A Controllability Investigation of Magnetic Properties for FePt Alloy Nanocomposite Thin Films
Abstract
:1. Introduction
2. Experimental
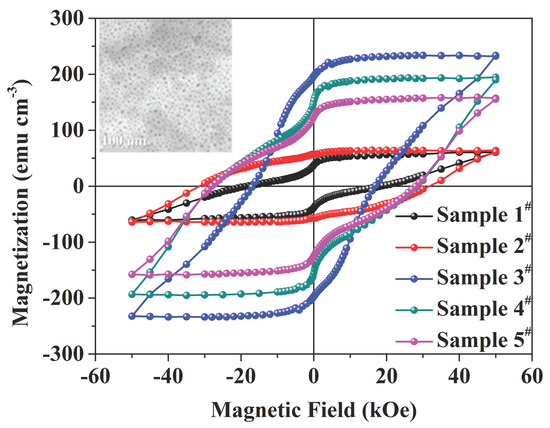
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, J.S.; Lim, B.C.; Ding, Y.F.; Chow, G.M. Low-temperature deposition of L1(0) FePt films for ultra-high density magnetic recording. J. Magn. Magn. Mater. 2006, 303, 309–317. [Google Scholar] [CrossRef]
- Chen, J.S.; Hu, J.F.; Lim, B.C.; Ding, Y.F.; Chow, G.M.; Ju, G. Development of L1(0) FePt:C (001) Thin Films With High Coercivity and Small Grain Size for Ultra-High-Density Magnetic Recording Media. IEEE Trans. Magn. 2009, 45, 839–844. [Google Scholar] [CrossRef]
- Giannopoulos, G.; Speliotis, T.; Li, W.F.; Hadjipanayis, G.; Niarchos, D. Structural and magnetic properties of L10/A1, FePt nanocomposites. J. Magn. Magn. Mater. 2013, 325, 75–81. [Google Scholar] [CrossRef]
- Xia, A.; Ren, S.; Lin, J.; Ma, Y.; Xu, C.; Li, J.; Jin, C.; Liu, X. Magnetic properties of sintered SrFe12O19-CoFe2O4 nanocomposites with exchange coupling. J. Alloys Compd. 2015, 653, 108–116. [Google Scholar] [CrossRef]
- Song, F.; Shen, X.; Liu, M.; Xiang, J. Microstructure, magnetic properties and exchange-coupling interactions for one-dimensional hard/soft ferrite nanofibers. J. Solid State Chem. 2012, 185, 31–36. [Google Scholar] [CrossRef]
- Xiong, Z.W.; Cao, L.H. Red-ultraviolet photoluminescence tuning by Ni nanocrystals in epitaxial SrTiO3 matrix. Appl. Surf. Sci. 2018, 445, 65–70. [Google Scholar] [CrossRef]
- Joseyphus, R.J.; Shinoda, K.; Sato, Y.; Tohji, K.; Jeyadevan, B. Composition controlled synthesis of fcc-FePt nanoparticles using a modified polyol process. J. Mater. Sci. 2008, 43, 2402–2406. [Google Scholar] [CrossRef]
- Lu, S. On the tetragonality of martensites in ferrous shape memory alloy Fe3Pt: A first-principles study. Acta Mater. 2016, 111, 56–65. [Google Scholar] [CrossRef]
- Maat, S.; Kellock, A.J.; Weller, D.; Baglin, J.E.E.; Fullerton, E.E. Ferromagnetism of FePt3 films induced by ion-beam irradiation. J. Magn. Magn. Mater. 2003, 265, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Li, S.; Liu, M.; Feng, M.; Li, H. Microstructure and magnetic properties of fcc-FePt/L1(0)-FePt exchange-coupled composite films. Appl. Phys. A: Mater. Sci. Process. 2018, 124. [Google Scholar] [CrossRef]
- Sun, S.H.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.W.; Guo, J.Y.; Liu, C.Y.; Ouyang, H.; van Lierop, J.; Phuoc, N.N.; Suzuki, T. Exchange coupling in FePt-FePt3 nanocomposite films. Phys. Status Solidi A 2007, 204, 3991–3994. [Google Scholar] [CrossRef]
- Suber, L.; Imperatori, P.; Bauer, E.M.; Porwal, R.; Peddis, D.; Cannas, C.; Ardu, A.; Mezzi, A.; Kaciulis, S.; Notargiacomo, A.; et al. Tuning hard and soft magnetic FePt nanocomposites. J. Alloys Compd. 2016, 663, 601–609. [Google Scholar] [CrossRef]
- Zeynali, H.; Sebt, S.A.; Arabi, H.; Akbari, H.; Hosseinpour-Mashkani, S.M.; Rao, K.V. Synthesis and Characterization of FePt/NiO Core-Shell Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2012, 22, 1314–1319. [Google Scholar] [CrossRef]
- Nguyen Hoang, N.; Nguyen Thi Thanh, V.; Nguyen Dang, P.; Tran Thi, H.; Nguyen Hoang, H.; Nguyen Hoang, L. Magnetic Properties of FePt Nanoparticles Prepared by Sonoelectrodeposition. J. Nanomater. 2012. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Gong, Y.-P.; Li, X.-F.; Li, A.-D.; Zhang, J.-L.; Yan, Q.-Y.; Wu, D. Magnetic properties of FePt nanoparticle assemblies embedded in atomic-layer-deposited Al2O3. J. Mater. Chem. 2011, 21, 5046–5050. [Google Scholar] [CrossRef]
- Zheng, B.J.; Lian, J.S.; Zhao, L.; Jiang, Q. Optical and electrical properties of In-doped CdO thin films fabricated by pulse laser deposition. Appl. Surf. Sci. 2010, 256, 2910–2914. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.W.; Yin, Z.G.; Qu, S.; You, J.B.; Chen, N.F. Magnetic Properties of FePt Nanoparticles Prepared by a Micellar Method. Nanoscale Res. Lett. 2010, 5, 1–6. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, T.; Wang, X.; Zhao, Y.; Li, X.; Xu, X.; Xiong, Z.; Wang, X.; Peng, L.; Wang, J.; et al. Splitting of the ultraviolet plasmon resonance from controlling FePt nanoparticles morphology. Appl. Surf. Sci. 2018, 435, 1–6. [Google Scholar] [CrossRef]
- Traub, M.C.; Biteen, J.S.; Michalak, D.J.; Webb, L.J.; Brunschwig, B.S.; Lewis, N.S. Phosphine Functionalization of GaAs(111)A Surfaces. J. Phys. Chem. C 2008, 112, 18467–18473. [Google Scholar] [CrossRef]
- Christodoulides, J.A.; Farber, P.; Daniil, M.; Okumura, H.; Hadjipanayis, G.C.; Skumryev, V.; Simopoulos, A.; Weller, D. Magnetic, structural and microstructural properties of FePt/M (M = C, BN) granular films. IEEE Trans. Magn. 2001, 37, 1292–1294. [Google Scholar] [CrossRef]
- Kim, J.S.; Koo, Y.M. Thickness dependence of (001) texture evolution in FePt thin films on an amorphous substrate. Thin Solid Films 2008, 516, 1147–1154. [Google Scholar] [CrossRef]
- Barmak, K.; Wang, B.C.; Jesanis, A.T.; Berry, D.C.; Rickman, J.M. L1(0) FePt: Ordering, Anisotropy Constant and Their Relation to Film Composition. IEEE Trans. Magn. 2013, 49, 3284–3291. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, X.; Yu, J.; Peng, L.; Zhao, Y.; Xiong, Z.; Shen, C.; Jiang, T.; Yang, Q.; Wang, X.; et al. The microstructure, strain state and optical properties of FePt nano-clusters in MgO matrix. J. Alloys Compd. 2018, 731, 554–559. [Google Scholar] [CrossRef]
- Rellinghaus, B.; Stappert, S.; Acet, M.; Wassermann, E.F. Magnetic properties of FePt nanoparticles. J. Magn. Magn. Mater. 2003, 266, 142–154. [Google Scholar] [CrossRef]
- Tamada, Y.; Morimoto, Y.; Yamamoto, S.; Takano, M.; Nasu, S.; Ono, T. Effects of annealing time on structural and magnetic properties of L1(0)-FePt nanoparticles synthesized by the SiO2-nanoreactor method. J. Magn. Magn. Mater. 2007, 310, 2381–2383. [Google Scholar] [CrossRef]
- Padmanapan, S. Magnetic properties of FePt based nanocomposite thin films grown on low cost substrates. Phys. Procedia 2014, 54, 23–29. [Google Scholar] [CrossRef]
- Yan, M.L.; Li, X.Z.; Gao, L.; Liou, S.H.; Sellmyer, D.J.; van de Veerdonk, R.J.M.; Wierman, K.W. Fabrication of nonepitaxially grown double-layered FePt: C/FeCoNi thin films for perpendicular recording. Appl. Phys. Lett. 2003, 83, 3332–3334. [Google Scholar] [CrossRef]
- Tsai, J.-L.; Huang, J.-C.; Tai, H.-W.; Tsai, W.-C.; Lin, Y.-C. Magnetic properties and microstructure of FePtB, FePt(B-Ag) granular films. J. Magn. Magn. Mater. 2013, 329, 6–13. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, K.; Tian, Y.; Li, X.Z.; Kramer, M.J.; Sellmyer, D.J.; Shield, J.E.; Sun, S. One-Pot Synthesis of Urchin-like FePd-Fe3O4 and Their Conversion into Exchange-Coupled L1(0)-FePd-Fe Nanocomposite Magnets. Nano Lett. 2013, 13, 4975–4979. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Cao, L. Interparticle spacing dependence of magnetic anisotropy and dipolar interaction of Ni nanocrystals embedded in epitaxial BaTiO3 matrix. Ceram. Int. 2018, 44, 8155–8160. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Yu, J.; Xiao, T.; Peng, L.; Fan, L.; Wang, C.; Shen, Q.; Wu, W. Tailoring the Grain Size of Bi-Layer Graphene by Pulsed Laser Deposition. Nanomaterials 2018, 8, 885. [Google Scholar] [CrossRef] [PubMed]






| Experiment Conditions | Experiment Parameters |
|---|---|
| background vacuum | 3.0 × 10−6 Pa |
| working vacuum | 5.0 × 10−5 Pa |
| target | MgO purity > 99.99% FePt purity > 99.99% |
| substrate | MgO (100) |
| laser pulse frequency | 2 Hz |
| number of pulses | MgO: 1200 pulses |
| distance between the target and substrate | 5 cm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Xiao, T.; Wang, X.; Zhou, X.; Wang, X.; Peng, L.; Zhao, Y.; Wang, J.; Chen, J.; Yin, H.; et al. A Controllability Investigation of Magnetic Properties for FePt Alloy Nanocomposite Thin Films. Nanomaterials 2019, 9, 53. https://doi.org/10.3390/nano9010053
Yu J, Xiao T, Wang X, Zhou X, Wang X, Peng L, Zhao Y, Wang J, Chen J, Yin H, et al. A Controllability Investigation of Magnetic Properties for FePt Alloy Nanocomposite Thin Films. Nanomaterials. 2019; 9(1):53. https://doi.org/10.3390/nano9010053
Chicago/Turabian StyleYu, Jian, Tingting Xiao, Xuemin Wang, Xiuwen Zhou, Xinming Wang, Liping Peng, Yan Zhao, Jin Wang, Jie Chen, Hongbu Yin, and et al. 2019. "A Controllability Investigation of Magnetic Properties for FePt Alloy Nanocomposite Thin Films" Nanomaterials 9, no. 1: 53. https://doi.org/10.3390/nano9010053