A Magnetic Adsorbent for the Removal of Cationic Dyes from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedures
2.3. Synthesis
2.4. Crystallography
2.5. The Experimental and Procedures Adopted for the Adsorption
3. Results and Discussion
3.1. Crystal Structure Description of Compound 1
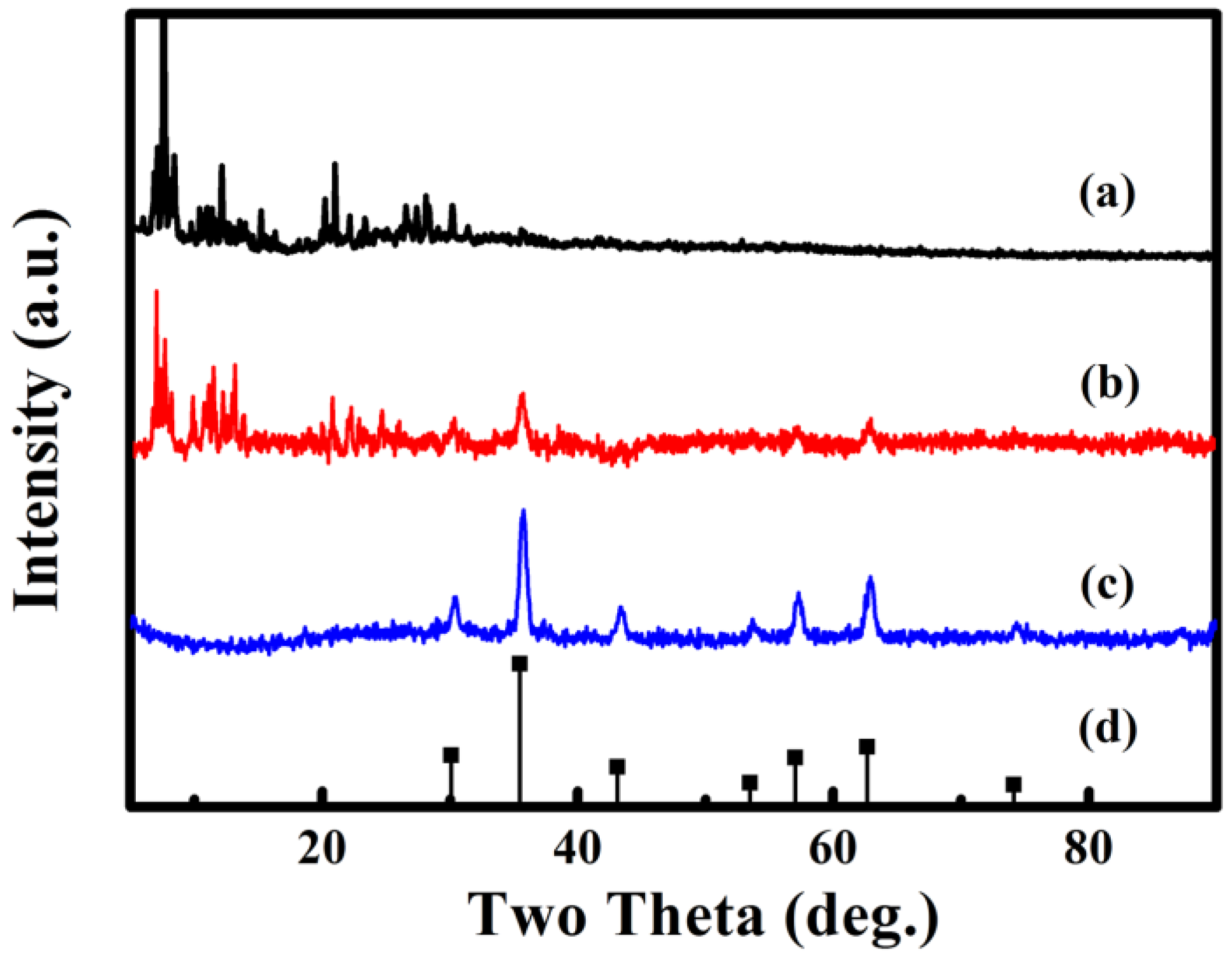
3.2. XRD Patterns
3.3. IR Spectroscopy
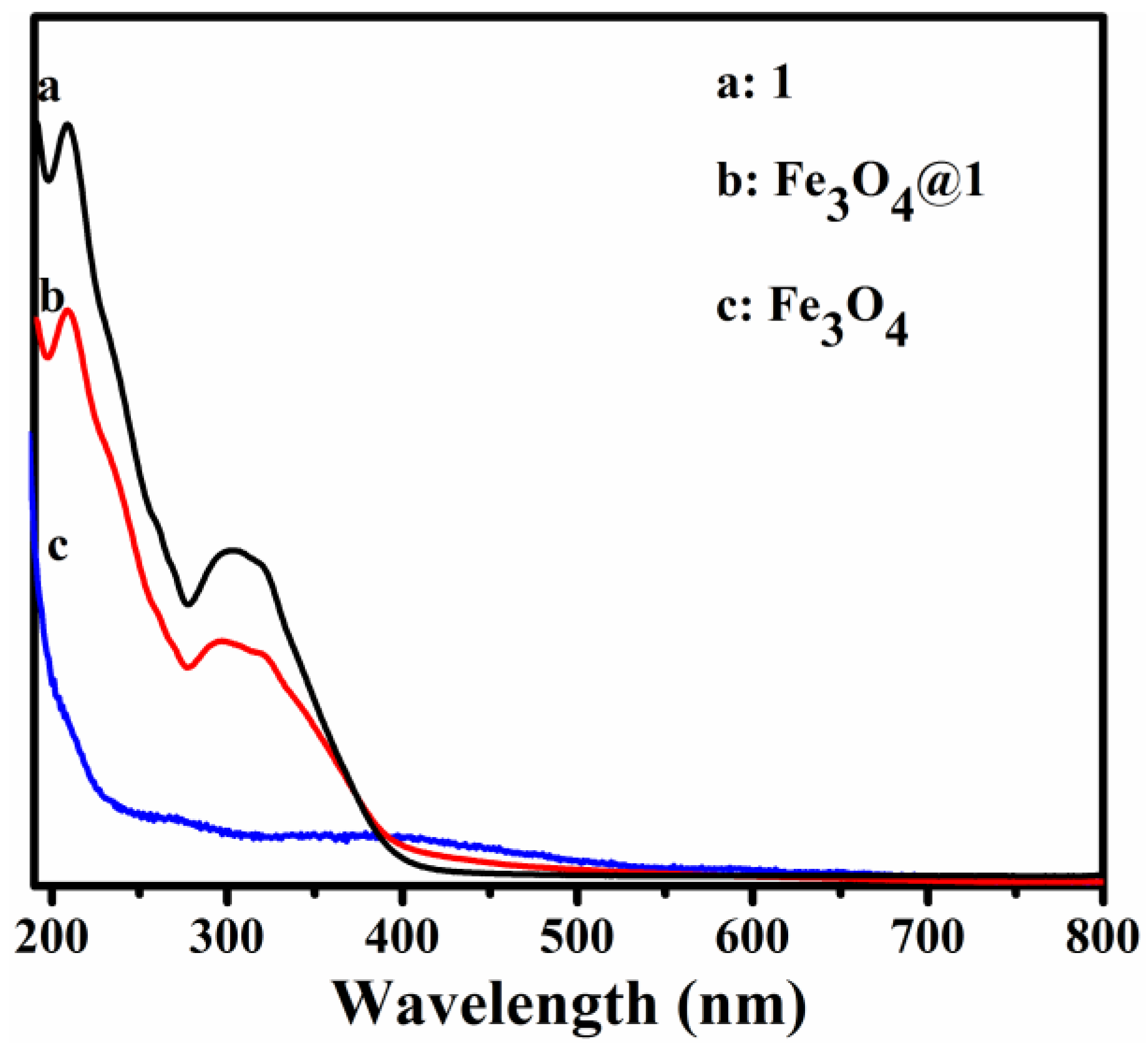
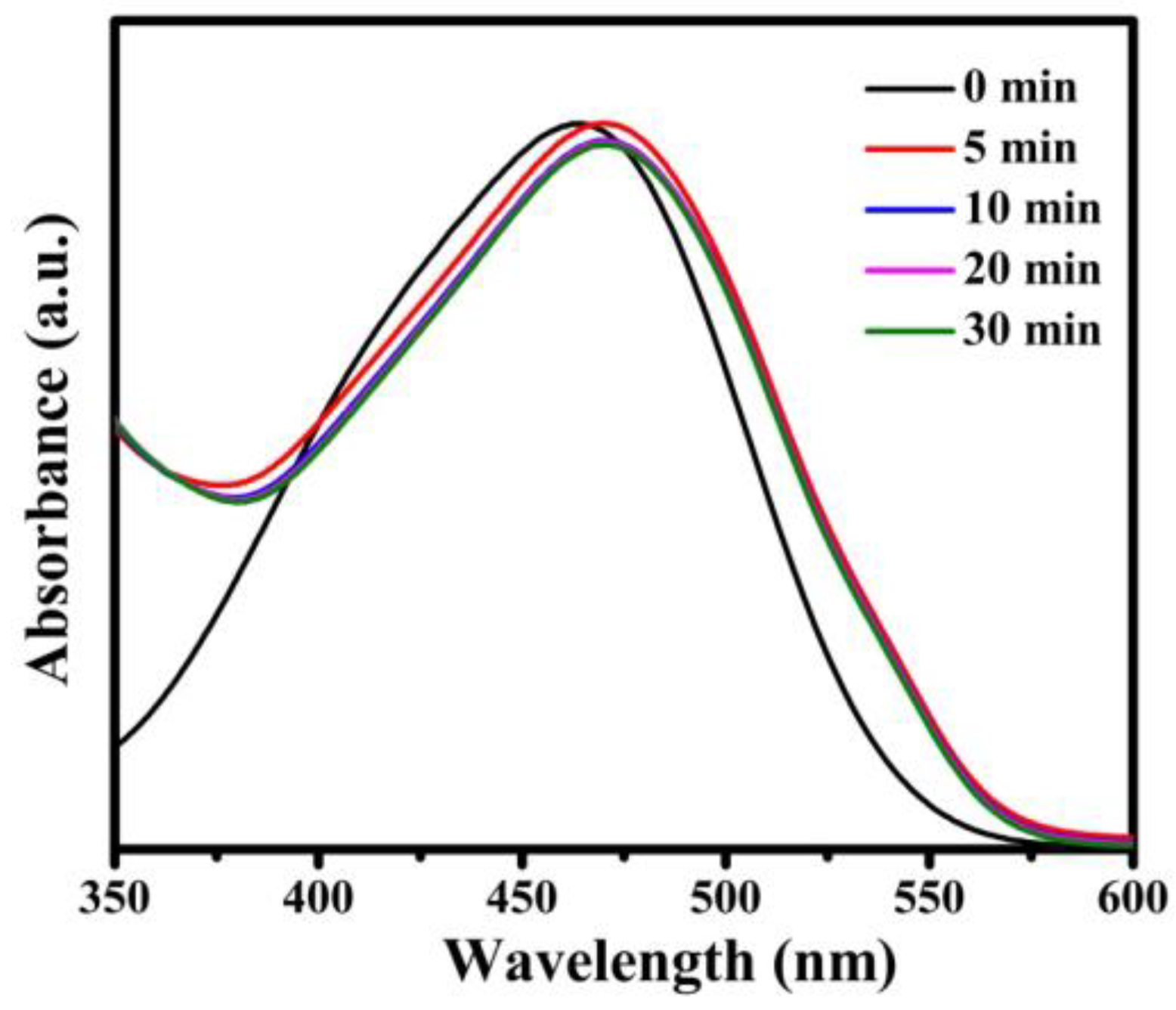
3.4. UV-Vis Spectroscopy
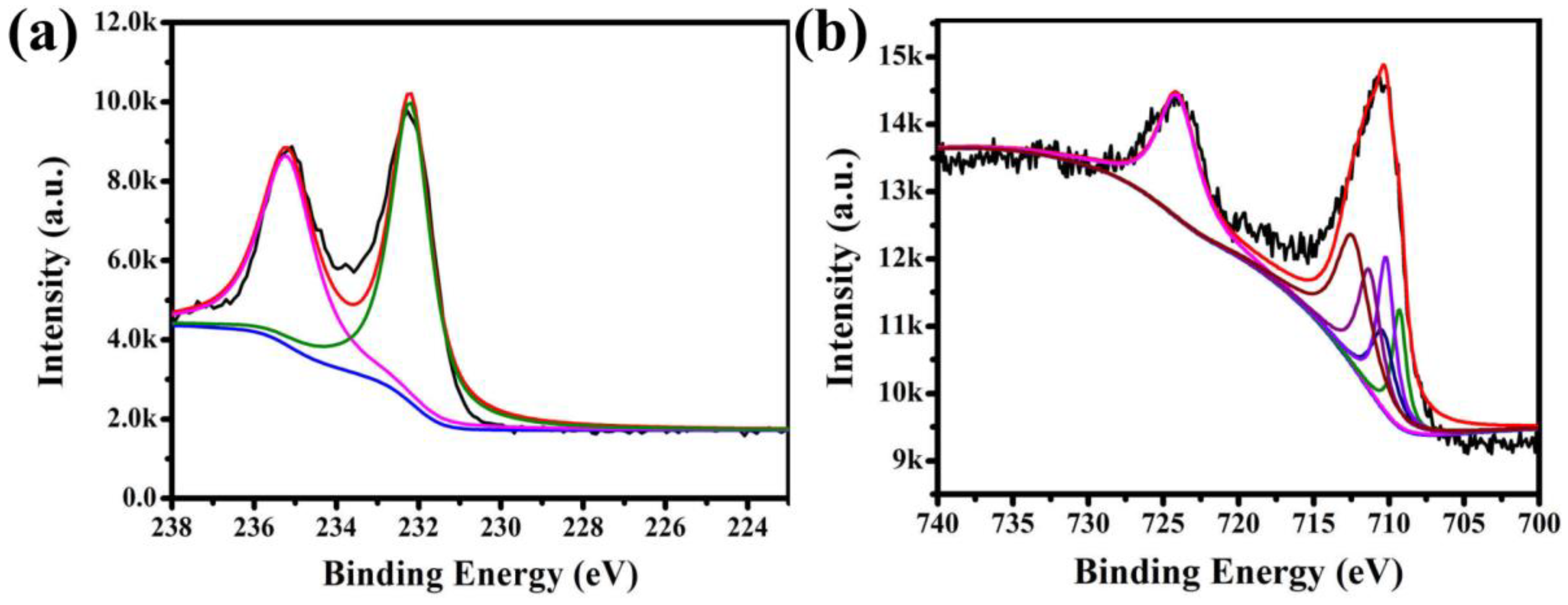
3.5. XPS Characterization
3.6. TEM Morphology and Particle Size Distribution of Fe3O4 and Fe3O4@1
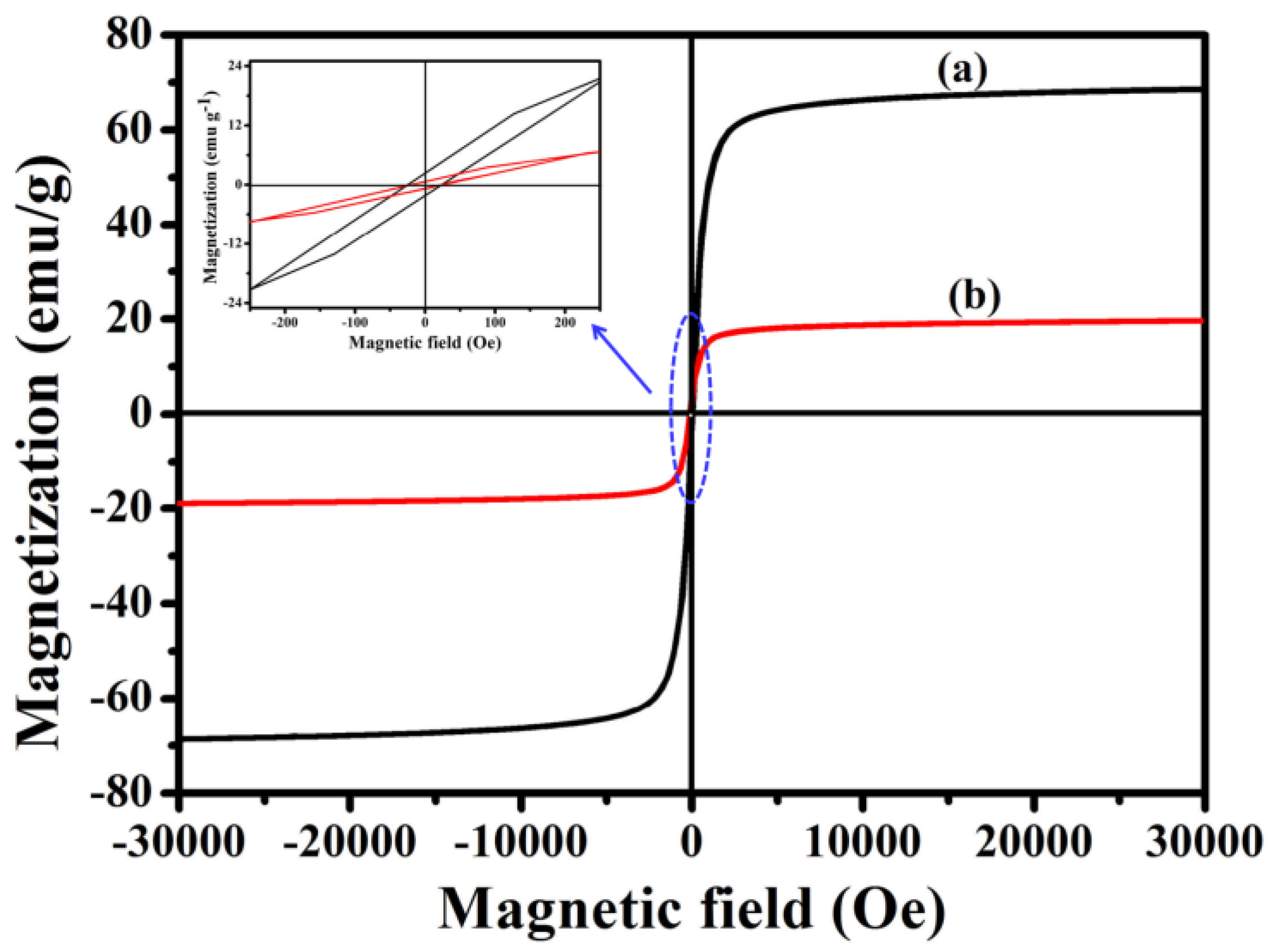
3.7. Magnetic Properties of Fe3O4@1
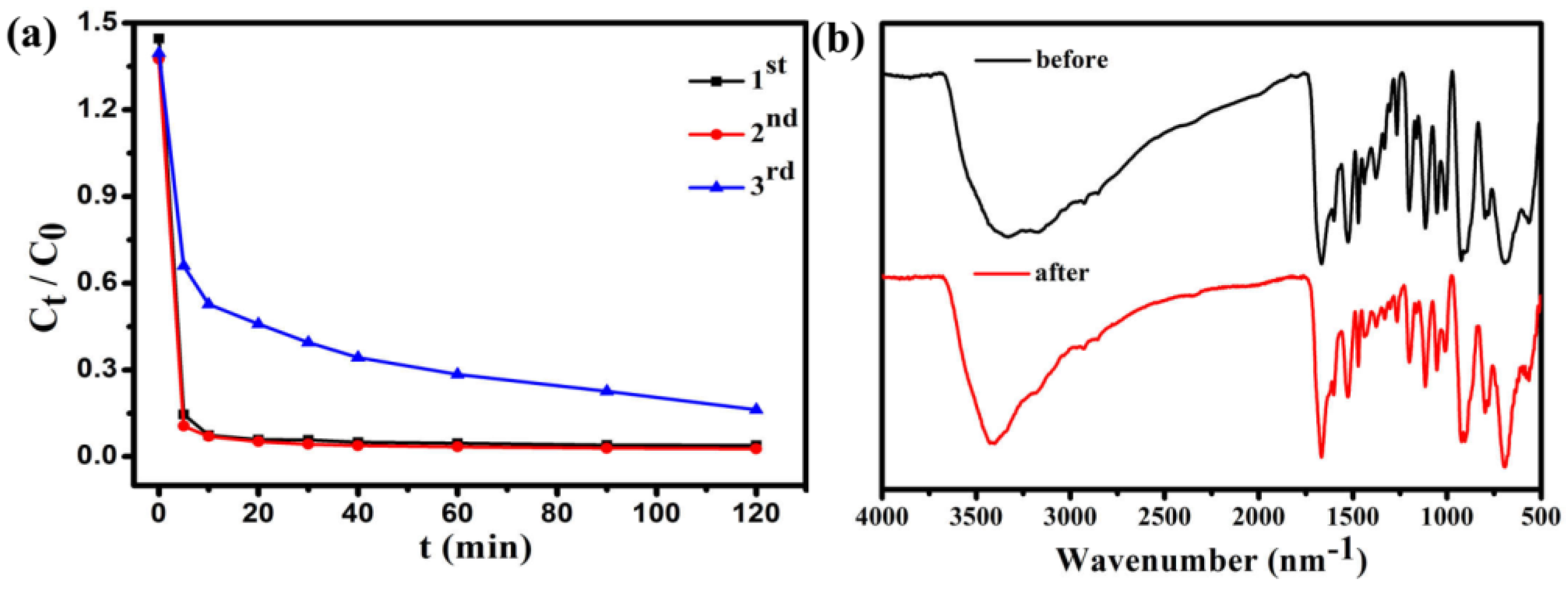
3.8. Separation and Redispersion Process of Fe3O4@1
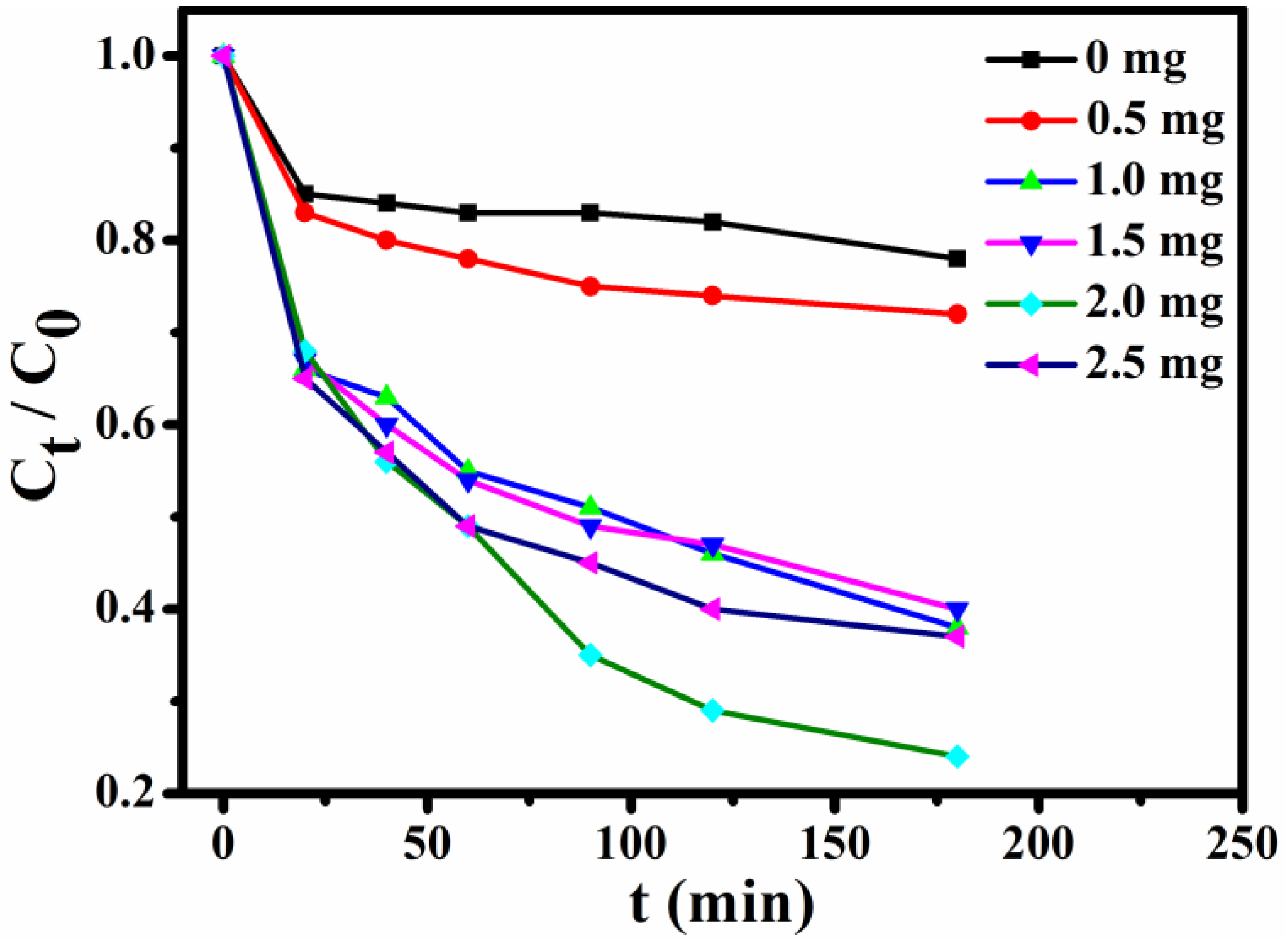
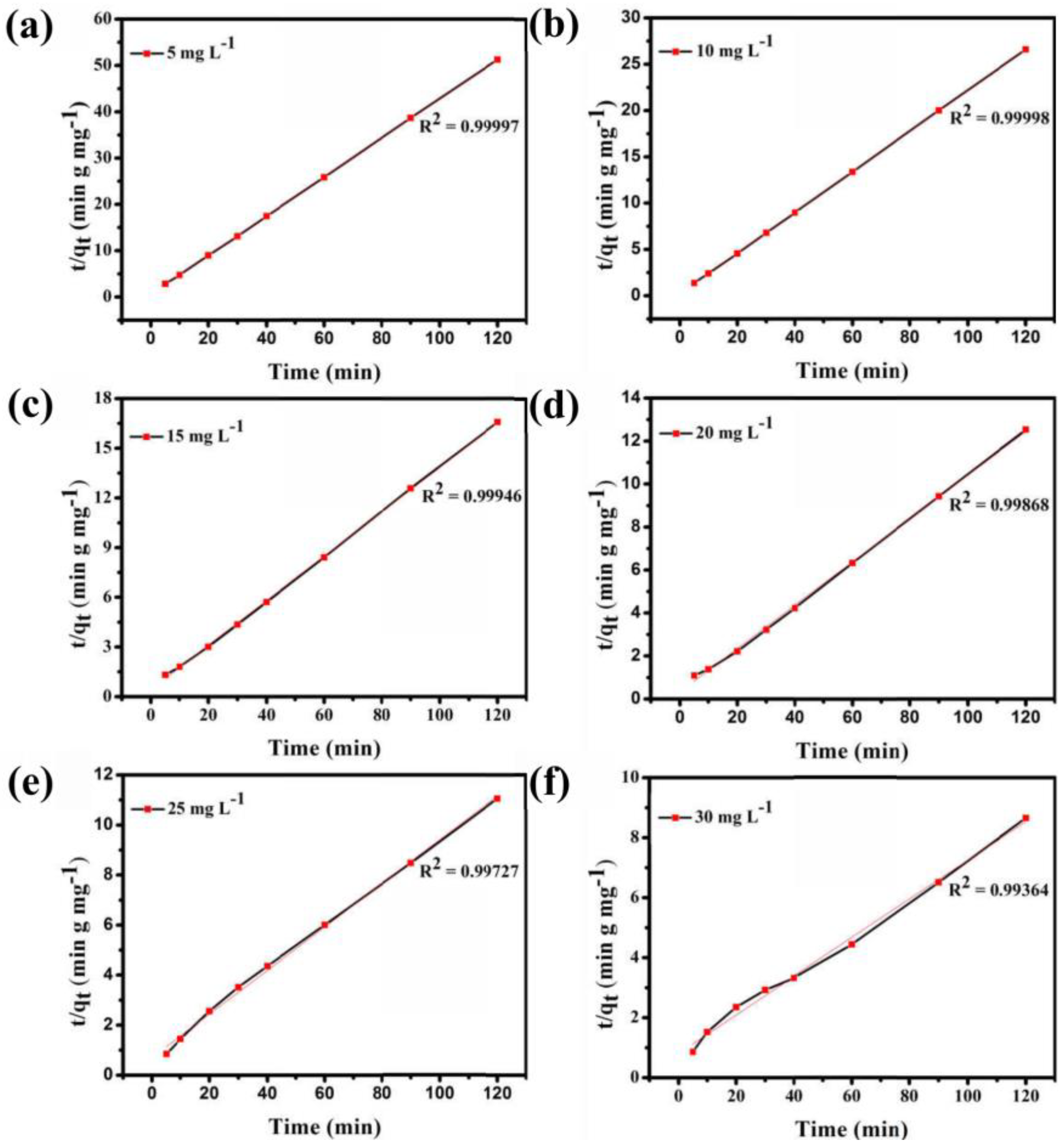
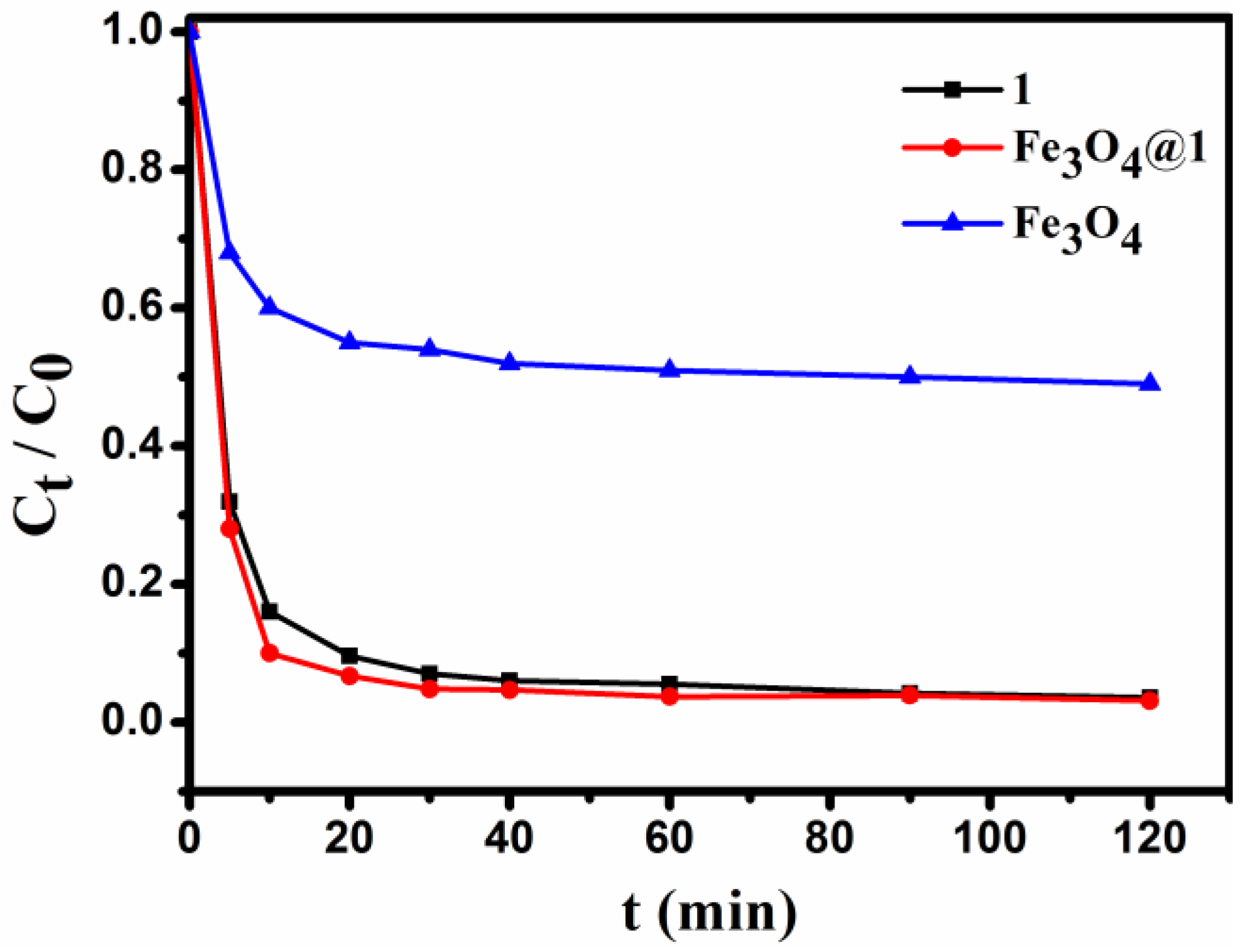
3.9. Dye Adsorption Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef] [Green Version]
- Yi, F.Y.; Zhu, W.; Dang, S.; Li, J.P.; Wu, D.; Li, Y.H.; Sun, Z.M. Polyoxometalates-based heterometallic organic–inorganic hybrid materials for rapid adsorption and selective separation of methylene blue from aqueous solutions. Chem. Commun. 2015, 51, 3336–3339. [Google Scholar] [CrossRef] [PubMed]
- Oyanedel-Craver, V.A.; Smith, J.A. Sustainable colloidal-silver-impregnated ceramic filter for point-of-use water treatment. Environ. Sci. Technol. 2008, 42, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.P.; Thiruvenkatachari, R.; Shim, W.G.; Moon, H. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigments 2006, 69, 196–203. [Google Scholar] [CrossRef]
- Lu, H.D.; Lim, T.L.; Javitt, S.; Heinmiller, A.; Prud’homme, R.K. Assembly of macrocycle dye derivatives into particles for fluorescence and photoacoustic applications. ACS Comb. Sci. 2017, 19, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, A.D.; Perera, S.D.; Tan, K.; Chabal, Y.; Balkus, K.J., Jr. Synthesis, characterization, and photocatalytic activity of Y-doped CeO2 nanorods. ACS Catal. 2014, 4, 577–584. [Google Scholar] [CrossRef]
- Mian, F.; Bottaro, G.; Rancan, M.; Pezzato, L.; Gombac, V.; Fornasiero, P.; Armelao, L. Bi12O17Cl2/(BiO)2CO3 nanocomposite materials for pollutant adsorption and degradation: Modulation of the functional properties by composition tailoring. ACS Omega 2017, 2, 6298–6308. [Google Scholar] [CrossRef]
- Petrella, A.; Mascolo, G.; Murgolo, S.; Petruzzelli, V.; Ranieri, E.; Spasiano, D.; Petruzzelli, D. Photocatalytic oxidation of organic micro-pollutants: Pilot plant investigation and mechanistic aspects of the degradation reaction. Chem. Eng. Commun. 2016, 203, 1298–1307. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Y.M.; Tang, H.B.; Zhang, P.; Wang, J.J. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic activity of BiPO4 nanoparticles. Appl. Surf. Sci. 2017, 425, 100–106. [Google Scholar] [CrossRef]
- Karimifard, S.; Moghaddam, M.R.A. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018, 640–641, 772–797. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Palui, G.; Banerjee, A. Self-assembling tripeptide based hydrogels and their use in removal of dyes from waste-water. Soft Matter 2009, 5, 3452–3460. [Google Scholar] [CrossRef]
- Dang, T.D.; Banerjee, A.N.; Cheney, M.A.; Qian, S.; Joo, S.W.; Min, B.K. Bio-silica coated with amorphous manganese oxide as an efficient catalyst for rapid degradation of organic pollutant. Colloid Surf. B 2013, 106, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Jameel, U.; Zhu, M.Q.; Chen, X.Z.; Tong, Z.F. Recent progress of synthesis and applications in polyoxometalate and nanogold hybrid materials. J. Mater. Sci. 2016, 51, 2181–2198. [Google Scholar] [CrossRef]
- Du, D.Y.; Yan, L.K.; Su, Z.M.; Li, S.L.; Lan, Y.Q.; Wang, E.B. Chiral polyoxometalate-based materials: From design syntheses to functional applications. Coord. Chem. Rev. 2013, 257, 702–717. [Google Scholar] [CrossRef]
- Sathya, A.; Ravindran, T.R.; Philip, J. Superior thermal stability of polymer capped Fe3O4 magnetic nanoclusters. J. Am. Ceram. Soc. 2018, 101, 483–491. [Google Scholar] [CrossRef]
- Liu, H.L.; Wu, J.H.; Min, J.H.; Zhang, X.Y.; Kim, Y.K. Tunable synthesis and multifunctionalities of Fe3O4–ZnO hybrid core-shell nanocrystals. Mater. Res. Bull. 2013, 48, 551–558. [Google Scholar] [CrossRef]
- Xia, M.S.; Jiang, Y.S.; Zhao, L.; Li, F.F.; Xue, B.; Sun, M.M.; Liu, D.R.; Zhang, X.G. Wet grinding of montmorillonite and its effect on the properties of mesoporous montmorillonite. Colloid Surf. A 2010, 356, 1–9. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, R. The molecular and crystal structure of Na6Mo5P2O23(H2O)13, a compound containing sodium-coordinated pentamolybdodiphosphate anions. Acta Chem. Scand. 1973, 27, 1004–1018. [Google Scholar] [CrossRef]
- Li, Z.L.; Wang, Y.; Zhang, L.C.; Wang, J.P.; You, W.S.; Zhu, Z.M. Three molybdophosphates based on Strandbergtype anions and Zn(II)-H2biim/H2O subunits: Syntheses, structures and catalytic properties. Dalton Trans. 2014, 43, 5840–5846. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, J.P.; Jia, J.G.; Ma, P.T.; Zhang, D.D.; Wang, J.P.; Niu, J.Y. Isopentatungstate-supported metal carbonyl derivative: Synthesis, characterization, and catalytic properties for alkene epoxidation. Dalton Trans. 2016, 45, 6726–6731. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.A.; Perdigão, G.M.C.; Rodrigues, L.E.; Da Silva, J.G.; Souza-Fagundes, E.M.; Takahashi, J.A.; Rocha, W.R.; Beraldo, H. Cytotoxic and antimicrobial effects of indium(III) complexes with 2-acetylpyridine-derived thiosemicarbazones. Dalton Trans. 2017, 46, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, K.S.O.; Da Silva, J.G.; Costa, F.M.; Mendes, B.M.; Rodrigues, B.L.; Dos Santos, R.G.; Beraldo, H. N(4)-Tolyl-2-acetylpyridine thiosemicarbazones and their platinum(II,IV) and gold(III) complexes: Cytotoxicity against human glioma cells and studies on the mode of action. Biometals 2013, 26, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Wu, C.X.; Feng, X.J.; Tan, H.Q.; Yan, L.K.; Liu, Y.; Kang, Z.H.; Wang, E.B.; Li, Y.G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Liu, H.L.; Wu, J.H.; Min, J.H.; Lee, J.H.; Kim, Y.K. Monosized core–shell Fe3O4(Fe)/Au multifunctional nanocrystals. J. Nanosci. Nanotechnol. 2009, 9, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Shi, Y.L.; Qiu, W.; Zheng, Y. Synthesis and characterization of a POM-based nanocomposite as a novel magnetic photocatalyst. J. Phys. Chem. Solids 2006, 67, 2409–2418. [Google Scholar] [CrossRef]
- Zhu, W.H.; Zeng, M.; Wang, J.; Li, C.Y.; Tian, L.H.; Yin, J.C.; Liu, Y.K. A multifunctional lanthanide metal–organic framework supported by Keggin type polyoxometalates. Dalton Trans. 2016, 45, 10141–10145. [Google Scholar] [CrossRef] [PubMed]
- Schaepertoens, M.; Didaskalou, C.; Kim, J.F.; Livingston, A.G.; Szekely, G. Solvent recycle with imperfect membranes: A semi-continuous workaround for diafiltration. J. Membr. Sci. 2016, 514, 646–658. [Google Scholar] [CrossRef] [Green Version]
- Fodi, T.; Didaskalou, C.; Kupai, J.; Balogh, G.T.; Huszthy, P.; Szekely, G. Nanofiltration-enabled in situ solvent and reagent recycle for sustainable continuous-flow synthesis. ChemSusChem 2017, 10, 3435–3444. [Google Scholar] [CrossRef] [PubMed]



| Crystal Data | 1 |
|---|---|
| Empirical formula Formula weight | C32H58Cu2Mo5N16O37P2 1909.52 |
| crystal system | Triclinic |
| space group | P-1 |
| Temperature (K) | 296(2) |
| a (Å) | 14.0430(7) |
| b (Å) c (Å) α (deg) | 14.6752(7) 18.3928(9) 71.4980(10) |
| β (deg) γ (deg) Volume (Å3) Z | 69.5040(10) 63.3060(10) 3113.5(3) 2 |
| Calculated density (g cm−3) | 2.037 |
| Absorption coefficient (mm−1) Crystal size (mm3) | 1.804 0.41 × 0.33 × 0.25 |
| Theta range for data collection (deg) | 1.58–25.00 |
| F(000) | 1876 |
| Limiting indices | −16 ≤ h ≤ 16, −14 ≤ k ≤ 17, −14 ≤ l ≤ 21 |
| Rint | 0.0165 |
| parameters | 833 |
| Reflections collected/unique | 10869/9512 |
| Final R indices [I ≥ 2σ(I)] | R1 = 0.0318, wR2 = 0.0902 |
| R indices (all data) | R1 = 0.0379, wR2 = 0.0931 |
| Largest diff. peak and hole (e Å−3) | 2.103, −0.677 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Ma, C.; Li, J.; Zhao, H.; Chen, Q.; Li, M.; Liu, H. A Magnetic Adsorbent for the Removal of Cationic Dyes from Wastewater. Nanomaterials 2018, 8, 710. https://doi.org/10.3390/nano8090710
Ji Y, Ma C, Li J, Zhao H, Chen Q, Li M, Liu H. A Magnetic Adsorbent for the Removal of Cationic Dyes from Wastewater. Nanomaterials. 2018; 8(9):710. https://doi.org/10.3390/nano8090710
Chicago/Turabian StyleJi, Yumei, Chenguang Ma, Jie Li, Haiyan Zhao, Qianqian Chen, Mingxue Li, and Hongling Liu. 2018. "A Magnetic Adsorbent for the Removal of Cationic Dyes from Wastewater" Nanomaterials 8, no. 9: 710. https://doi.org/10.3390/nano8090710
APA StyleJi, Y., Ma, C., Li, J., Zhao, H., Chen, Q., Li, M., & Liu, H. (2018). A Magnetic Adsorbent for the Removal of Cationic Dyes from Wastewater. Nanomaterials, 8(9), 710. https://doi.org/10.3390/nano8090710





