Physicochemical Properties and Storage Stability of Food Protein-Stabilized Nanoemulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoemulsions Preparation
2.3. Droplet Size, Zeta Potential and Apparent Viscosity Measurements
2.4. Confocal Laser Scanning Microscopy
2.5. Storage Stability
2.5.1. Physical Stability Measurement
2.5.2. Oxidative Stability Measurement
2.6. Fourier Transform Infrared (FTIR) Spectroscopy of Protein
2.7. Interfacial Tension (IT) Measurement of Protein
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effects of Protein Concentrations on Nanoemulsions MDD and ZP
3.2. Effects of Oil Phase Fraction on Nanoeumlsions MDD and ZP
3.3. Effects of Ultrasonic Power and Time on Nanoemulsions MDD and ZP
3.4. Apparent Viscosity of Nanoemulsions
3.5. Microstructure of Nanoemulsions
3.6. Storage Stability of Nanoemulsions
3.6.1. Physical Stability
3.6.2. Oxidative Stability
3.7. Characteristics of Proteins
3.7.1. Secondary Structure
3.7.2. Interfacial Characteristics
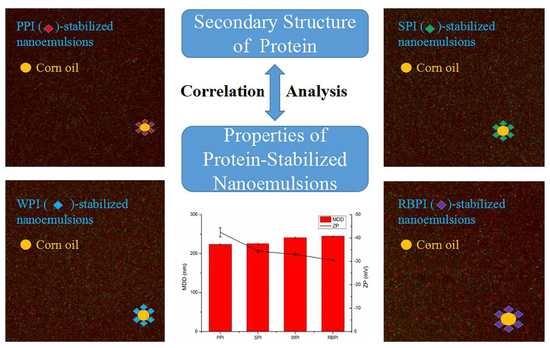
3.8. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guerra-Rosas, M.I.; Morales-Castro, J.; Ochoa-Martínez, L.A.; Salvia-Trujillo, L.; Martín-Belloso, O. Long-term stability of food-grade nanoemulsions from high methoxyl pectin containing essential oils. Food Hydrocoll. 2016, 52, 438–446. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Nanoemulsion-based delivery systems to improve functionality of lipophilic components. Front. Nutr. 2014, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J. Nanoemulsion-based oral delivery systems for lipophilic bioactive components: Nutraceuticals and pharmaceuticals. Ther. Deliv. 2013, 4, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for food applications: Development and characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation techniques for food bioactive components: A review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Mcclements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef]
- Peshkovsky, A.S.; Peshkovsky, S.L.; Bystryak, S. Scalable high-power ultrasonic technology for the production of translucent nanoemulsions. Chem. Eng. Process. Process Intensif. 2013, 69, 77–82. [Google Scholar] [CrossRef]
- Kaltsa, O.; Michon, C.; Yanniotis, S.; Mandala, I. Ultrasonic energy input influence οn the production of sub-micron o/w emulsions containing whey protein and common stabilizers. Ultrason. Sonochem. 2013, 20, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Gordon, L.; Pilosof, A.M.R. Application of high-intensity ultrasounds to control the size of whey proteins particles. Food Biophys. 2010, 5, 203–210. [Google Scholar] [CrossRef]
- Kumar, R.; Kaur, K.; Uppal, S.; Mehta, S.K. Ultrasound processed nanoemulsion: A comparative approach between resveratrol and resveratrol cyclodextrin inclusion complex to study its binding interactions, antioxidant activity and UV light stability. Ultrason. Sonochem. 2017, 37, 478–489. [Google Scholar] [CrossRef]
- Sivakumar, M.; Tang, S.Y.; Tan, K.W. Cavitation technology—A greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason. Sonochem. 2014, 21, 2069–2083. [Google Scholar] [CrossRef] [PubMed]
- Alzorqi, I.; Ketabchi, M.R.; Sudheer, S.; Manickam, S. Optimization of ultrasound induced emulsification on the formulation of palm-olein based nanoemulsions for the incorporation of antioxidant β-d-glucan polysaccharides. Ultrason. Sonochem. 2016, 31, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Manickam, S.; Wei, T.K.; Nashiru, B. Formulation development and optimization of a novel Cremophore EL-based nanoemulsion using ultrasound cavitation. Ultrason. Sonochem. 2012, 19, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Sivakumar, M.; Nashiru, B. Impact of osmotic pressure and gelling in the generation of highly stable single core water-in-oil-in-water (W/O/W) nano multiple emulsions of aspirin assisted by two-stage ultrasonic cavitational emulsification. Colloid Surf. B 2013, 102, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Sivakumar, M.; Ng, A.M.; Shridharan, P. Anti-inflammatory and analgesic activity of novel oral aspirin-loaded nanoemulsion and nano multiple emulsion formulations generated using ultrasound cavitation. Int. J. Pharm. 2012, 430, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shi, M.; Li, W.; Zhao, L.; Wang, Z.; Yan, X.; Norde, W.; Li, Y. Pickering emulsions stabilized by whey protein nanoparticles prepared by thermal cross-linking. Colloid Surf. B 2015, 127, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Structuring of colloidal particles at interfaces and the relationship to food emulsion and foam stability. J. Colloid Interface Sci. 2015, 449, 38–45. [Google Scholar] [CrossRef]
- Relkin, P.; Shukat, R.; Bourgaux, C.; Meneau, F. Nanostructures and polymorphisms in protein stabilised lipid nanoparticles, as food bioactive carriers: Contribution of particle size and adsorbed materials. Procedia Food Sci. 2011, 1, 246–250. [Google Scholar] [CrossRef]
- Donsi, F.; Senatore, B.; Huang, Q.; Ferrari, G. Development of novel pea protein-based nanoemulsions for delivery of nutraceuticals. J. Agric. Food Chem. 2010, 58, 10653–10660. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Beliciu, C.M.; Moraru, C.I. The effect of protein concentration and heat treatment temperature on micellar casein–soy protein mixtures. Food Hydrocoll. 2011, 25, 1448–1460. [Google Scholar] [CrossRef]
- Amine, C.; Dreher, J.; Helgason, T.; Tadros, T. Investigation of emulsifying properties and emulsion stability of plant and milk proteins using interfacial tension and interfacial elasticity. Food Hydrocoll. 2014, 39, 180–186. [Google Scholar] [CrossRef]
- Han, S.W.; Chee, K.M.; Cho, S.J. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. 2015, 172, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Lu, Q.Y. Characterizing the structural and surface properties of proteins isolated before and after enzymatic demulsification of the aqueous extract emulsion of peanut seeds. Food Hydrocoll. 2015, 47, 51–60. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Z.; Shen, K.; Cai, X.; Zheng, B.; Miao, S. Influence of ultrasound-assisted alkali treatment on the structural properties and functionalities of rice protein. J. Cereal Sci. 2018, 79, 204–209. [Google Scholar] [CrossRef]
- Nejadmansouri, M.; Hosseini, S.M.H.; Niakosari, M.; Yousefi, G.H.; Golmakani, M.T. Physicochemical properties and storage stability of ultrasound-mediated wpi-stabilized fish oil nanoemulsions. Food Hydrocoll. 2016, 61, 801–811. [Google Scholar] [CrossRef]
- Wang, X.B.; Chi, Y.J. Microwave-assisted phosphorylation of soybean protein isolates and their physicochemical properties. Czech J. Food Sci. 2012, 30, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Ziani, K.; Barish, J.A.; McClementsm, D.J.; Goddard, J.M. Manipulating interactions between functional colloidal particles and polyethylene surfaces using interfacial engineering. J. Colloid Interface Sci. 2011, 360, 31–38. [Google Scholar] [CrossRef]
- Tcholakova, S.; Denkov, N.D.; Danner, T. Role of surfactant type and concentration for the mean drop size during emulsification in turbulent flow. Langmuir 2004, 20, 7444–7458. [Google Scholar] [CrossRef]
- Primozic, M.; Duchek, A.; Nickerson, M.; Ghosh, S. Effect of lentil proteins isolate concentration on the formation, stability and rheological behavior of oil-in-water nanoemulsions. Food Chem. 2017, 237, 65–74. [Google Scholar] [CrossRef]
- Chen, E.; Cao, L.; Mcclements, D.J.; Liu, S.; Li, B.; Li, Y. Enhancement of physicochemical properties of whey protein-stabilized nanoemulsions by interfacial cross-linking using cinnamaldehyde. Food Hydrocoll. 2017, 77, 976–985. [Google Scholar] [CrossRef]
- Abbas, S.; Bashari, M.; Akhtar, W.; Li, W.W.; Zhang, X. Process optimization of ultrasound-assisted curcumin nanoemulsions stabilized by OSA-modified starch. Ultrason. Sonochem. 2014, 21, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Charoen, R.; Jangchud, A.; Jangchud, K.; Harnsilawat, T.; Decker, E.A.; McClements, D.J. Influence of interfacial composition on oxidative stability of oil-in-water emulsions stabilized by biopolymer emulsifiers. Food Chem. 2012, 131, 1340–1346. [Google Scholar] [CrossRef]
- Guo, Q.; Mu, T.H. Emulsifying properties of sweet potato protein: Effect of protein concentration and oil volume fraction. Food Hydrocoll. 2011, 25, 98–106. [Google Scholar] [CrossRef]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; McClements, D.J. Core-shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein-pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Pinjari, D.V.; Pandit, A.B.; Mhaske, S.T. Synthesis of titanium dioxide by ultrasound assisted sol-gel technique: Effect of amplitude (power density) variation. Ultrason. Sonochem. 2010, 17, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Handa, C.L.; Xu, J. Effects of ultrasound pre-treatment on the structure of β-conglycinin and glycinin and the antioxidant activity of their hydrolysates. Food Chem. 2017, 218, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.J.; Holkar, C.R.; Karekar, S.E.; Pinjari, D.V.; Pandit, A.B. Ultrasound assisted manufacturing of paraffin wax nanoemulsions: Process optimization. Ultrason. Sonochem. 2015, 23, 201–207. [Google Scholar] [CrossRef]
- Khalloufi, S.; Alexander, M.; Goff, H.D.; Corredig, M. Physicochemical properties of whey protein isolate stabilized oil-in-water emulsions when mixed with flaxseed gum at neutral pH. Food Res. Int. 2008, 41, 964–972. [Google Scholar] [CrossRef]
- Rodea-González, D.A.; Cruz-Olivares, J.; Román-Guerrero, A.; Rodríguez-Huezo, M.E.; Vernon-Carter, E.J.; Pérez-Alonso, C. Spray-dried encapsulation of chia essential oil (Salvia hispanica L.) in whey protein concentrate-polysaccharide matrices. J. Food Eng. 2012, 111, 102–109. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, Y.; Bai, L.; Liu, F.; Deng, Y.; Mcclements, D.J. Fabrication of β-carotene nanoemulsion-based delivery systems using dual-channel microfluidization: Physical and chemical stability. J. Colloid Interface Sci. 2017, 490, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Wooster, T.J.; Ashokkumar, M.; Balachandran, S.; Mawson, R.; Simons, L. The use of ultrasonics for nanoemulsion preparation. Innov. Food Sci. Emerg. 2008, 9, 170–175. [Google Scholar] [CrossRef]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Formation of whey protein isolate hydrolysate stabilised nanoemulsion. Food Hydrocoll. 2014, 41, 169–177. [Google Scholar] [CrossRef]
- Chityala, P.K.; Khouryieh, H.; Williams, K.; Conte, E. Effect of xanthan/enzyme-modified guar gum mixtures on the stability of whey protein isolate stabilized fish oil-in-water emulsions. Food Chem. 2016, 212, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhao, M.; Mcclements, D.J. Improving the stability of wheat protein-stabilized emulsions: Effect of pectin and xanthan gum addition. Food Hydrocoll. 2015, 43, 377–387. [Google Scholar] [CrossRef]
- Mu, L.; Zhao, M.; Yang, B.; Zhao, H.; Cui, C.; Zhao, Q. Effect of ultrasonic treatment on the graft reaction between soy protein isolate and gum acacia and on the physicochemical properties of conjugates. J. Agric. Food Chem. 2010, 58, 4494–4499. [Google Scholar] [CrossRef]
- Xiang, L.; Zhou, Y.; Long, B.; Liu, F.; Zhang, R.; Zhang, Z.; Zheng, B.; Deng, Y.; McClements, D.J. Production of highly concentrated oil-in-water emulsions using dual-channel microfluidization: Use of individual and mixed natural emulsifiers (saponin and lecithin). Food Res. Int. 2017, 96, 103–112. [Google Scholar]
- Gu, L.; Su, Y.; Zhang, M.; Chang, C.; Li, J.; McClements, D.J.; Yang, Y. Protection of β-carotene from chemical degradation in emulsion-based delivery systems using antioxidant interfacial complexes: Catechin-egg white protein conjugates. Food Res. Int. 2017, 96, 84–93. [Google Scholar] [CrossRef]








| Sample | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| PPI | 12.01 ± 0.03 a | 30.17 ± 0.03 a | 41.76 ± 0.03 d | 16.06 ± 0.03 d |
| RBPI | 16.90 ± 0.01 d | 34.63 ± 0.03 c | 34.64 ± 0.02 b | 13.83 ± 0.01 a |
| SPI | 12.84 ± 0.02 b | 31.44 ± 0.03 b | 40.99 ± 0.04 c | 14.72 ± 0.02 c |
| WPI | 15.68 ± 0.02 c | 35.65 ± 0.02 d | 34.11 ± 0.04 a | 14.56 ± 0.03 b |
| α-Helix | β-Sheet | β-Turn | Random | MDD | ZP | |
|---|---|---|---|---|---|---|
| α-Helix | 1 | |||||
| β-Sheet | 0.928 | 1 | ||||
| β-Turn | −0.961 * | −0.986 * | 1 | |||
| Random | −0.872 | −0.784 | 0.767 | 1 | ||
| MDD | 0.994 ** | 0.942 | −0.979 * | −0.817 | 1 | |
| ZP | 0.824 | 0.808 | −0.761 | −0.980 * | 0.773 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Jin, H.; Sun, X.; Sun, J.; Liu, C.; Liu, C.; Xu, J. Physicochemical Properties and Storage Stability of Food Protein-Stabilized Nanoemulsions. Nanomaterials 2019, 9, 25. https://doi.org/10.3390/nano9010025
Li Y, Jin H, Sun X, Sun J, Liu C, Liu C, Xu J. Physicochemical Properties and Storage Stability of Food Protein-Stabilized Nanoemulsions. Nanomaterials. 2019; 9(1):25. https://doi.org/10.3390/nano9010025
Chicago/Turabian StyleLi, Yangyang, Hua Jin, Xiaotong Sun, Jingying Sun, Chang Liu, Chunhong Liu, and Jing Xu. 2019. "Physicochemical Properties and Storage Stability of Food Protein-Stabilized Nanoemulsions" Nanomaterials 9, no. 1: 25. https://doi.org/10.3390/nano9010025