MoO3-Doped MnCo2O4 Microspheres Consisting of Nanosheets: An Inexpensive Nanostructured Catalyst to Hydrolyze Ammonia Borane for Hydrogen Generation
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Catalysts
2.2. Characterizations
2.3. Catalytic Experiments
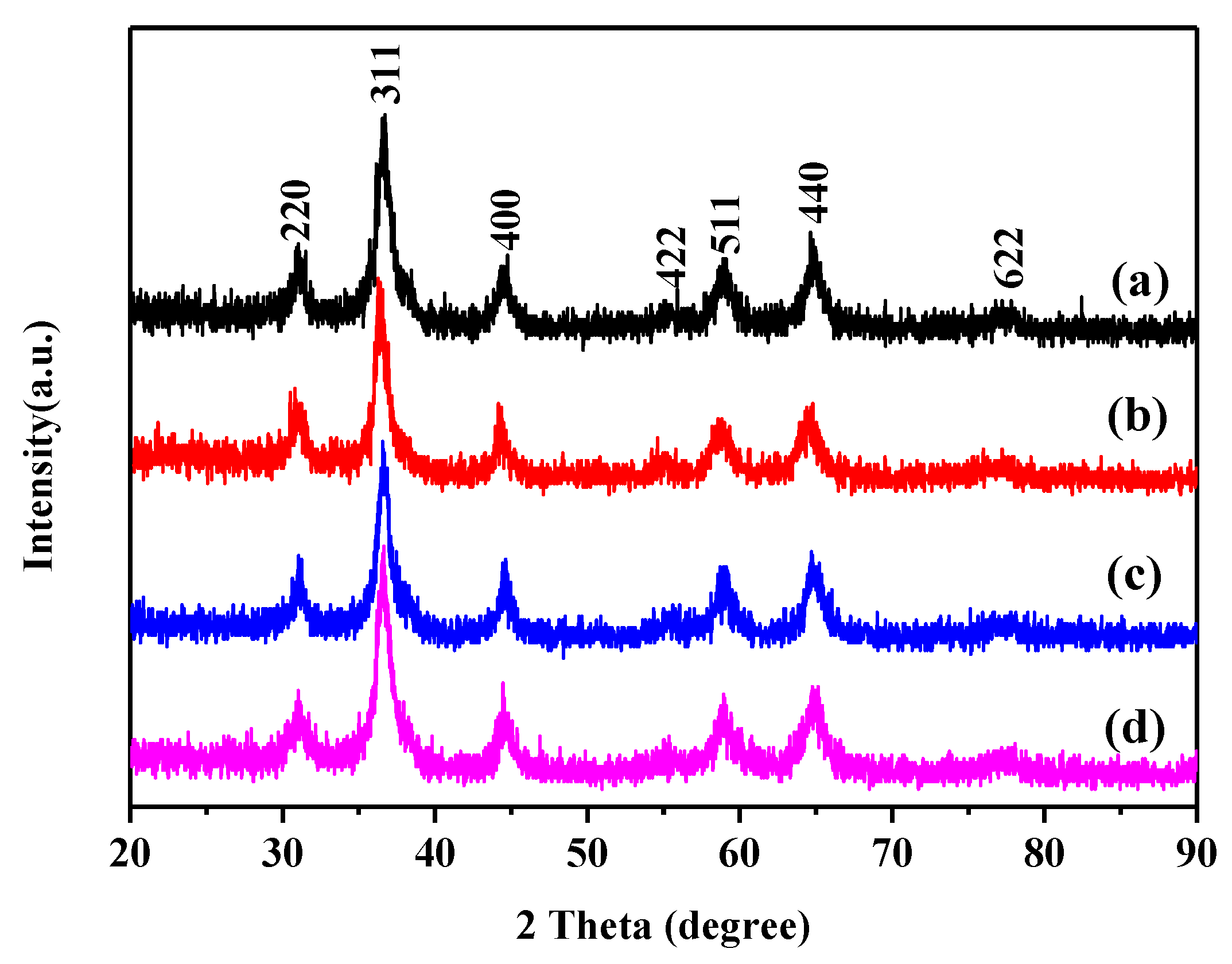
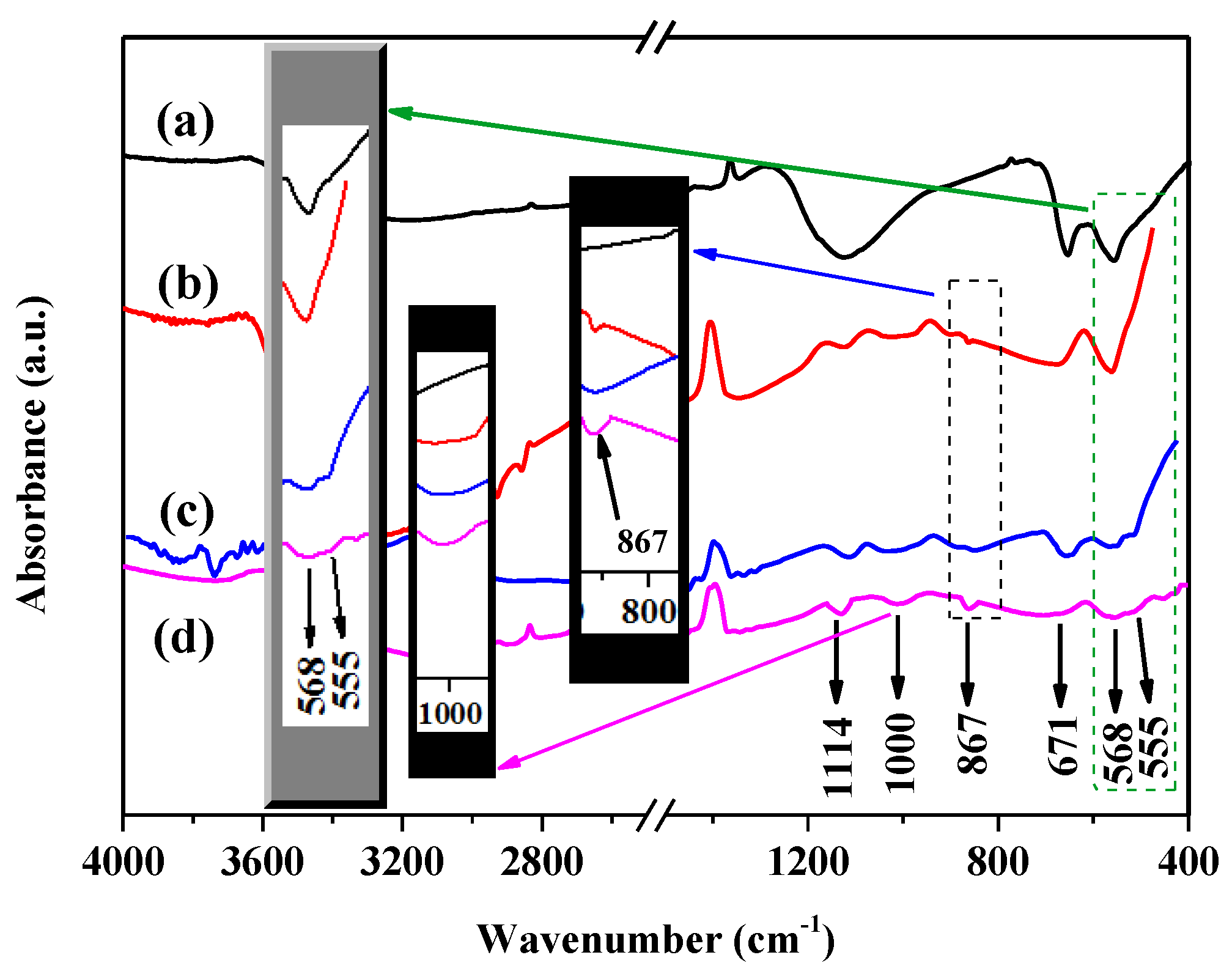
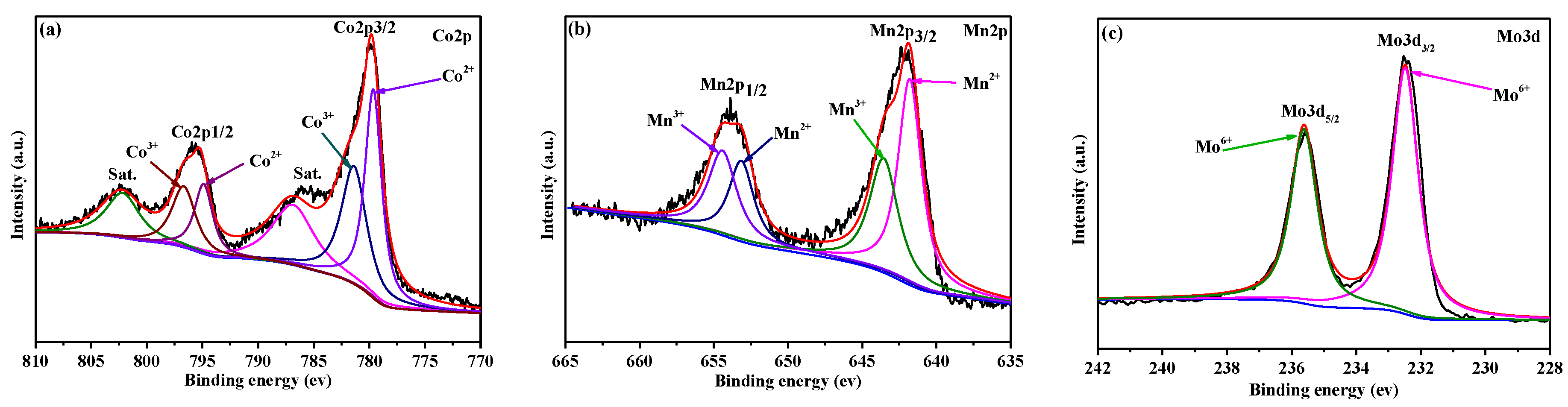
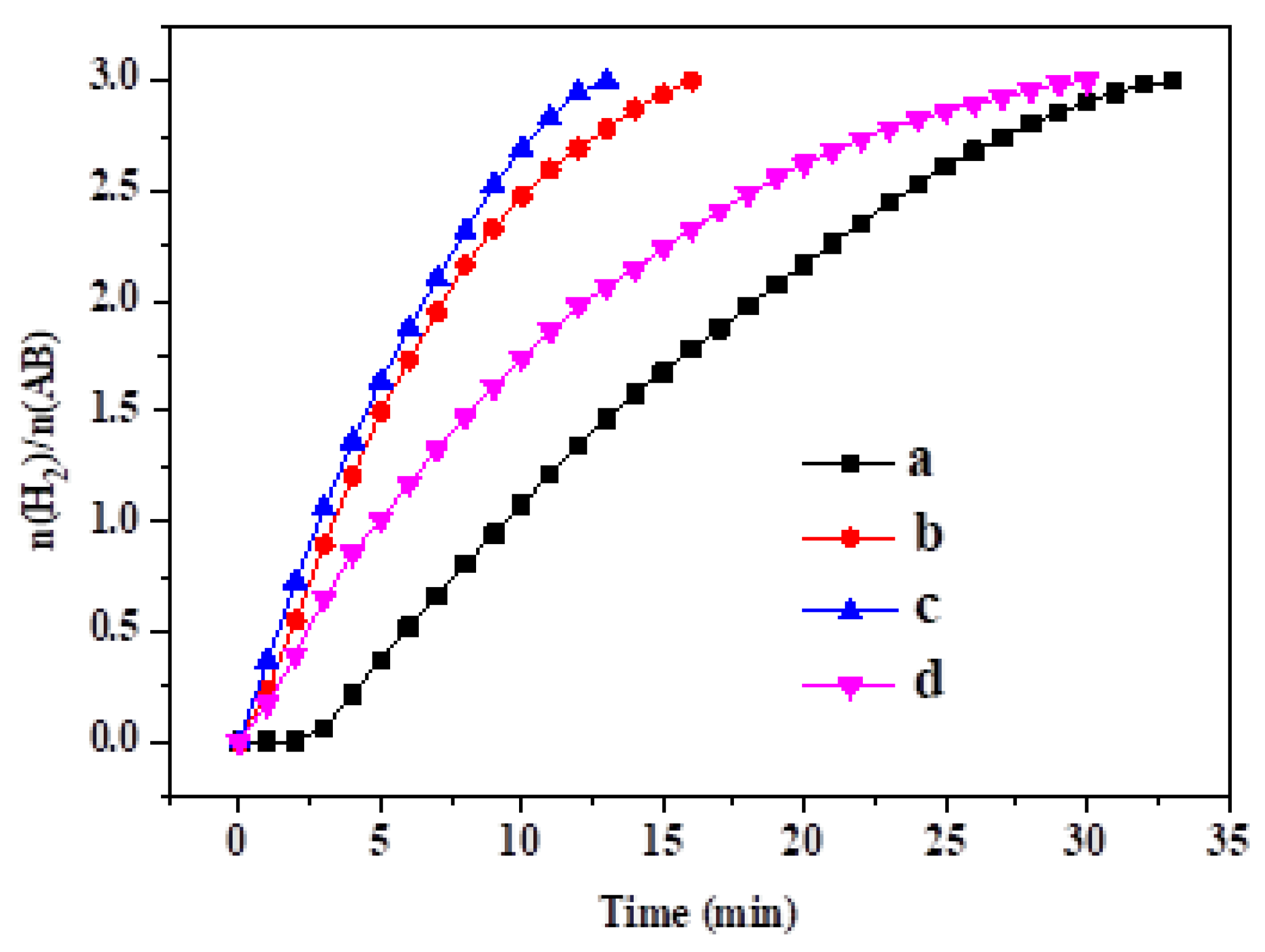
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cipriani, G.; Dio, V.D.; Genduso, F.; Cascia, D.; Liga, R.; Miceli, R.; Galluzzo, G. Perspective on hydrogen energy carrier and its automotive applications. Int. J. Hydrog. Energy 2014, 39, 8482–8494. [Google Scholar] [CrossRef]
- Reshak, A.H. Sulfide Oxide XZnSO (X = Ca or Sr) as Novel Active Photocatalytic Water Splitting Solar-to-Hydrogen Energy Conversion. Appl. Catal. B Environ. 2018, 225, 273–283. [Google Scholar] [CrossRef]
- Sadaghiani, M.S.; Mehrpooya, M. Introducing and energy analysis of a novel cryogenic hydrogen liquefaction process configuration. Int. J. Hydrog. Energy 2017, 42, 6033–6050. [Google Scholar] [CrossRef]
- Monde, M.; Woodfield, P.; Takano, T.; Kosaka, M. Estimation of temperature change in practical hydrogen pressure tanks being filled at high pressures of 35 and 70 MPa. Int. J. Hydrog. Energy 2012, 37, 5723–5734. [Google Scholar] [CrossRef]
- Germain, J.; Hradil, J.; Fréchet, J.M.J.; Svec, F. High Surface Area Nanoporous Polymers for Reversible Hydrogen Storage. Chem. Mater. 2006, 18, 4430–4435. [Google Scholar] [CrossRef]
- Béguin, F.; Kierzek, K.; Friebe, M.; Jankowska, A.; Machnikowski, J.; Jurewiczm, K.; Frackowiak, E. Effect of various porous nanotextures on the reversible electrochemical sorption of hydrogen in activated carbons. Electrochim. Acta 2006, 51, 2161–2167. [Google Scholar] [CrossRef]
- Ikuhara, Y.H.; Saito, T.; Sasaki, Y.; Takahashi, S.; Hirayama, T. Determination of reversible hydrogen adsorption site in Ni-nanoparticle-dispersed amorphous silica for hydrogenseparation at high temperature. J. Mater. Res. 2010, 25, 2008–2014. [Google Scholar] [CrossRef]
- Ye, X.J.; Liu, C.S.; Zhong, W.; Zeng, Z.; Du, Y.W. Metalized T graphene: A reversible hydrogen storage material at room temperature. J. Appl. Phys. 2014, 116, 114304. [Google Scholar] [CrossRef]
- Peng, B.; Chen, J. Ammonia borane as an efficient and lightweight hydrogen storage medium. Energy Environ. Sci. 2008, 1, 479–483. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. A high-performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia–borane. J. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Rakap, M.; Kalu, E.E.; Özkar, S. Hydrogen generation from hydrolysis of ammonia-borane using Pd-PVB-TiO2 and Co-Ni-P/Pd-TiO2 under stirred conditions. J. Power Sources 2012, 210, 184–190. [Google Scholar] [CrossRef]
- Shang, N.Z.; Feng, C.; Gao, S.T.; Wang, C. Ag/Pd nanoparticles supported on amine-functionalized metal–organic framework for catalytic hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2016, 41, 944–950. [Google Scholar] [CrossRef]
- Zhong, W.D.; Tian, X.K.; Yang, C.; Zhou, Z.X.; Liu, X.W.; Li, Y. Active 3D Pd/graphene aerogel catalyst for hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrog. Energy 2016, 41, 15225–15235. [Google Scholar] [CrossRef]
- Göksu, H.; Yıldız, Y.; Çelik, B.; Yazıcı, M.; Kılbas, B.; Sen, F. Highly Efficient and Monodisperse Graphene Oxide Furnished Ru/Pd Nanoparticles for the Dehalogenation of Aryl Halides via Ammonia Borane. Chemistryselect 2016, 1, 953–958. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, T.J.; Kim, T.Y.; Lee, G.; Park, J.; Nam, S.; Kang, S. Tetraglyme-mediated synthesis of Pd nanoparticles for dehydrogenation of ammonia borane. Chem. Commun. 2012, 48, 2021–2023. [Google Scholar] [CrossRef] [PubMed]
- Nan, C.; Su, J.; Wei, L.; Cheng, G. Hydrolytic dehydrogenation of ammonia borane and methylamine borane catalyzed by graphene supported Ru@Ni core–shell nanoparticles. Int. J. Hydrog. Energy 2014, 39, 426–435. [Google Scholar]
- Qiu, F.; Li, L.; Liu, G.; Xu, C.; An, C.; Xu, Y.; Wang, Y.; Huang, Y.; Chen, C.; Wang, Y. Synthesis of size-controlled Ag@Co@Ni/graphene core-shell nanoparticles for the catalytic hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2014, 9, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, T.; Yan, J.-M.; Zhang, X.-B.; Shioyama, H.; Kuriyama, N.; Xu, Q. Co–SiO2 nanosphere-catalyzed hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage. J. Power Sources 2010, 195, 8209–8214. [Google Scholar] [CrossRef]
- Bulut, A.; Yurderi, M.; Ertas, İ.E.; Celebi, M.; Kaya, M.; Zahmakiran, M. Carbon dispersed copper-cobalt alloy nanoparticles: A cost-effective heterogeneous catalyst with exceptional performance in the hydrolytic dehydrogenation of ammonia-borane. Appl. Catal. B Environ. 2016, 180, 121–129. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Yan, W.; Duan, F.; Zhang, B.; Gao, Z.; Qin, Y. Ni nanoparticles supported on CNTs with excellent activity produced by atomic layer deposition for hydrogen generation from the hydrolysis of ammonia borane. Catal. Sci. Technol. 2016, 6, 2112–2119. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Li, X.; Qian, Y. A facile route to synthesize multiporous MnCo2O4 and CoMn2O4 spinel quasi-hollow spheres with improved lithium storage properties. Nanoscale 2013, 5, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Cai, X.; Song, Y.; Sun, X.; Liu, X.X. VOx@MoO3 Nanorod Composite for High-Performance Supercapacitors. Adv. Funct. Mater 2018, 28, 1803901. [Google Scholar] [CrossRef]
- Ma, S.; Sun, L.; Cong, L.; Gao, X.; Yao, C.; Guo, X.; Tai, L.; Mei, P.; Zeng, Y.; Xie, H.; et al. Multiporous MnCo2O4 Microspheres as an Efficient Bifunctional Catalyst for Nonaqueous Li-O2 Batteries. J. Phys. Chem. C 2013, 117, 25890–25897. [Google Scholar] [CrossRef]
- Luo, W.; Hu, X.; Sun, Y.; Huang, Y. Electrospun porous ZnCo2O4 nanotubes as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. 2012, 22, 8916–8921. [Google Scholar] [CrossRef]
- Jin, R.; Meng, Y.; Ma, Y.; Li, H.; Sun, Y.; Chen, G. Hierarchical MnCo2O4 constructed by mesoporous nanosheets@polypyrrole composites as anodes for lithium ion batteries. Electrochim. Acta 2016, 209, 163–170. [Google Scholar] [CrossRef]
- Reddy, C.V.S.; Jr, E.H.W.; Wen, C.; Mho, S.I. Hydrothermal synthesis of MoO3 nanobelts utilizing poly(ethylene glycol). J. Power Sources 2008, 183, 330–333. [Google Scholar] [CrossRef]
- Mai, L.Q.; Hu, B.; Chen, W.; Qi, Y.Y.; Lao, C.S.; Yang, R.S.; Dai, Y.; Wang, Z.L. Lithiated MoO3 Nanobelts with Greatly Improved Performance for Lithium Batteries. Adv. Mater. 2007, 19, 3712–3716. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.J.; Wang, R.L.; Mi, R.; Li, S.M.; Cui, Y.H.; Deng, Y.F.; Mei, J.; Liu, H. Coating of a-MoO3 on nitrogen-doped carbon nanotubes by electrodeposition as a high-performance cathode material for lithium-ion batteries. J. Power Sources 2015, 274, 1063–1069. [Google Scholar] [CrossRef]
- Venkatachalam, V.; Alsalme, A.; Alghamdi, A.; Jayavel, R. High performance electrochemical capacitor based on MnCo2O4 nanostructured electrode. J. Electroanal. Chem. 2015, 756, 94–100. [Google Scholar] [CrossRef]
- Herrmann, A.K.; Formanek, P.; Borchardt, L.; Klose, M.; Giebeler, L.; Eckert, J.; Kaskel, S.; Gaponik, N.; Eychmuller, A. Multimetallic Aerogels by Template-Free Self-Assembly of Au, Ag, Pt, and Pd Nanoparticles. Chem. Mater. 2013, 26, 1074–1083. [Google Scholar] [CrossRef]
- Fernandes, R.; Patel, N.; Miotello, A.; Calliar, L. Co–Mo–B–P Alloy with Enhanced Catalytic Properties for H2 Production by Hydrolysis of Ammonia Borane. Top. Catal. 2012, 55, 1032–1039. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Lee, S.H.; Ryu, K.S. Hierarchical CuCo2O4 nanobelts as a supercapacitor electrode with high areal and specific capacitance. Electrochim. Acta 2015, 182, 979–986. [Google Scholar] [CrossRef]
- Liu, S.; Hui, K.S.; Hui, K.N.; Yun, J.M.; Kim, K.H. Vertically stacked bilayer CuCo2O4/MnCo2O4 heterostructures on functionalized graphite paper for high-performance electrochemical capacitors. J. Mater. Chem. A 2016, 4, 8061–8071. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Liu, X.; Li, B.; Tang, D.; Liu, W.; Qin, W. Synthesis of magnetic core-shell carbon dots@MFe2O4 (M = Mn, Zn and Cu) hybrid materials and their catalytic properties. J. Mater. Chem. A 2016, 4, 4044–4055. [Google Scholar] [CrossRef]
- Fu, C.; Li, G.; Luo, D.; Huang, X.; Zheng, J.; Li, L. One-step calcination-free synthesis of multicomponent spinel assembled microspheres for high-performance anodes of li-ion batteries: A case study of MnCo2O4. ACS Appl. Mater. Interfaces 2014, 6, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Kamegawa, T.; Mori, K.; Yamashita, H. Surfactant-free nonaqueous synthesis of plasmonic molybdenum oxide nanosheets with enhanced catalytic activity for hydrogen generation from ammonia borane under visible light. Angew. Chem. 2014, 126, 2954–2958. [Google Scholar] [CrossRef]
- Qiu, M.; Zhan, S.; Yu, H.; Zhu, D.; Wang, S. Facile preparation of ordered mesoporous MnCo2O4 for low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2015, 7, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Yang, K.; Hong, X.; Chen, X.; Lu, Z.H. Base-promoted hydrolytic dehydrogenation of ammonia borane catalyzed by noble-metal-free nanoparticles. Catal. Sci. Technol. 2018, 8, 870–877. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Li, Y. Synthesis and catalytic properties of bimetallic nanomaterials with various architectures. Nano Today 2012, 7, 448–466. [Google Scholar] [CrossRef]
- Men, Y.; Su, J.; Du, X.; Liang, L.; Cheng, G.; Luo, W. CoBP nanoparticles supported on three-dimensional nitrogen-doped graphene hydrogel and their superior catalysis for hydrogen generation from hydrolysis of ammonia borane. J. Alloy. Compd. 2018, 735, 1271–1276. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Guan, H.; Zhao, Y.; Yang, J.H.; Zhang, B. Preparation of bimetallic Cu-Co nanocatalysts on poly (diallyldimethylammonium chloride) functionalized halloysite nanotubes for hydrolytic dehydrogenation of ammonia borane. Appl. Surf. Sci. 2018, 427, 106–113. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Zhou, L.; Yang, K. CuNi NPs supported on MIL-101 as highly active catalysts for the hydrolysis of ammonia borane. Appl. Surf. Sci. 2017, 427, 114–122. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Q.L.; Xu, Q. Non-noble bimetallic CuCo nanoparticles encapsulated in the pores of metal–organic frameworks: Synergetic catalysis in the hydrolysis of ammonia borane for hydrogen generation. Catal. Sci. Technol. 2014, 5, 525–530. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, Y.; Zheng, Z.; Huang, B. Covalently-terminated germanane GeH and GeCH3 for hydrogen generation from catalytic hydrolysis of ammonia borane under visible light irradiation. Catal. Commun. 2019, 118, 46–50. [Google Scholar] [CrossRef]
- Feng, W.; Lan, Y.; Nan, C.; Cheng, D.; Dai, H.; Wei, L.; Cheng, G. In situ facile synthesis of bimetallic CoNi catalyst supported on graphene for hydrolytic dehydrogenation of amine borane. Int. J. Hydrog. Energy 2014, 39, 3371–3380. [Google Scholar] [CrossRef]
- Wang, C.; Sun, D.; Yu, X.; Zhang, X.; Lu, Z.; Wang, X.; Zhao, J.L.; Li, L.; Yang, X. Cu/Ni nanoparticles supported on TiO2(B) nanotubes as hydrogen generation photocatalysts via hydrolysis of ammonia borane. Inorg. Chem. Front. 2018, 5, 2038–2044. [Google Scholar] [CrossRef]
- Yang, L.; Cao, N.; Cheng, D.; Dai, H.; Hu, K.; Luo, W.; Cheng, G. Graphene supported cobalt (0) nanoparticles for hydrolysis of ammonia borane. Mater. Lett. 2014, 115, 113–116. [Google Scholar] [CrossRef]
- Du, X.; Yang, C.; Zeng, X.; Wu, T.; Zhou, Y.; Cai, P.; Cheng, G.; Luo, W. Amorphous NiP supported on rGO for superior hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2017, 42, 14181–14187. [Google Scholar] [CrossRef]
- Cui, L.; Cao, X.; Sun, X.; Yang, W.; Liu, J. A Bunch-like Copper Oxide Nanowire Array as an Efficient, Durable, and Economical Catalyst for the Methanolysis of Ammonia Borane. Chemcatchem 2018, 10, 710–715. [Google Scholar] [CrossRef]
- Zacho, S.L.; Mielby, J.; Kegnæs, S. Hydrolytic dehydrogenation of ammonia borane over ZIF-67 derived Co nanoparticle catalysts. Catal. Sci. Technol. 2018, 8, 4741–4746. [Google Scholar] [CrossRef]
- Lou, Y.; He, J.; Liu, G.; Qi, S.; Cheng, L.; Chen, J.; Zhao, Y.; Zhu, J. Efficient hydrogen evolution from the hydrolysis of ammonia borane using bilateral-like WO3-x nanorods coupled with Ni2P nanoparticles. Chem. Commun. 2018, 54, 6188–6191. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yano, K.; Xu, Q.; Fukuzumi, S. Cu/Co3O4 Nanoparticles as Catalysts for Hydrogen Evolution from Ammonia Borane by Hydrolysis. J. Phys. Chem. C 2012, 114, 16456–16462. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. Size-Dependent Catalytic Activity of Monodispersed Nickel Nanoparticles for the Hydrolytic Dehydrogenation of Ammonia Borane. ACS Appl. Mater. Interfaces 2018, 10, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Cheng, F.; Tao, Z.; Chen, J. Cobalt nanoparticles embedded in porous N-doped carbon as long-life catalysts for hydrolysis of ammonia borane. Catal. Sci. Technol. 2016, 6, 3443–3448. [Google Scholar] [CrossRef]
- Lu, H.; Li, M.; Hu, J. A stable, efficient 3D Cobalt-graphene composite catalyst for the hydrolysis of ammonia borane. Catal. Sci. Technol. 2016, 6, 7186–7192. [Google Scholar]
- Zheng, H.; Feng, K.; Shang, Y.; Kang, Z.; Sun, X.; Zhong, J. Cube-Like CuCoO Nanostructures on Reduced Graphene Oxide for H2 Generation from Ammonia Borane. Inorg. Chem. Front. 2018, 5, 1180–1187. [Google Scholar] [CrossRef]


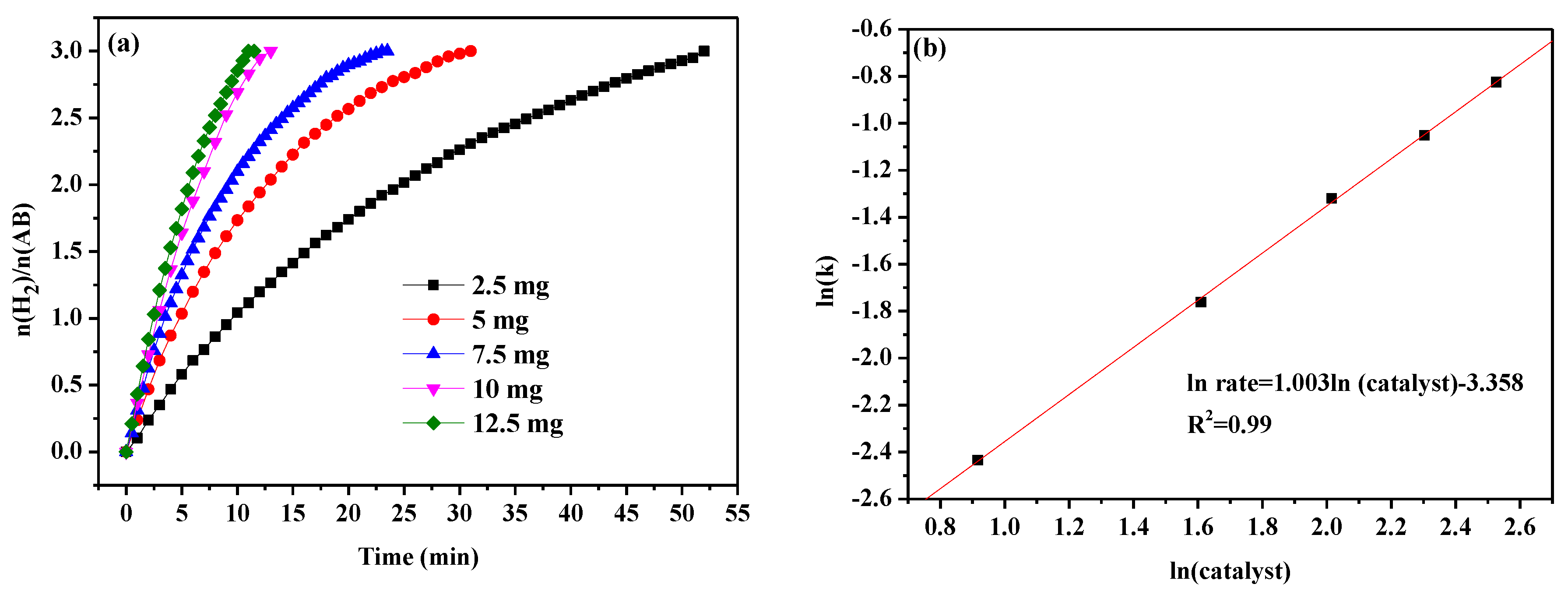
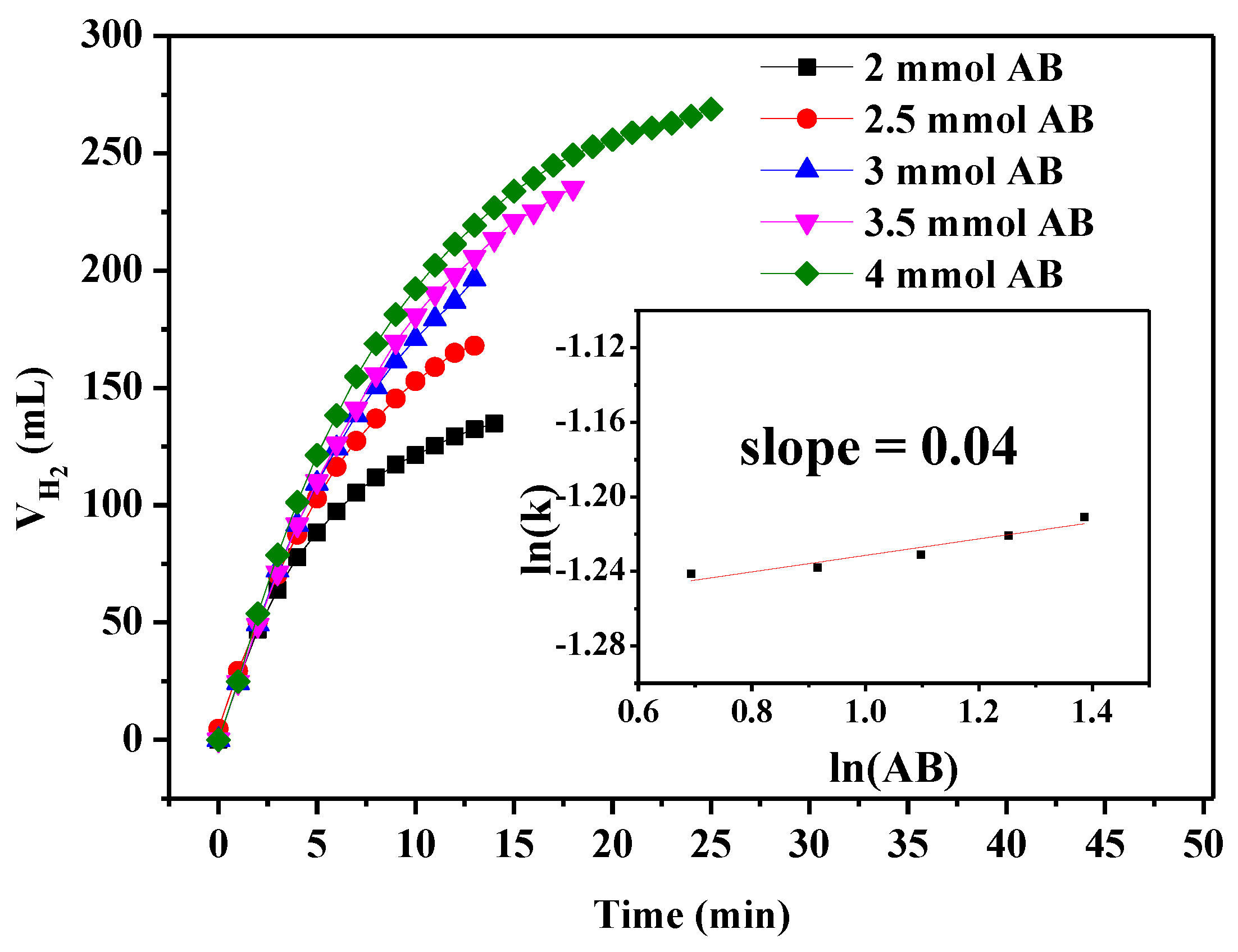
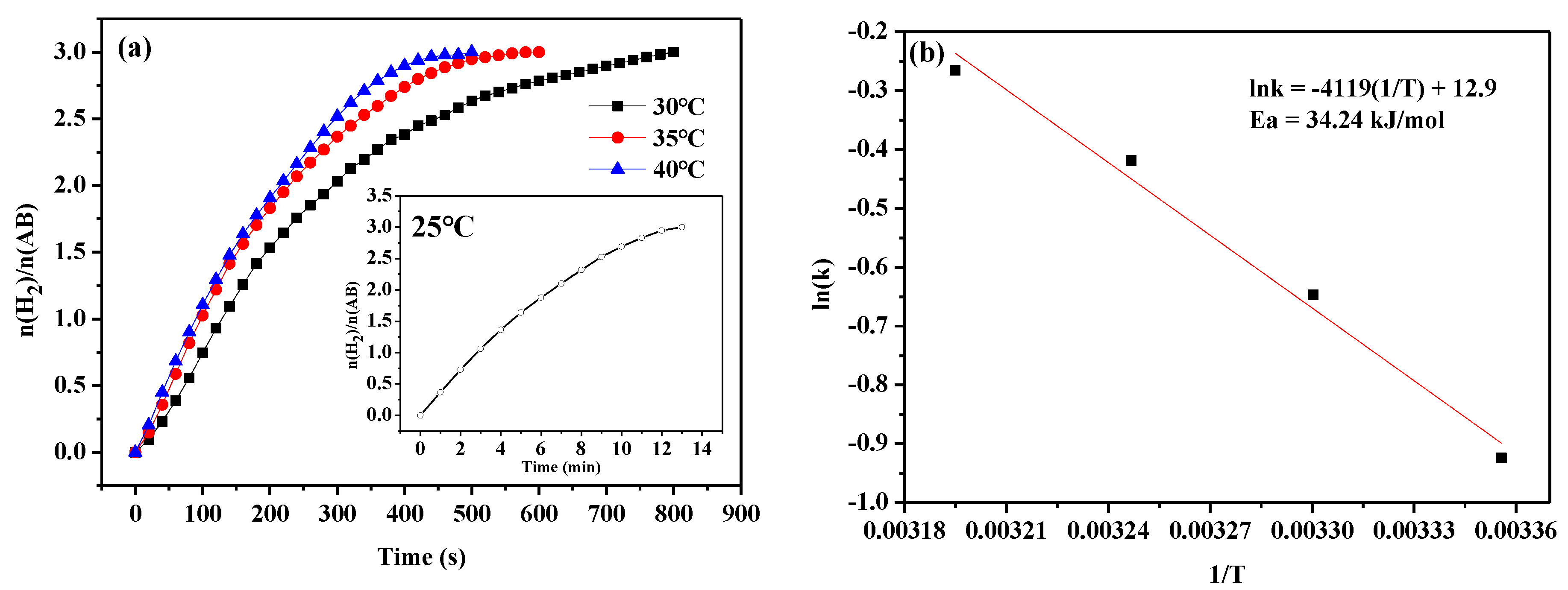
| Catalyst | BET (m² g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| MoO3-doped MnCo2O4 (0) | 13.24 | 0.069 | 3.81 |
| MoO3-doped MnCo2O4 (0.04) | 37.29 | 0.123 | 3.71 |
| MoO3-doped MnCo2O4 (0.10) | 43.26 | 0.193 | 3.48 |
| MoO3-doped MnCo2O4 (0.12) | 62.06 | 0.254 | 3.48 |
| Catalysts | TOF (molhydrogenmin−1molcat−1) | Reference |
|---|---|---|
| Co0.79B0.15P0.06/NGH | 32.8 | [40] |
| Cu-Co/PDDA-HNTs | 30.8 | [41] |
| Cu0.49Co0.51/C | 28.7 | [19] |
| MoO3-doped MnCo2O4 (0.10) | 26.4 | This work |
| Ni/CNTs | 26.2 | [20] |
| Cu2Ni1@MIL-101 | 20.9 | [42] |
| CuCo@MIL-101 | 19.6 | [43] |
| GeCH3 | 18.16 | [44] |
| CoNi/Graphene | 16.8 | [45] |
| Cu0.64Ni0.36-TiO2 | 15.9 | [46] |
| Co/Graphene | 13.8 | [47] |
| Ni91P9/rGO | 13.3 | [48] |
| b-CuO NA/CF | 13.3 | [49] |
| Co/NC-50 | 12.7 | [50] |
| Ni2P | 8.16 | [51] |
| Cu/Co3O4 | 7.0 | [52] |
| Ni/KB | 5.9 | [53] |
| Co@N-C | 5.6 | [54] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Feng, Y.; Ding, Z.; Liao, J.; Zhang, X.; Liu, H.-R.; Li, H. MoO3-Doped MnCo2O4 Microspheres Consisting of Nanosheets: An Inexpensive Nanostructured Catalyst to Hydrolyze Ammonia Borane for Hydrogen Generation. Nanomaterials 2019, 9, 21. https://doi.org/10.3390/nano9010021
Lu D, Feng Y, Ding Z, Liao J, Zhang X, Liu H-R, Li H. MoO3-Doped MnCo2O4 Microspheres Consisting of Nanosheets: An Inexpensive Nanostructured Catalyst to Hydrolyze Ammonia Borane for Hydrogen Generation. Nanomaterials. 2019; 9(1):21. https://doi.org/10.3390/nano9010021
Chicago/Turabian StyleLu, Dongsheng, Yufa Feng, Zitian Ding, Jinyun Liao, Xibin Zhang, Hui-Ru Liu, and Hao Li. 2019. "MoO3-Doped MnCo2O4 Microspheres Consisting of Nanosheets: An Inexpensive Nanostructured Catalyst to Hydrolyze Ammonia Borane for Hydrogen Generation" Nanomaterials 9, no. 1: 21. https://doi.org/10.3390/nano9010021





