Self-Cleaning Properties of Electrospun PVA/TiO2 and PVA/ZnO Nanofibers Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.3. Characterizations
3. Results and Discussion
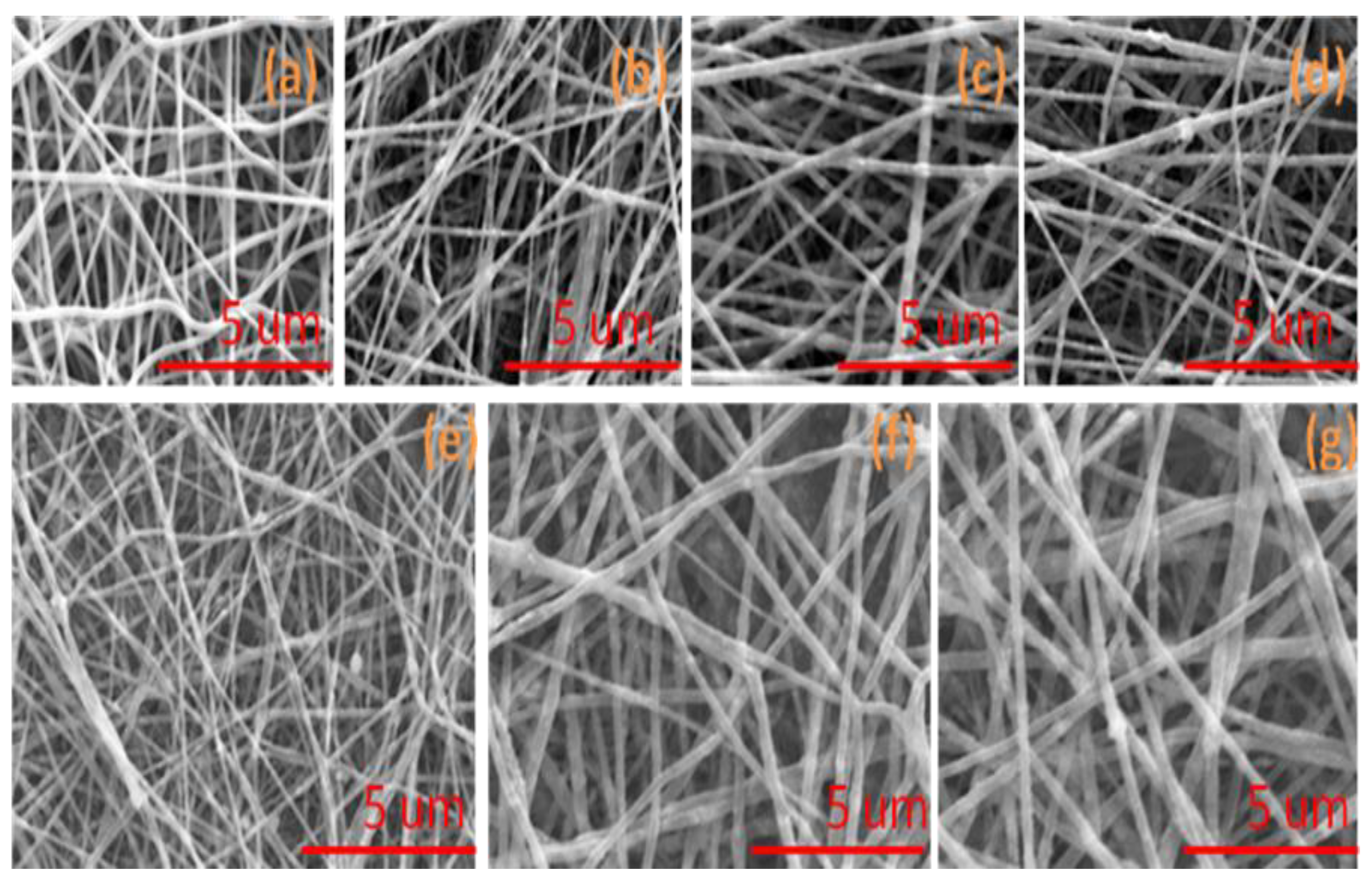
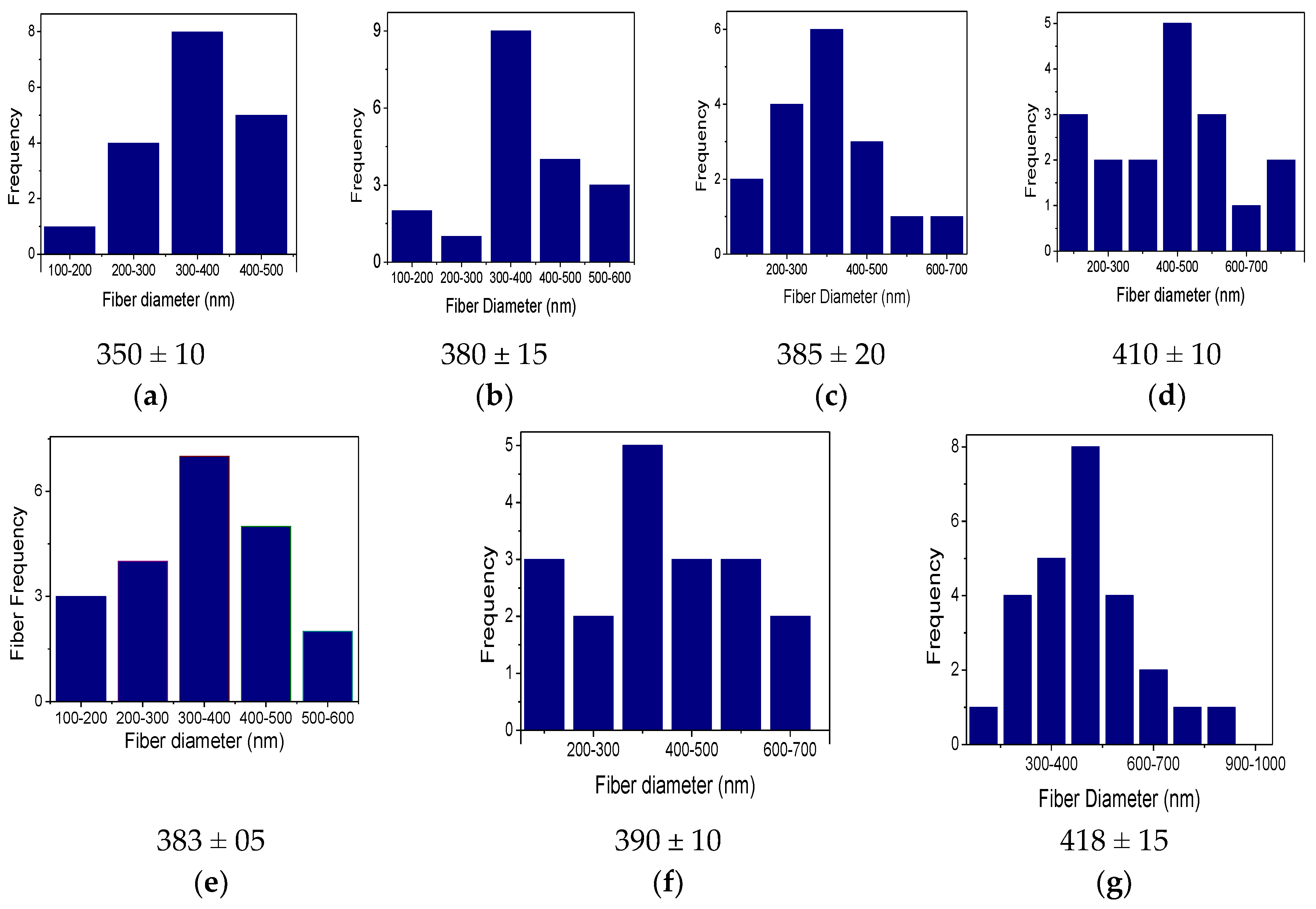
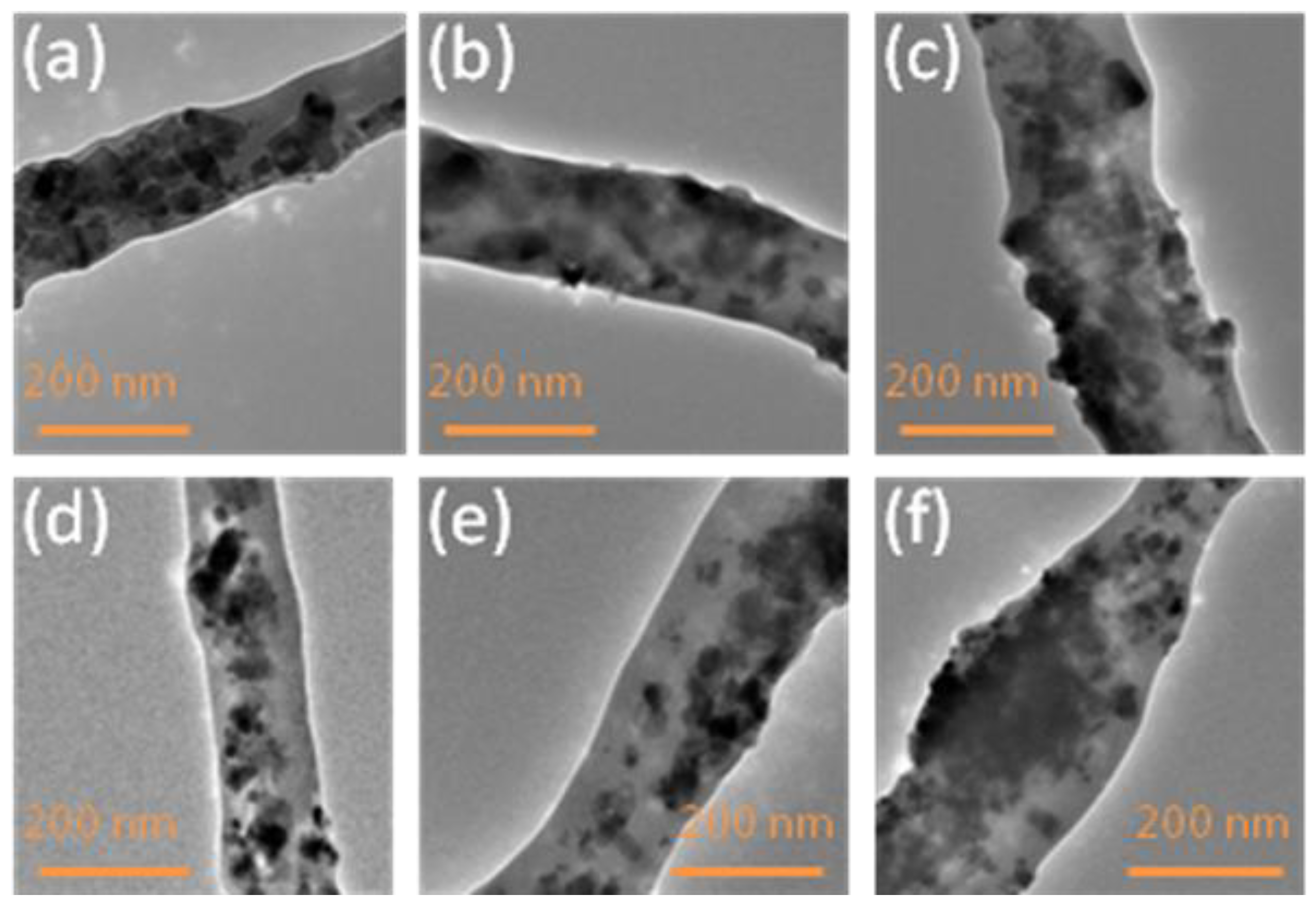
3.1. Morphology of Nanofibers
3.2. Chemical Structure Analysis
3.3. XRD Study
3.4. Water Contact Angle Measurements
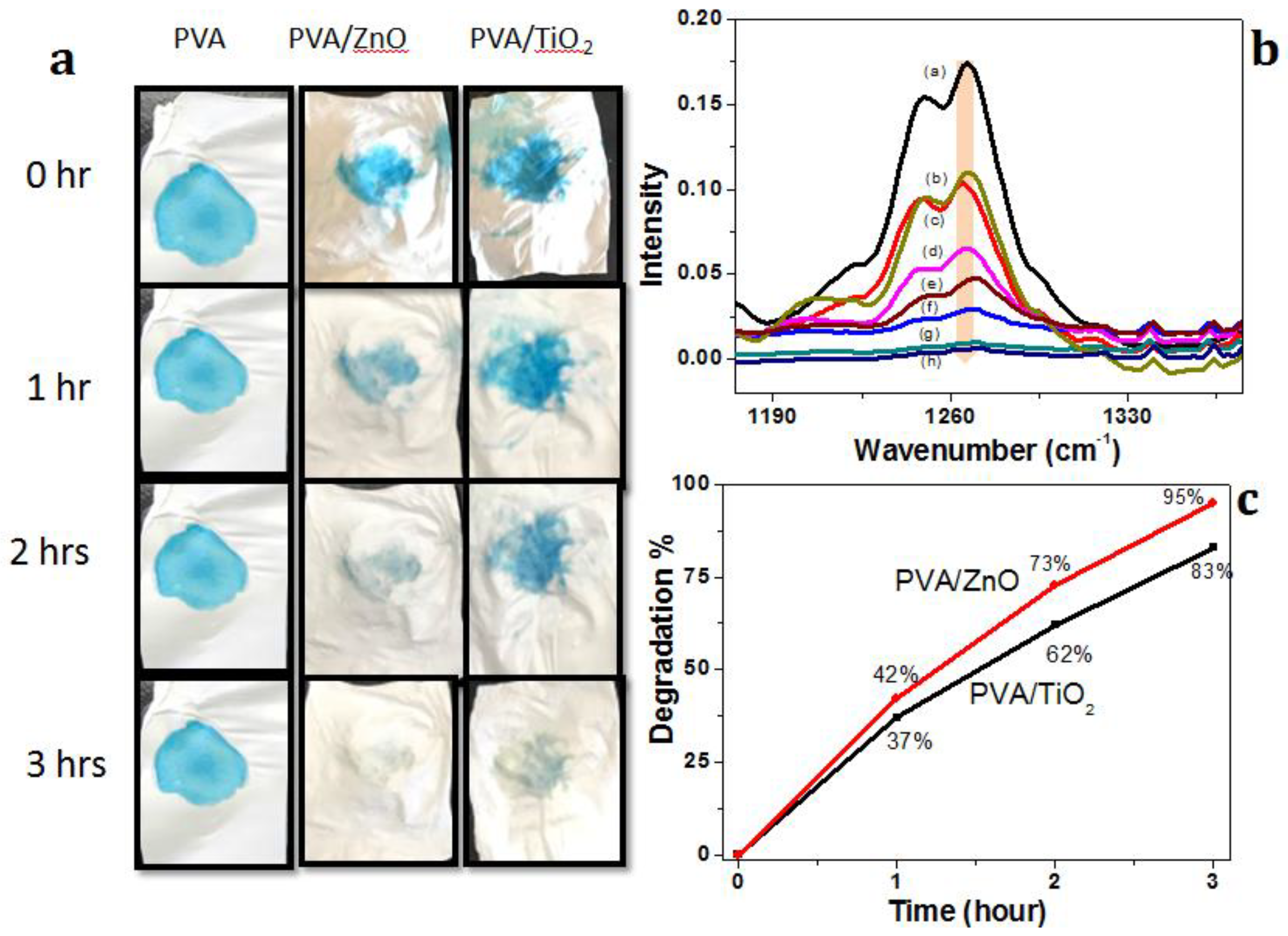
3.4.1. Photo-Catalysis Study
3.4.2. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Greiner, A.; Wendorff, J.H.; Bognitzki, M. Nanostructured Fibers via Electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar]
- Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z.; Ramakrishna, S. An Introduction to Nanofibers and Electrospinning; World Scientific Publishing Co. Pte. Ltd.: Majulah, Singapore, 2005. [Google Scholar]
- Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L.; Barnes, C.P. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Sci. Dir. 2007, 59, 1413–1433. [Google Scholar]
- Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S.; Subbiah, T. Electrospinning of Nanofibers. Wiley Intersci. 2004, 96, 557–569. [Google Scholar]
- Wei, Q. Functional Nanofibers and Their Applications; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Kundu, S.C.; Bhardwaj, N. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar]
- Bedford, N.M.; Steckl, A.J. Photocatalytic Self Cleaning Textile Fibers by coaxial electrospinning. Appl. Mater. Interfaces 2010, 2, 2448–2455. [Google Scholar] [CrossRef]
- Lyua, S.; Leea, S.; Kimb, Y.S.; Hwang, W.; Leea, K. Characteristics and self-cleaning effect of the transparent super-hydrophobic film having nanofibers array structures. Appl. Surf. Sci. 2010, 256, 6729–6735. [Google Scholar]
- Tang, J.; Wang, R.; Lu, H.; Li, L.; Kong, Y.; Qi, K.; Xin, J.H.; Liu, Y. Artificial lotus leaf structures from assembling carbon nanotubes and their application in hydrophobic textile. J. Mater. Chem. 2007, 17, 1071–1078. [Google Scholar]
- Guan, K. Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf. Coat. Technol. 2005, 191, 155–160. [Google Scholar] [CrossRef]
- Dehn, F.; Quaas, J.; Orgass, M.; Benedix, R. Application of Titanium Dioxide Photocatalysis to Create Self-Cleaning Building Materials. In Leipzig Annual Civil Engineering Report Lacer No. 5; Univ., Wirtschaftliche Fakultät, Fachgruppe Bau- und Wirtschaftsingenieure: Leipzig, Germany, 2005; pp. 157–167. [Google Scholar]
- Zhang, Y.-Z.; Khotki, M.; Ramakrishna, S.; Huang, Z.-M. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar]
- Luo, Z.; Rabenberg, L.; Heller, A.; Paz, Y. Photooxidative self-cleaning transparent titanium dioxide films on glass. J. Mater. Res. 1995, 10, 2842–2848. [Google Scholar]
- Pyrgiotakis, G.; Sigmund, W.; Woan, K. Photocatalytic Carbon-Nanotube–TiO2 Composites. Adv. Mater. 2009, 21, 2223–2239. [Google Scholar]
- Jiang, X.; Wang, T. Influence of Preparation Method on Morphology and Photocatalysis Activity of Nanostructured TiO2. Environ. Sci. Technol. 2007, 41, 4441–4446. [Google Scholar] [CrossRef] [PubMed]
- Jimmy, C.Y.; Yu, J.-G.; Kwok, Y.-C.; Che, Y.-K.; Zhao, J.-C.; Ding, L.; Ge, W.-K.; Wong, P.-K.; Yu, Y. Enhancement of photocatalytic activity of mesoporous TiO2 by using carbon nanotubes. Appl. Catal. A 2005, 289, 186–196. [Google Scholar]
- Lee, H.; Koo, J.M.; Khatri, Z.; Sui, J.; Im, S.S.; Zhu, C.; Kim, I.S.; Khan, M.Q. Self-cleaning effect of electrospun poly (1,4-cyclohexanedimethylene isosorbide terephthalate) nanofibers embedded with zinc oxide nanoparticles. Text. Res. J. 2017. [Google Scholar] [CrossRef]
- Kharaghani, D.; Lee, H.; Ishikawa, T.; Nagaishi, T.; Kim, S.H.; Kim, I.S. Comparison of fabrication methods for the effective loading of Ag onto PVA nanofibers. Text. Res. J. 2018. [Google Scholar] [CrossRef]
- Boyadjiev, S.I.; Kéri, O.; Bárdos, P.; Firkala, T.; Gáber, F.; Nagy, Z.K.; Baji, Z.; Takács, M.; Szilágyi, I.M. TiO2/ZnO and ZnO/TiO2 core/shell nanofibers prepared by electrospinning and atomic layer deposition for photocatalysis and gas sensing. Appl. Surf. Sci. 2017, 424, 190–197. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Geetha, N.; Kanimozhi, K.; Magdalane, C.M.; Sivaranjani, S.; Ayeshamariam, A.; Kennedy, J.; Maaza, M. In vitro cytotoxicity effect and antibacterial performance of human lung epithelial cells A549 activity of zinc oxide doped TiO2 nanocrystals: Investigation of bio-medical application by chemical method. Mater. Sci. Eng. C 2017, 74, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Boccuzzi, F.; Chiorino, A.; Tsubota, S.; Haruta, M. FTIR study of carbon monoxide oxidation and scrambling at room temperature over gold supported on ZnO and TiO2. 2. J. Phys. Chem. 1996, 100, 3625–3631. [Google Scholar] [CrossRef]
- Ognibene, G.; Cristaldi, D.A.; Fiorenza, R.; Blanco, I.; Cicala, G.; Scire, S.; Fragala, M.E. Photoactivity of hierarchically nanostructured ZnO–PES fibre mats for water treatments. RSC Adv. 2016, 6, 42778–42785. [Google Scholar] [CrossRef]
- Juby, K.A.; Dwivedi, C.; Kumar, M.; Kota, S.; Misra, H.S.; Bajaj, P.N. Silver nanoparticle-loaded PVA/gum acacia hydrogel: Synthesis, characterization and antibacterial study. Carbohydr. Polym. 2012, 89, 906–913. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.Q.; Kharaghani, D.; Ullah, S.; Waqas, M.; Abbasi, A.M.R.; Saito, Y.; Zhu, C.; Kim, I.S. Self-Cleaning Properties of Electrospun PVA/TiO2 and PVA/ZnO Nanofibers Composites. Nanomaterials 2018, 8, 644. https://doi.org/10.3390/nano8090644
Khan MQ, Kharaghani D, Ullah S, Waqas M, Abbasi AMR, Saito Y, Zhu C, Kim IS. Self-Cleaning Properties of Electrospun PVA/TiO2 and PVA/ZnO Nanofibers Composites. Nanomaterials. 2018; 8(9):644. https://doi.org/10.3390/nano8090644
Chicago/Turabian StyleKhan, Muhammad Qamar, Davood Kharaghani, Sana Ullah, Muhammad Waqas, Abdul Malik Rehan Abbasi, Yusuke Saito, Chunhong Zhu, and Ick Soo Kim. 2018. "Self-Cleaning Properties of Electrospun PVA/TiO2 and PVA/ZnO Nanofibers Composites" Nanomaterials 8, no. 9: 644. https://doi.org/10.3390/nano8090644





