Three-Dimensional Hierarchical Porous Structure of PPy/Porous-Graphene to Encapsulate Polysulfides for Lithium/Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
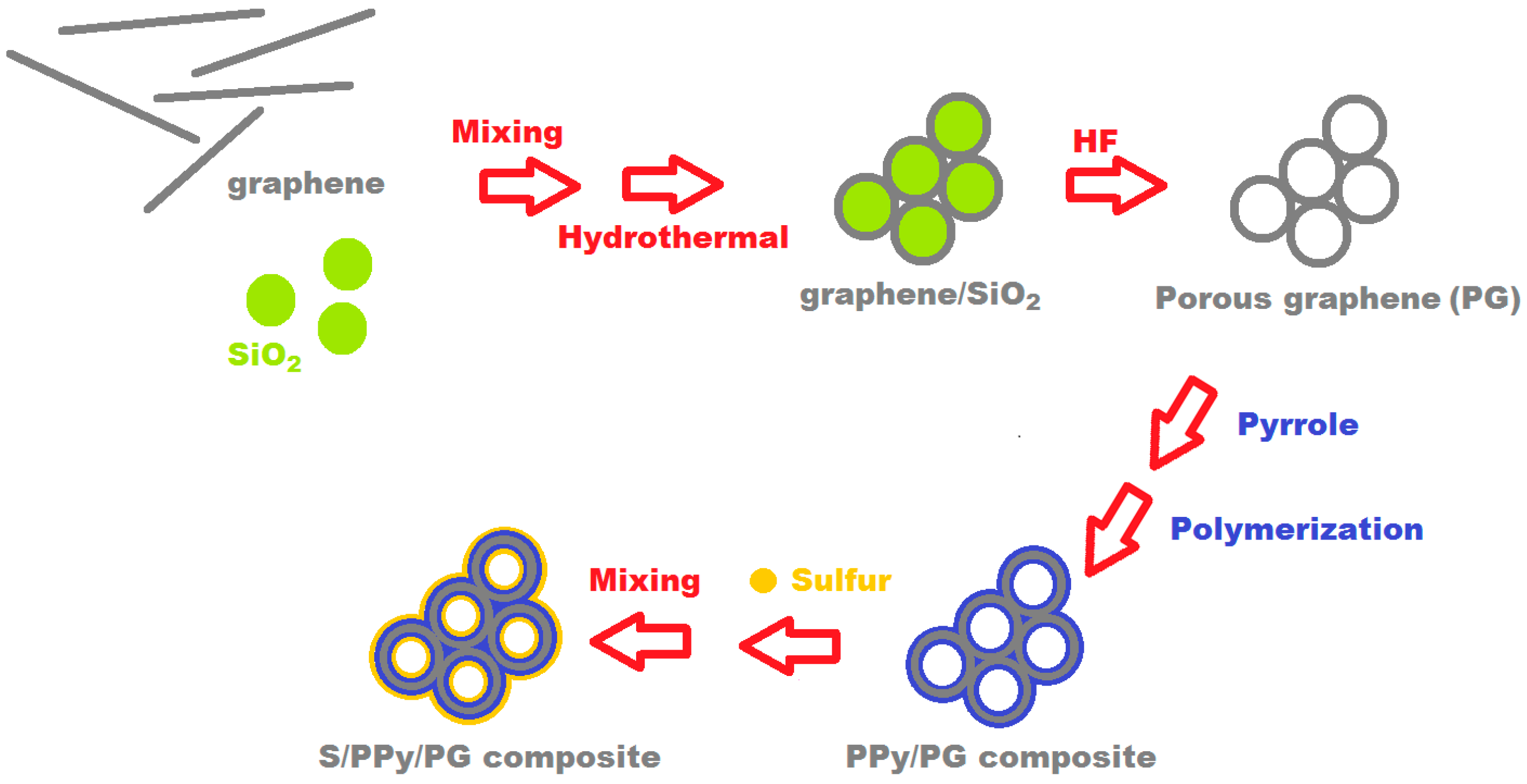
2.1. Material Synthesis
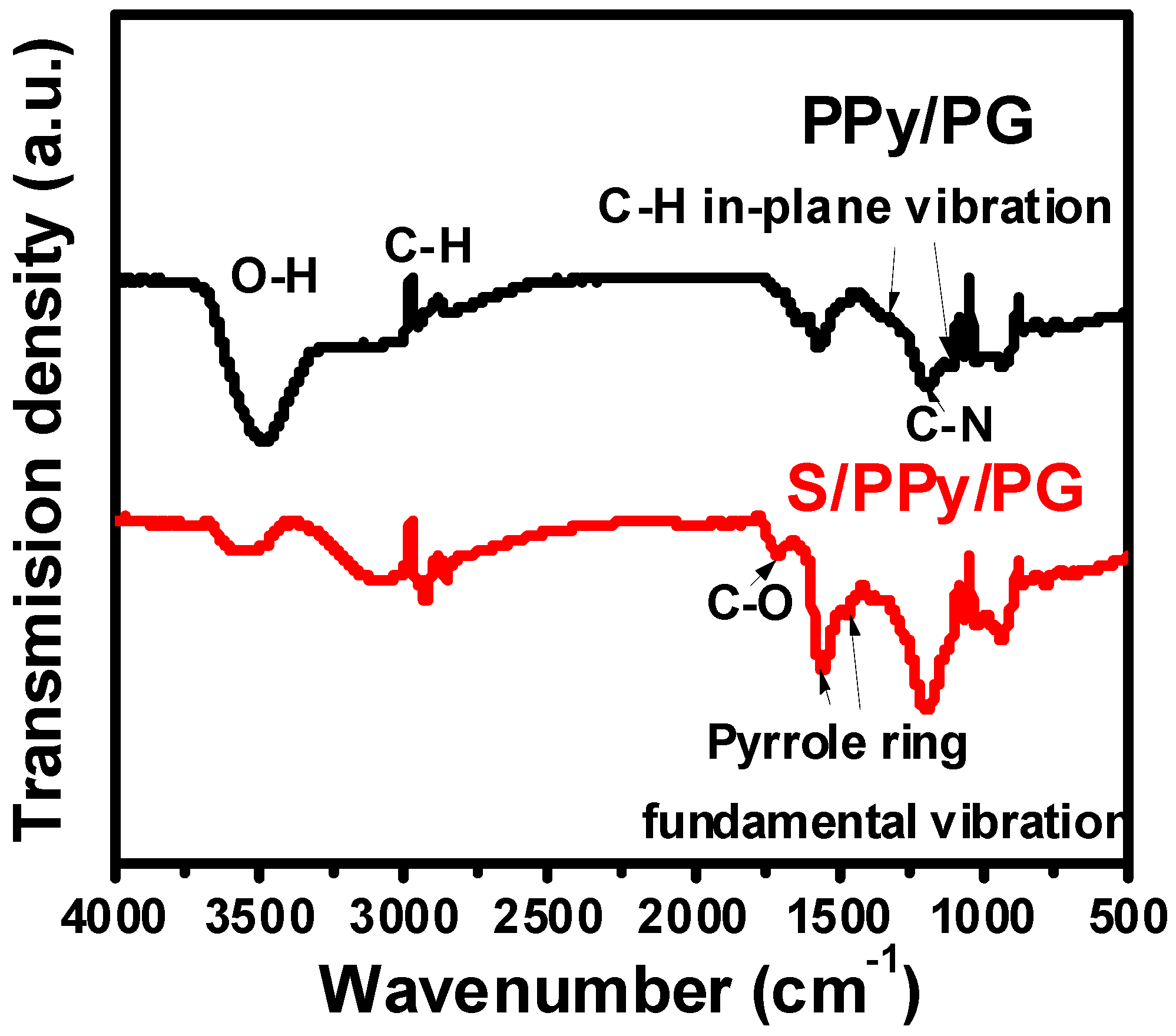
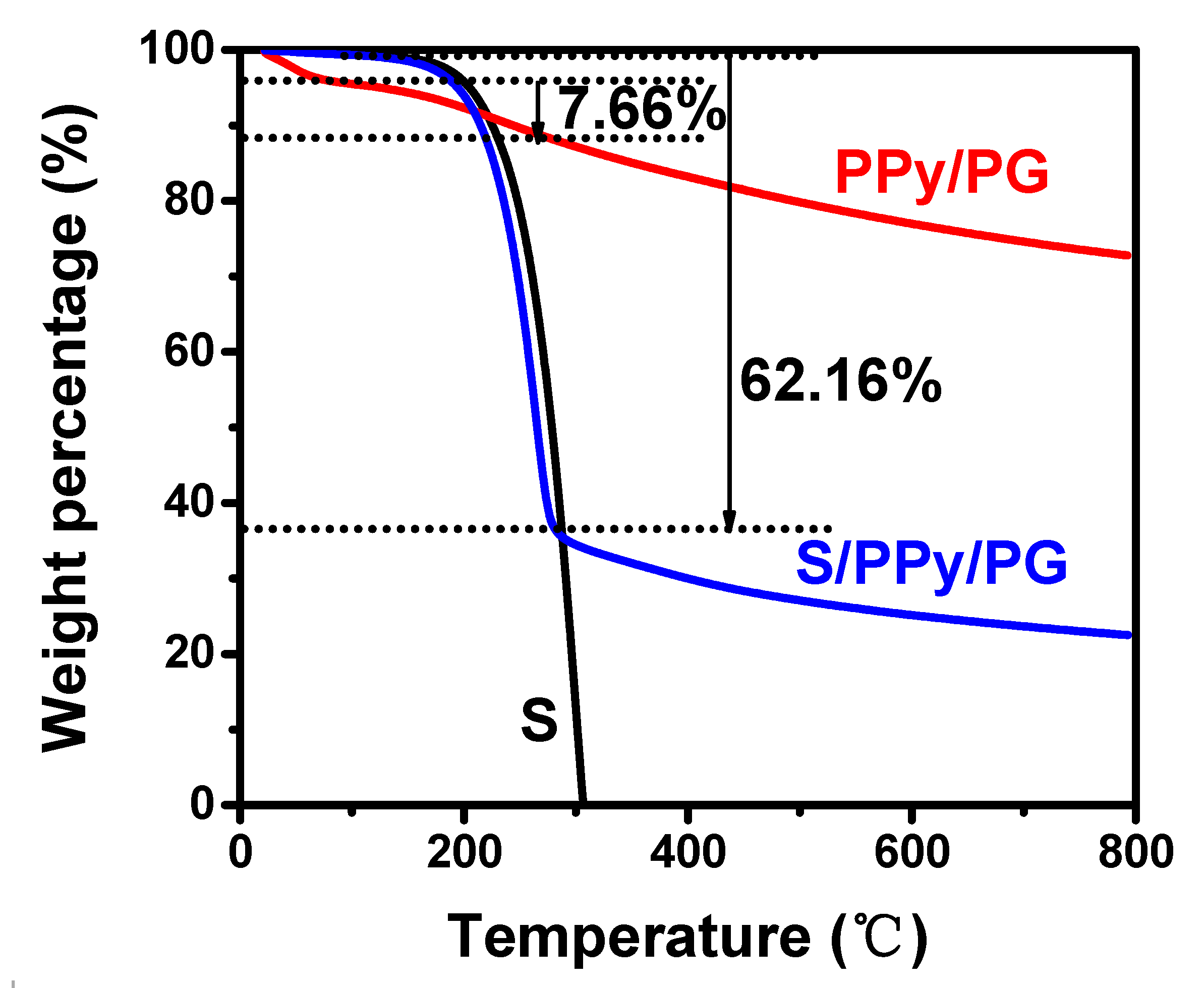
2.2. Material Characterization
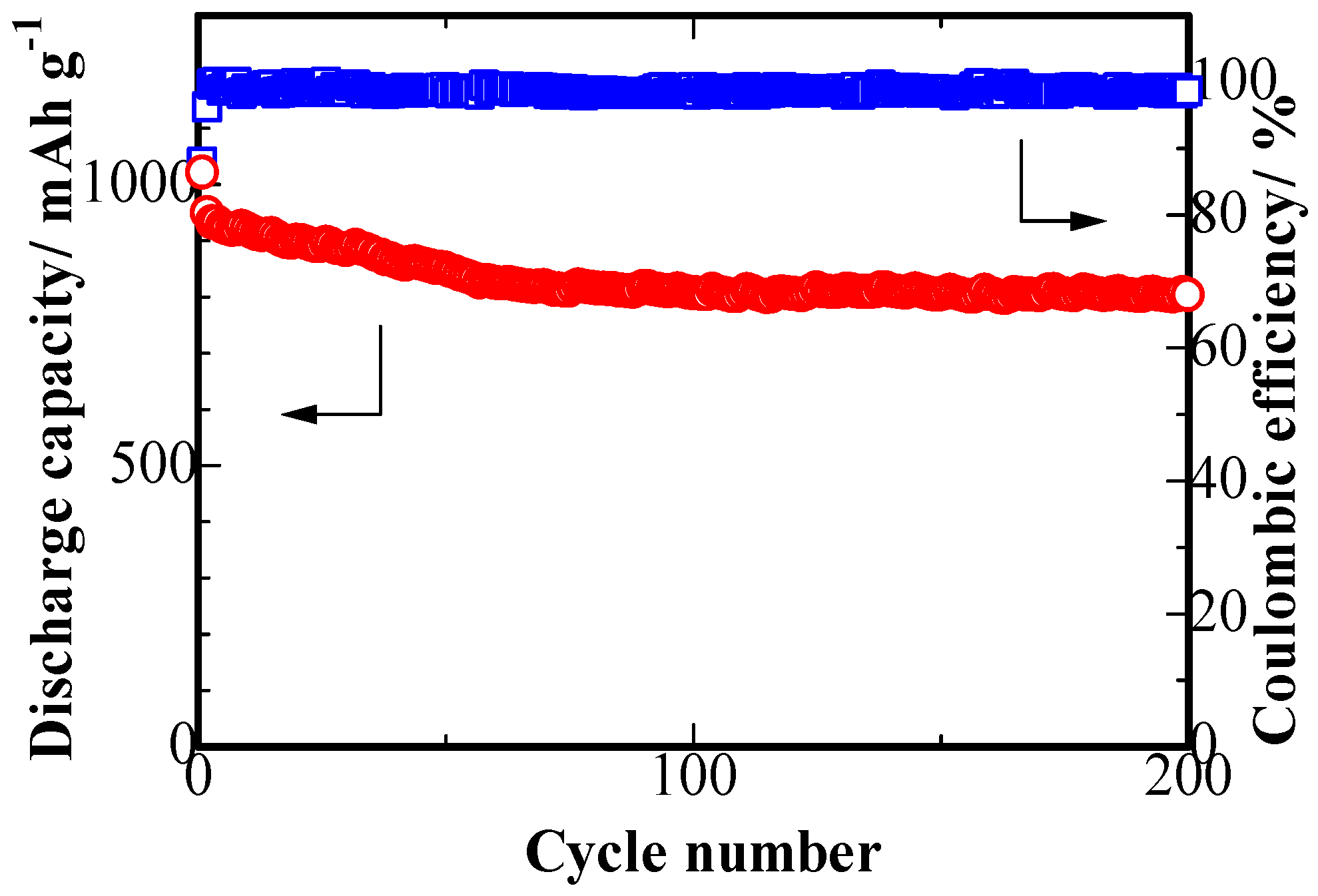
2.3. Electrochemical Characterization
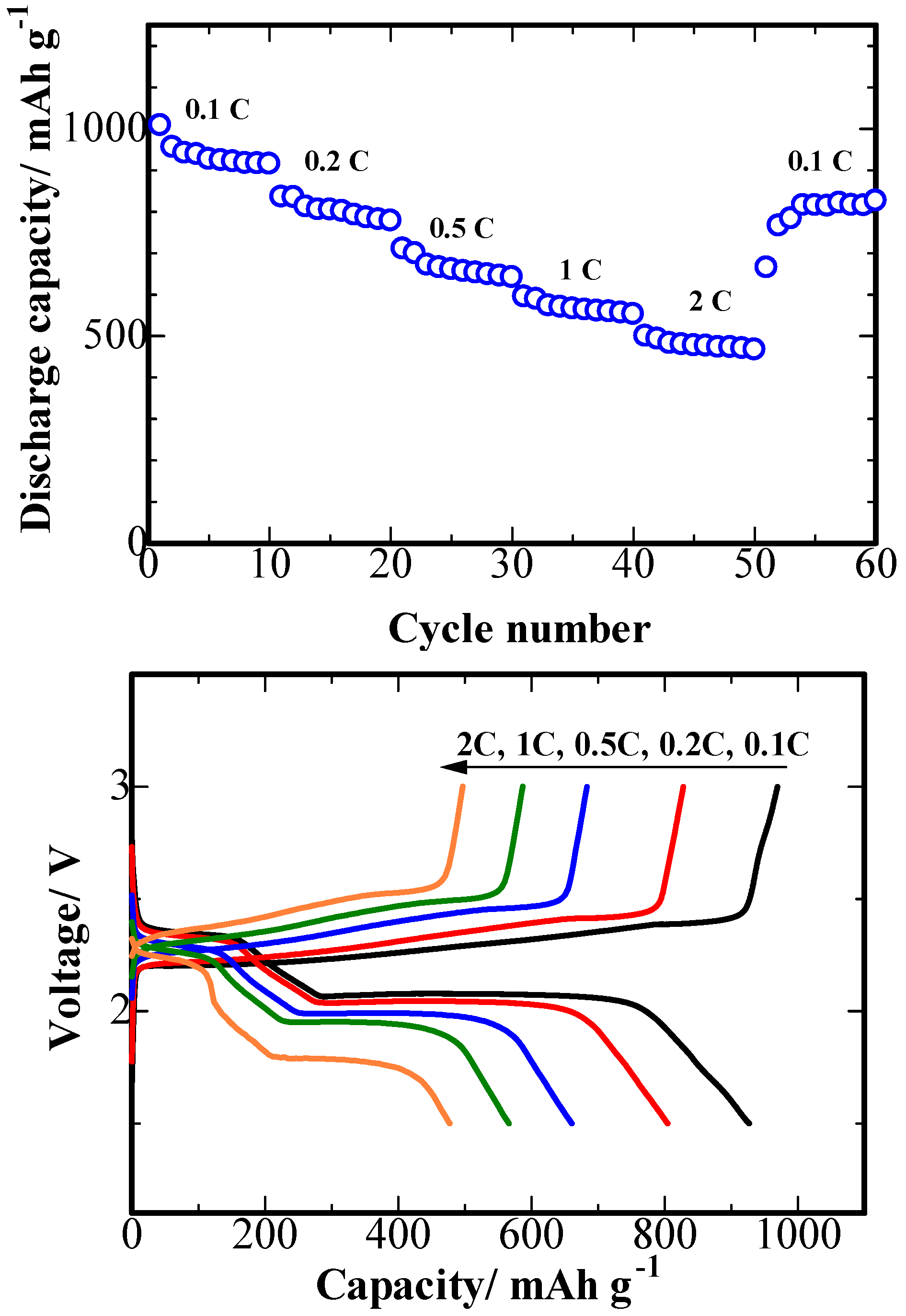
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Odani, A.; Nimberger, A.; Markovsky, B.; Sominski, E.; Levi, E.; Kumar, V.G.; Motiei, M.; Gedanken, A.; Dan, P.; Aurbach, D. Development and testing of nanomaterials for rechargeable lithium batteries. J. Power Sources 2003, 119–121, 517–521. [Google Scholar] [CrossRef]
- Li, H.P.; Sun, L.C.; Zhang, Y.G.; Tan, T.Z.; Wang, G.K.; Bakenov, Z. Enhanced cycle performance of Li/S battery with the reduced graphene oxide/activated carbon functional interlayer. J. Energy Chem. 2017, 26, 1276–1281. [Google Scholar] [CrossRef]
- Kim, J.K. Hybrid gel polymer electrolyte for high-safety lithium-sulfur batteries. Mater. Lett. 2017, 187, 40–43. [Google Scholar] [CrossRef]
- Xu, H.H.; Qie, L.; Manthiram, A. An integrally-designed, flexible polysulfide host for high-performance lithium-sulfur batteries with stabilized lithium-metal anode. Nano Energy 2016, 26, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Griebel, J.J.; Glass, R.S.; Char, K. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability, energy and defense. Prog. Polym. Sci. 2016, 58, 90–125. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Krüner, B.; Massuti-Balleter, P.; Tolosa, A.; Prehal, C.; Grobelsek, I.; Paris, O.; Borchardt, L.; Presser, V. Microporous novolac-derived carbon beads/sulfur hybrid cathode for lithium-sulfur batteries. J. Power Sources 2017, 357, 198–208. [Google Scholar] [CrossRef]
- Zellmer, S.; Titscher, P.; Wienken, E.; Kwade, A.; Garnweitner, G. Fabrication of carbon-sulphur composites via a vibration mill process as cathode material for lithium sulphur batteries. Energy Storage Mater. 2017, 9, 70–77. [Google Scholar] [CrossRef]
- Chiochan, P.; Phattharasupakun, N.; Wutthiprom, J.; Suksomboon, M.; Kaewruang, S.; Suktha, P.; Sawangphruk, M. Core-double shell sulfur@carbon black nanosphere@oxidized carbon nanosheet composites as the cathode materials for Li-S batteries. Electrochim. Acta 2017, 237, 78–86. [Google Scholar] [CrossRef]
- Agrawal, M.; Choudhry, S.; Gruber, K.; Simon, F.; Fischer, D.; Albrecht, V.; Göbel, M.; Koller, S.; Stamm, M.; Ionov, L. Porous carbon materials for Li–S batteries based on resorcinol–formaldehyde resin with inverse opal structure. J. Power Sources 2014, 261, 363–370. [Google Scholar] [CrossRef]
- Yu, M.; Li, R.; Wu, M.; Shi, G. Graphene materials for lithium–sulfur batteries. Energy Storage Mater. 2015, 1, 51–73. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.G.; Bakenov, Z.; Chen, P. Electrochemical performance of lithium gel polymer battery with nanostructured sulfur/carbon composite cathode. Solid State Ion. 2013, 234, 40–45. [Google Scholar] [CrossRef]
- Rehman, S.; Tang, T.Y.; Ali, Z.; Huang, X.X.; Hou, Y.L. Integrated Design of MnO2@Carbon Hollow Nanoboxes to Synergistically Encapsulate Polysulfides for Empowering Lithium Sulfur Batteries. Small 2017, 13, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.X.; Liu, X.; Zhao, Y.; Zhang, Y.G.; Menbayeva, A.; Bakenov, Z.; Wang, X. Well-dispersed sulfur anchored on interconnected polypyrrole nanofiber network as high performance cathode for lithium-sulfur batteries. Solid State Sci. 2017, 66, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Sohn, H.; Gordin, M.L.; Regula, M.; Kim, D.H.; Song, J.; Wang, D. Porous spherical polyacrylonitrile-carbon nanocomposite with high loading of sulfur for lithium–sulfur batteries. J. Power Sources 2016, 302, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Erhardt, C.; Sörgel, S.; Meinhard, S.; Sörgel, T. Proof of concept for a novel, binder-free and conducting carbon-free sulfur battery cathode: Composite electroformation of copper foil with incorporated polythiophene wrapped sulfur particles. J. Power Sources 2015, 296, 70–77. [Google Scholar] [CrossRef]
- Rao, J.; Xu, R.; Zhou, T.; Zhang, D.; Zhang, C. Rational design of self-supporting graphene-Polypyrrole/sulfur-Graphene sandwich as structural paper electrode for lithium sulfur batteries. J. Alloys Compd. 2017, 728, 376–382. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Percec, S.; Howe, L.; Li, J.; Bagshaw, A.; Peacock, S.; Brill, D. Pyrrole polymerization on polyimide surfaces creates conductive nano-domains. Polymer 2013, 54, 5754–5761. [Google Scholar] [CrossRef]
- Qian, W.; Gao, Q.; Zhang, H.; Tian, W.; Li, Z.; Tan, Y. Crosslinked Polypyrrole Grafted Reduced Graphene Oxide-Sulfur Nanocomposite Cathode for High Performance Li-S Battery. Electrochim. Acta 2017, 235, 32–41. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Hu, N.; Yang, Z.; Wei, H.; Zhang, Y. Reduced graphene oxide/polypyrrole nanotube papers for flexible all-solid-state supercapacitors with excellent rate capability and high energy density. J. Power Sources 2016, 302, 39–45. [Google Scholar] [CrossRef]
- Han, P.; Yue, Y.; Liu, Z.; Xu, W.; Zhang, L.; Xu, H.; Dong, S.; Cui, G. Graphene oxide nanosheets/multi-walled carbon nanotubes hybrid as an excellent electrocatalytic material towards VO2+/VO2+ redox couples for vanadium redox flow batteries. Energy Environ. Sci. 2011, 4, 4710–4717. [Google Scholar] [CrossRef]
- Xu, L.; Yang, W.; Neoh, K.; Kang, E.; Fu, G. Dopamine-Induced Reduction and Functionalization of Graphene Oxide Nanosheets. Macromolecules 2010, 43, 8336–8339. [Google Scholar] [CrossRef]
- Xue, K.; Zhou, S.; Shi, H.; Feng, X.; Xin, H.; Song, W. A novel amperometric glucose biosensor based on ternary gold nanoparticles/polypyrrole/reduced graphene oxide nanocomposite. Sens. Actuators B Chem. 2014, 203, 412–416. [Google Scholar] [CrossRef]
- Li, X.; Lushington, A.; Liu, J.; Li, R.; Sun, X. Superior stable sulfur cathodes of Li-S batteries enabled by molecular layer deposition. Chem. Commun. 2014, 50, 9757–9760. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Yuan, C.; Shen, L.; Xu, G.; Nie, P.; Zhang, X. Encapsulating Sulfur into Hierarchically Ordered Porous Carbon as a High-Performance Cathode for Lithium–Sulfur Batteries. J. Mater. Chem. A 2013, 19, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Zhao, Y.; Bakenov, Z.; Tuiyebayeva, M.; Konarov, A.; Chen, P. Synthesis of Hierarchical Porous Sulfur/Polypyrrole/Multiwalled Carbon Nanotube Composite Cathode for Lithium Batteries. Electrochim. Acta 2014, 143, 49–55. [Google Scholar] [CrossRef]
- Yamin, H.; Gorenshtain, A.; Penciner, J.; Sternberg, Y.; Peled, E. Lithium Sulphur Battery Oxidation/Reduction Mechanisms of Polysulphides in THF solution. J. Electrochem. Soc. 1988, 135, 1045–1048. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, Y.; Konarov, A.; Gosselink, D.; Soboleski, H.G.; Chen, P. A novel nano-sulfur/polypyrrole/graphene nanocomposite cathode with a dual-layered structure for lithium rechargeable batteries. J. Power Sources 2013, 241, 517–521. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Bakenov, Z.; Tan, T.; Huang, J. Three-Dimensional Hierarchical Porous Structure of PPy/Porous-Graphene to Encapsulate Polysulfides for Lithium/Sulfur Batteries. Nanomaterials 2018, 8, 606. https://doi.org/10.3390/nano8080606
Zhang Y, Bakenov Z, Tan T, Huang J. Three-Dimensional Hierarchical Porous Structure of PPy/Porous-Graphene to Encapsulate Polysulfides for Lithium/Sulfur Batteries. Nanomaterials. 2018; 8(8):606. https://doi.org/10.3390/nano8080606
Chicago/Turabian StyleZhang, Yongguang, Zhumabay Bakenov, Taizhe Tan, and Jin Huang. 2018. "Three-Dimensional Hierarchical Porous Structure of PPy/Porous-Graphene to Encapsulate Polysulfides for Lithium/Sulfur Batteries" Nanomaterials 8, no. 8: 606. https://doi.org/10.3390/nano8080606
APA StyleZhang, Y., Bakenov, Z., Tan, T., & Huang, J. (2018). Three-Dimensional Hierarchical Porous Structure of PPy/Porous-Graphene to Encapsulate Polysulfides for Lithium/Sulfur Batteries. Nanomaterials, 8(8), 606. https://doi.org/10.3390/nano8080606





