induce neurodegeneration-like changes in behavior, neuropathology, and affect synapse through mapk activation. Part. Fibre Toxicol. 2018, 15, 28. [Google Scholar] [CrossRef]
- Zhou, M.; Xie, L.; Fang, C.-J.; Yang, H.; Wang, Y.-J.; Zhen, X.-Y.; Yan, C.-H.; Wang, Y.; Zhao, M.; Peng, S. Implications for blood-brain-barrier permeability, in vitro oxidative stress and neurotoxicity potential induced by mesoporous silica nanoparticles: Effects of surface modification. RSC Adv. 2016, 6, 2800–2809. [Google Scholar] [CrossRef]
- Xue, Y. Chapter 11—Carbon nanotubes for biomedical applications. In Industrial Applications of Carbon Nanotubes; Peng, H., Li, Q., Chen, T., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 323–346. [Google Scholar]
- Ajitha, A.; Akhina, H.; Aswathi, M.; P, L.M.; Sabu, T. Carbon nanotubes: An ideal candidate for biomedical applications. JSM Nanotechnol. Nanomed. 2018, 6, 1065. [Google Scholar]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon nanotubes in biomedical applications: Factors, mechanisms, and remedies of toxicity. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Mi, G.; Webster, T.J. Chapter 11—The synthesis, application, and related neurotoxicity of carbon nanotubes. In Neurotoxicity of Nanomaterials and Nanomedicine; Jiang, X., Gao, H., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 259–284. [Google Scholar]
- Gholamine, B.; Karimi, I.; Salimi, A.; Mazdarani, P.; Becker, L.A. Neurobehavioral toxicity of carbon nanotubes in mice:Focus on brain-derived neurotrophic factor messenger rna and protein. Toxicol. Ind. Health 2017, 33, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Granada-Ramírez, D.A.; Arias-Cerón, J.S.; Rodriguez-Fragoso, P.; Vázquez-Hernández, F.; Luna-Arias, J.P.; Herrera-Perez, J.L.; Mendoza-Álvarez, J.G. Chapter 16—Quantum dots for biomedical applications. In Nanobiomaterials; Narayan, R., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 411–436. [Google Scholar]
- Aswathi, M.; Ajitha, A.; Akhina, H.; Lovely, M.; Thomas, S. Quantum dots: A promising tool for biomedical application. JSM Nanotechnol. Nanomed. 2018, 6, 1066. [Google Scholar]
- Zhao, Y.; Wang, X.; Wu, Q.; Li, Y.; Wang, D. Translocation and neurotoxicity of cdte quantum dots in rmes motor neurons in nematode caenorhabditis elegans. J. Hazard. Mater. 2015, 283, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, T.; Chen, Y.; Tang, M. Research advances on potential neurotoxicity of quantum dots. J. Appl. Toxicol. JAT 2016, 36, 345–351. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neurotoxicity of Nanomaterials: An Up-to-Date Overview. Nanomaterials 2019, 9, 96. https://doi.org/10.3390/nano9010096
Teleanu DM, Chircov C, Grumezescu AM, Teleanu RI. Neurotoxicity of Nanomaterials: An Up-to-Date Overview. Nanomaterials. 2019; 9(1):96. https://doi.org/10.3390/nano9010096
Chicago/Turabian StyleTeleanu, Daniel Mihai, Cristina Chircov, Alexandru Mihai Grumezescu, and Raluca Ioana Teleanu. 2019. "Neurotoxicity of Nanomaterials: An Up-to-Date Overview" Nanomaterials 9, no. 1: 96. https://doi.org/10.3390/nano9010096







