Nanocrystalline Cr-Ni Alloying Layer Induced by High-Current Pulsed Electron Beam
Abstract
:1. Introduction
2. Experiments
3. Results
3.1. Phase Identification
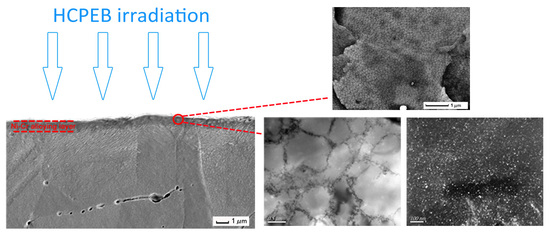
3.2. Microstructure Analysis
3.3. Surface Hardness
3.4. Tribological Properties
3.5. Electrochemical Property
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rebak, R.B.; Crook, P. Nickel alloys for corrosive environments. Adv. Mater. Process. 2000, 157, 37–42. [Google Scholar]
- Deb, D.; Iyer, S.R.; Radhakrishnan, V.M. A comparative study of oxidation and hot corrosion of a cast nickel base superalloy in different corrosive environments. Mater. Lett. 1996, 29, 19–23. [Google Scholar] [CrossRef]
- Pollock, T.M.; Tin, S. Nickel-based superalloys for advanced turbine engines: Chemistry, microstructure and properties. J. Propul Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Massalski, T.B.; Subramanian, P.R.; Okamoto, H.; Kacprzak, L. Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Kang, N.; Coddet, P.; Liao, H.; Baur, T.; Coddet, C. Wear behavior and microstructure of hypereutectic Al-Si alloys prepared by selective laser melting. Appl. Surf. Sci. 2016, 378, 142–149. [Google Scholar] [CrossRef]
- Li, Q.; Song, G.M.; Zhang, Y.Z.; Lei, T.C.; Chen, W.Z. Microstructure and dry sliding wear behavior of laser clad Ni-based alloy coating with the addition of SiC. Wear 2003, 254, 222–229. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, X.; Wang, S.; Shian, Z. Effect of CeO2 on microstructure and corrosive wear behavior of laser-cladded Ni/WC coating. Thin Solid Films 2000, 379, 128–132. [Google Scholar]
- Dong, C.; Wu, A.; Hao, S.; Zou, J.X.; Liu, Z.M.; Zhong, P.; Zhang, A.M.; Xu, T.; Chen, J.; Xu, J.; et al. Surface treatment by high current pulsed electron beam. Surf. Coat. Technol. 2003, 163, 620–624. [Google Scholar] [CrossRef]
- Hao, S.; Wu, P.; Zou, J.; Grosdidier, T.; Dong, C. Microstructure evolution occurring in the modified surface of 316L stainless steel under high current pulsed electron beam treatment. Appl. Surf. Sci. 2007, 253, 5349–5354. [Google Scholar] [CrossRef]
- Zou, J.; Grosdidier, T.; Zhang, K.; Dong, C. Mechanisms of nanostructure and metastable phase formations in the surface melted layers of a HCPEB-treated D2 steel. Acta Mater. 2006, 54, 5409–5419. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, J.; Lv, P.; Zhang, Y.; Xia, H.; Guan, Q. Surface microstructure and properties of Cu-C powder metallurgical alloy induced by high-current pulsed electron beam. J. Alloy Compd. 2016, 697, 96–103. [Google Scholar] [CrossRef]
- Zou, J.; Wu, A.; Dong, C.; Hao, S.; Liu, Z.; Ma, H. Oxidation protection of AISI H13 steel by high current pulsed electron beam treatment. Surf. Coat. Technol. 2004, 183, 261–267. [Google Scholar] [CrossRef]
- Zhang, K.M.; Zou, J.X.; Grosdidier, T.; Gey, N.; Weber, S.; Yang, D.Z.; Dong, C. Mechanisms of structural evolutions associated with the high current pulsed electron beam treatment of a NiTi shape memory alloy. J. Vac. Sci. Technol. A 2007, 25, 28–36. [Google Scholar] [CrossRef]
- Pearson, W.B. A Handbook of Lattice Spacings and Structures of Metals and Alloys; Pergamon Press: Oxford, UK, 1958; Volume 115, pp. 319–320. [Google Scholar]
- Zou, J.X.; Grosdidier, T.; Dong, C.; Weber, S. Rapid surface alloying by Ti of AISI 316L stainless steel using low energy high current pulsed electron beam. Eur. Phys. J.-Appl. Phys. 2008, 43, 343–347. [Google Scholar] [CrossRef]
- Proskurovsky, D.I.; Rotshtein, V.P.; Ozur, G.E.; Ivanov, Y.F.; Markov, A.B. Physical foundations for surface treatment of materials with low energy, high current electron beams. Surf. Coat. Technol. 2000, 125, 49–56. [Google Scholar] [CrossRef]
- Benjacob, E.; Garik, P. The formation of patterns in non-equilibrium growth. Nature 1990, 343, 523–530. [Google Scholar] [CrossRef]
- Qin, Y.; Dong, C.; Song, Z.; Hao, S.; Me, X.; Li, J.; Wang, X. Deep Modification of materials by thermal stress wave generated by irradiation of high-current pulsed electron beams. J. Vac. Sci. Technol. A 2009, 27, 430–435. [Google Scholar] [CrossRef]
- Hao, Y.; Gao, B.; Tu, G.F.; Li, S.W.; Hao, S.Z.; Dong, C. Surface modification of Al–20Si alloy by high current pulsed electron beam. Appl. Surf. Sci. 2011, 257, 3913–3919. [Google Scholar] [CrossRef]
- Zou, J.X.; Grosdidier, T.; Bolle, B.; Zhang, K.M.; Dong, C. Texture and microstructure at the surface of an AISI D2 steel treated by high current pulsed electron beam. Metall. Mater. Trans. A 2007, 38, 2061–2071. [Google Scholar] [CrossRef]
- Grosdidier, T.; Zou, J.X.; Stein, N.; Boulanger, C.; Hao, S.Z.; Dong, C. Texture modification, grain refinement and improved hardness/corrosion balance of a FeAl alloy by pulsed electron beam surface treatment in the “heating mode”. Scr. Mater. 2008, 58, 1058–1061. [Google Scholar] [CrossRef]
- Hao, S.; Gao, B.; Wu, A.; Zou, J.; Qin, Y.; Dong, C.; An, J.; Guan, Q. Surface modification of steels and magnesium alloy by high current pulsed electron beam. Nucl. Instrum. Meth. B 2005, 240, 646–652. [Google Scholar] [CrossRef]
- Cai, J.; Yang, S.Z.; Ji, L.; Guan, Q.F.; Wang, Z.P.; Han, Z.Y. Surface microstructure and high temperature oxidation resistance of thermal sprayed CoCrAlY coating irradiated by high current pulsed electron beam. Surf. Coat. Technol. 2014, 251, 217–225. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, J.; Grosdidier, T.; Dong, C. Formation and evolution of craters in carbon steels during low-energy high-current pulsed electron-beam treatment. J. Vac. Sci. Technol. A 2009, 27, 1217–1226. [Google Scholar] [CrossRef]
- Hao, S.Z.; Zhang, Y.; Xu, Y.; Gey, N.; Grosdidier, T.; Dong, C. WC/Co composite surface structure and nano graphite precipitate induced by high current pulsed electron beam irradiation. Appl. Surf. Sci. 2013, 285, 552–556. [Google Scholar] [CrossRef]
- Braunovic, M.; Konchits, V.V.; Myshkin, N.K. Electrical Contacts: Fundamentals, Applications and Technology; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Guan, Q.F.; Huang, W.; Li, H.F.; Gong, X.H.; Zhang, C.L.; Lv, P. Diffusion alloying of Cu-C induced by high current pulsed electron beam. J. Jilin Univ. 2016, 46, 1967–1973. [Google Scholar]
- Vinayak, S.; Vyas, H.P.; Vankar, V.D. Microstructure and electrical characteristics of Ni–Cr thin films. Thin Solid Films 2007, 515, 7109–7116. [Google Scholar] [CrossRef]
- Joint Committee on Powder Diffraction Standards. Powder Diffraction File; Card 26-04293; ASTM: Philadelphia, PA, USA, 2001. [Google Scholar]
- Maruyama, K.; Sawada, K.; Jun-Ichi, K. Strengthening mechanisms of creep resistant tempered martensitic steel. ISIJ Int. 2001, 41, 641–653. [Google Scholar] [CrossRef]
- Edalati, K.; Akama, D.; Nishio, A.; Lee, S.; Yonenaga, Y.; Cubero-Sesin, J.M.; Horita, Z. Influence of dislocation-solute atom interactions and stacking fault energy on grain size of single-phase alloys after severe plastic deformation using high-pressure torsion. Acta Mater. 2014, 69, 68–77. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Wang, S.Z.; Li, B.L.; Yin, Z.M.; Wan, Q.; Liu, P. Grain growth and microstructure evolution based mechanical property predicted by a modified Hall-Petch equation in hot worked Ni76Cr19AlTiCo alloy. Mater. Des. 2014, 55, 456–462. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.C.; Jiang, Q. Hall-Petch relationship in nanometer size range. J. Alloy Compd. 2003, 361, 160–164. [Google Scholar] [CrossRef]
- El-Sherik, A.M.; Erb, U.; Palumbo, G.; Aust, K.T. Deviations from Hall-Petch behaviour in as-prepared nanocrystalline nickel. Scr. Metall. Mater. 1992, 27, 1185–1188. [Google Scholar] [CrossRef]
- Ali, D.; Butt, M.Z. Structural characteristics and inverse Hall-Petch relation in high-purity nickel irradiated with nanosecond infrared laser pulses. Phys. B 2014, 444, 77–84. [Google Scholar] [CrossRef]
- Ren, F.; Zhu, W.; Chu, K.; Zhao, C. Tribological and corrosion behaviors of bulk Cu, W nanocomposites fabricated by mechanical alloying and warm pressing. J. Alloy Compd. 2016, 676, 164–172. [Google Scholar] [CrossRef]
- Zhang, K.M.; Yang, D.Z.; Zou, J.X.; Grosdidier, T.; Dong, C. Improved in vitro corrosion resistance of a NiTi alloy by high current pulsed electron beam treatment. Surf. Coat. Technol. 2006, 201, 3096–3102. [Google Scholar] [CrossRef]
- Li, P.; Li, L.; Wang, W.; Jin, W.; Liu, X.; Yeung, K.W.; Chu, P.K. Enhanced corrosion resistance and hemocompatibility of biomedical NiTi alloy by atmospheric-pressure plasma polymerized fluorine-rich coating. Appl. Surf. Sci. 2014, 297, 109–115. [Google Scholar] [CrossRef]
- Davis, G.D.; Fritz, T.L.; Rees, B.J.; Shaw, B.A.; Moshier, W.C. Study of the influence of alloying additions on the passivity of aluminum. Annu. Rep. 1991, 1, 89. [Google Scholar]
- Cai, L.X.; Wang, H.M.; Wang, C.M. Corrosion resistance of laser clad Cr-alloyed Ni2Si/NiSi intermetallic coatings. Surf. Coat. Technol. 2004, 182, 294–299. [Google Scholar] [CrossRef]









| Accelerated Voltage | Current Pulse Duration | Energy Density | Beam Diameter | Pulse Interval | Vacuum |
|---|---|---|---|---|---|
| 27 keV | 1.5 μs | 4 J cm−2 | 60 mm | 10 s | 5 × 10−3 Pa |
| Samples | icorr/(μA cm−2) | Ecorr/V |
|---|---|---|
| Initial | 12.59 | −0.768 |
| 10 pulses | 6.31 | −0.698 |
| 20 pulses | 3.98 | −0.679 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Peng, C.-T.; Guan, J.; Lv, P.; Guan, Q.; Lu, R. Nanocrystalline Cr-Ni Alloying Layer Induced by High-Current Pulsed Electron Beam. Nanomaterials 2019, 9, 74. https://doi.org/10.3390/nano9010074
Zhang L, Peng C-T, Guan J, Lv P, Guan Q, Lu R. Nanocrystalline Cr-Ni Alloying Layer Induced by High-Current Pulsed Electron Beam. Nanomaterials. 2019; 9(1):74. https://doi.org/10.3390/nano9010074
Chicago/Turabian StyleZhang, Lingyan, Ching-Tun Peng, Jintong Guan, Peng Lv, Qingfeng Guan, and Ruifeng Lu. 2019. "Nanocrystalline Cr-Ni Alloying Layer Induced by High-Current Pulsed Electron Beam" Nanomaterials 9, no. 1: 74. https://doi.org/10.3390/nano9010074