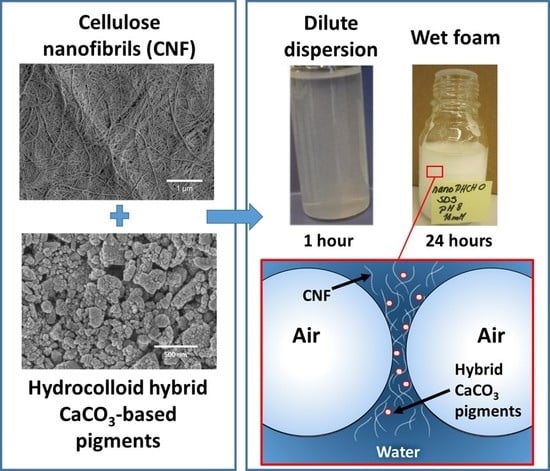
Enhancing the Stability of Aqueous Dispersions and Foams Comprising Cellulose Nanofibrils (CNF) with CaCO3 Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cellulose Nanofibrils (CNF)
2.2. Pigments
2.3. SEM Imaging of The CNF and Pigments
2.4. Other Chemicals
2.5. Preparation of Dilute Pure CNF Dispersions and Pigment-Containing CNF Dispersions
2.6. Preparation of CNF-stabilised Pigment-Containing Foams
2.7. Stability of Dilute CNF Dispersions and Pigment-Containing CNF Dispersions
2.8. Stability of CNF Stabilised Foams and Pigment-Containing CNF Foams
3. Results and Discussion
3.1. Assessment of Dispersion Stability
3.1.1. Stability of Dilute CNF Dispersions
3.1.2. Stability of Pigment-Containing CNF Dispersions
3.2. Assessment of Foam Stability
3.2.1. Stability of Reference Foams
3.2.2. Stability of Pigment-Containing CNF Foams
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jutila, E.; Koivunen, R.; Kiiski, I.; Bollström, R.; Sikanen, T.; Gane, P. Microfluidic lateral flow cytochrome P450 assay on a novel printed functionalised calcium carbonate-based platform for rapid screening of human xenobiotic metabolism. Adv. Funct. Mater. 2018, 1802793–1802804. [Google Scholar] [CrossRef]
- Farhadi-Khouzani, M.; Schütz, C.; Durak, G.M.; Fornell, J.; Sort, J.; Salazar-Alvarez, G.; Bergström, L.; Gebauer, D. A CaCO3/nanocellulose-based bioinspired nacre-like material. J. Mater. Chem. A 2017, 5, 16128–16133. [Google Scholar] [CrossRef]
- Mohammadkazemi, R.; Faria, M.; Cordeiro, N. In situ biosynthesis of bacterial nanocellulose-CaCO3 hybrid bionanocomposite: One-step process. Mater. Sci. Eng. C 2016, 65, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ma, Z.; Tian, H.; Gu, M. Biodegradable poly(vinyl alcohol) foams supported by cellulose nanofibrils: Processing, structure, and properties. Langmuir 2014, 30, 9544–9550. [Google Scholar] [CrossRef] [PubMed]
- Honorato, C.; Kumar, V.; Liu, J.; Koivula, H.; Xu, C.; Toivakka, M. Transparent nanocellulose-pigment composite films. J. Mater. Sci. 2015, 50, 7343–7352. [Google Scholar] [CrossRef]
- Liu, A.; Walther, A.; Ikkala, O.; Belova, L.; Berglund, L. Clay nanopaper with tough cellulose nanofiber matrix for fire retardancy and gas barrier functions. Biomacromolecules 2011, 12, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, J.; Saito, T.; Isogai, A. Simple freeze-drying procedure for producing nanocellulose aerogel-containing, high-performance air filters. ACS Appl. Mater. Interfaces 2015, 7, 19809–19815. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, B.; Aranda, P.; Ruiz-Hitzky, E.; Darder, M. Hierarchically structured bioactive foams based on polyvinyl alcohol-sepiolite nanocomposites. J. Mater. Chem. B 2013, 1, 2911–2920. [Google Scholar] [CrossRef]
- Radvan, B.; Gatward, A.P.J. The formation of wet-laid webs by a foaming process. Tappi 1972, 55, 748–751. [Google Scholar]
- Al-Qararah, A. Aqueous Foam as the Carrier Phase in the Deposition of Fibre Networks. Ph.D. Thesis, University of Jyväskylä, Jyväskylä, Finland, 2015. [Google Scholar]
- Al-Qararah, A.; Ekman, A.; Hjelt, T.; Ketoja, J.A.; Kiiskinen, H.; Koponen, A.; Timonen, J. A unique microstructure of the fiber networks deposited from foam-fiber suspensions. Colloids Surf. A 2015, 482, 544–553. [Google Scholar] [CrossRef]
- Lee, K.-Y. Nanocellulose and Sustainability. Production, Properties, Applications and Case Studies; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; ISBN 9781498761031. [Google Scholar]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Nonappa; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced materials through assembly of nanocelluloses. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Dimic-Misic, K.; Rantanen, J.; Maloney, T.C.; Gane, P.A.C. Gel structure phase behavior in micro nanofibrillated cellulose containing in situ precipitated calcium carbonate. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Liu, G.; Maloney, T.; Dimic-Misic, K.; Gane, P. Acid dissociation of surface bound water on cellulose nanofibrils in aqueous micro nanofibrillated cellulose (MNFC) gel revealed by adsorption of calcium carbonate nanoparticles under the application of ultralow shear. Cellulose 2017, 24, 3155–3178. [Google Scholar] [CrossRef] [Green Version]
- Tenhunen, T.-M.; Peresin, M.S.; Penttilä, P.A.; Pere, J.; Serimaa, R.; Tammelin, T. Significance of xylan on the stability and water interactions of cellulosic nanofibrils. React. Funct. Polym. 2014, 85. [Google Scholar] [CrossRef]
- Arola, S.; Malho, J.-M.; Laaksonen, P.; Lille, M.; Linder, M. The role of hemicellulose in nanofibrillated cellulose networks. Soft Matter 2013, 9, 1319–1326. [Google Scholar] [CrossRef]
- Tanaka, R.; Saito, T.; Hänninen, T.; Ono, Y.; Hakalahti, M.; Tammelin, T.; Isogai, A. Viscoelastic properties of core–shell-structured, hemicellulose-rich nanofibrillated cellulose in dispersion and wet-film states. Biomacromolecules 2016, 17, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Nypelö, T.; Pynnönen, H.; Österberg, M.; Paltakari, J.; Laine, J. Interactions between inorganic nanoparticles and cellulose nanofibrils. Cellulose 2012, 19, 779–792. [Google Scholar] [CrossRef]
- Schenker, M.; Schoelkopf, J.; Mangin, P.; Gane, P.A.C. Dewatering of micro and nano-fibrillated cellulose (MNFC) suspensions: Influence of additives and manufacturing process. In Proceedings of the Tappi PaperCon’16 Conference, Cincinnati, OH, USA, 15–18 May 2016; Tappi Press: Atlanta, GA, USA, 2016. [Google Scholar]
- Schenker, M.; Schoelkopf, J.; Mangin, P.; Gane, P. Rheological investigation of complex micro and nanofibrillated cellulose (MNFC) suspensions: Discussion of flow curves and gel stability. Tappi J. 2016, 15, 405–416. [Google Scholar]
- Bollström, R.; Ridgway, C.; Schenker, M.; Gane, P. Calcium carbonate pigment hydrocolloid hybrid enabling bonding with micro nanofibrillated cellulose. In Proceedings of the TAPPI International Conference on Nanotechnology for Renewable Materials 2016, Grenoble, France, 13–16 June 2016; Tappi Press: Atlanta, GA, USA, 2016. [Google Scholar]
- Rio, E.; Drenckhan, W.; Salonen, A.; Langevin, D. Unusually stable liquid foams. Adv. Colloid Interface Sci. 2014, 205, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Velikov, K.P.; Velev, O.D. Pickering stabilization of foams and emulsions with particles of biological origin. Curr. Opin. Colloid Interface Sci. 2014, 19, 490–500. [Google Scholar] [CrossRef]
- Gestranius, M.; Stenius, P.; Kontturi, E.; Sjöblom, J.; Tammelin, T. Phase behaviour and droplet size of oil-in-water pickering emulsions stabilised with plant-derived nanocellulosic materials. Colloids Surf. A 2017, 519, 60–70. [Google Scholar] [CrossRef]
- Cervin, N.T.; Johansson, E.; Benjamins, J.W.; Wågberg, L. Mechanisms behind the stabilizing action of Cellulose nanofibrils in wet-stable cellulose Foams. Biomacromolecules 2015, 16, 822–831. [Google Scholar] [CrossRef] [PubMed]
- SCAN-CM 71:09, Pulps—Carbohydrate Content; Scandinavian Pulp, Paper and Board Testing Committee: Stockholm, Sweden, 2009.
- Technical Association of the Pulp and Paper Industry. TAPPI-T 222 om-02, Acid-insoluble lignin in wood and pulp. In 2002–2003 TAPPI Test Methods; Tappi Press: Atlanta, GA, USA, 2002. [Google Scholar]
- SCAN-CM 49:03, Content of Acetone-Soluble Matter; Scandinavian Pulp, Paper and Board Testing Committee: Stockholm, Sweden, 2003.
- Swerin, A.; Odberg, L.; Lindström, T. Deswelling of hardwood kraft pulp fibers by cationic polymers the effect on wet pressing and sheet properties. Nord. Pulp Pap. Res. J. 1990, 5, 188–196. [Google Scholar] [CrossRef]
- Horvath, E.A.; Lindström, T.; Laine, J. On the indirect polyelectrolyte titration of cellulosic fibers. Conditions for charge stoichiometry and comparison with ESCA. Langmuir 2006, 22, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, P.; Liukkonen, S.; Pere, J.; Sneck, A.; Kangas, H. A Comparative study of fibrillated fibers from different mechanical and chemical pulps. BioResources 2014, 9, 2115–2127. [Google Scholar] [CrossRef]
- Hierrezuelo, J.; Sadeghpour, A.; Szilagyi, I.; Vaccaro, A.; Borkovec, M. Electrostatic stabilization of charged colloidal particles with adsorbed polyelectrolytes of opposite charge. Langmuir 2010, 26, 15109–15111. [Google Scholar] [CrossRef] [PubMed]
- Arzhavitina, A.; Steckel, H. Foams for pharmaceutical and cosmetic application. Int. J. Pharm. 2010, 394, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Wang, X. The effect of PVA foaming characteristics on foam forming. Cellulose 2017, 24, 4939–4948. [Google Scholar] [CrossRef]
- Hakalahti, M.; Salminen, A.; Seppälä, J.; Tammelin, T.; Hänninen, T. Effect of interfibrillar PVA bridging on water stability and mechanical properties of TEMPO/NaClO2oxidized cellulosic nanofibril films. Carbohydr. Polym. 2015, 126, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P. Particles as surfactants—Similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Du, Z.; Bilbao-Montoya, M.P.; Binks, B.P.; Dickinson, E.; Ettelaie, R.; Murray, B.S. Outstanding stability of particle-stabilized bubbles. Langmuir 2003, 19, 3106–3108. [Google Scholar] [CrossRef]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Stabilization of foams with inorganic colloidal particles. Langmuir 2006, 22, 10983–10988. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cao, J.; Liu, W.; Stoyanov, S. How rigid rods self-assemble at curved surfaces. Angew. Chem. Int. Ed. 2009, 48, 378–381. [Google Scholar] [CrossRef]
- Kadoi, K.; Nakae, H. Relationship between foam stabilization and Physical Properties of Particles on Aluminum Foam Production. Mater. Trans. 2011, 52, 1912–1919. [Google Scholar] [CrossRef]









| Dry solids content (w/w%) | 1.6 |
| pH of CNF gel | 6.7 |
| Conductivity of CNF gel (µS cm−1) | 45 |
| Conductivity of 0.05 w/w% CNF dispersion (µS cm−1) | 25 |
| Conductivity of 0.05 w/w% CNF dispersion, I = 5 mM (µS cm−1) | 465 at pH 8.0 370 at pH 8.5 |
| pH and Ionic Strengt | ζ-Potential |
|---|---|
| pH 8.0, 5 mM (mV) | −21.2 ± 0.2 |
| pH 8.0, 10 mM (mV) | −17.2 ± 0.2 |
| pH 8.5, 5 mM (mV) | −23.5 ± 1.1 |
| pH 8.5, 10 mM (mV) | −22.6 ± 1.8 |
| PHCH | nanoPHCH0 | nanoPHCH1 | nanoGCC | |
|---|---|---|---|---|
| Incorporated polyelectrolytes | 1.5 w/w% CMC + 0.6 w/w% pDADMAC | 13 w/w% CMC | 13 w/w% CMC + 5 w/w% pDADMAC | - |
| Median volume equivalent spherical diameter d50 (µm) * | 1.5 (by weight) | 0.14 (by number) 0.18 (by weight) | 0.08 (by number) 0.15 (by weight) | 0.11 (by number) 0.17 (by weight) |
| Specific surface area by BET (m2 g−1) | 4 | 16 * | 12 * | 32 |
| Charge by PET (µVal g−1) | −54 | −711 | −599 | −34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenhunen, T.-M.; Pöhler, T.; Kokko, A.; Orelma, H.; Schenker, M.; Gane, P.; Tammelin, T. Enhancing the Stability of Aqueous Dispersions and Foams Comprising Cellulose Nanofibrils (CNF) with CaCO3 Particles. Nanomaterials 2018, 8, 651. https://doi.org/10.3390/nano8090651
Tenhunen T-M, Pöhler T, Kokko A, Orelma H, Schenker M, Gane P, Tammelin T. Enhancing the Stability of Aqueous Dispersions and Foams Comprising Cellulose Nanofibrils (CNF) with CaCO3 Particles. Nanomaterials. 2018; 8(9):651. https://doi.org/10.3390/nano8090651
Chicago/Turabian StyleTenhunen, Tiia-Maria, Tiina Pöhler, Annaleena Kokko, Hannes Orelma, Michel Schenker, Patrick Gane, and Tekla Tammelin. 2018. "Enhancing the Stability of Aqueous Dispersions and Foams Comprising Cellulose Nanofibrils (CNF) with CaCO3 Particles" Nanomaterials 8, no. 9: 651. https://doi.org/10.3390/nano8090651