Evolution of Nanoporous Surface Layers on Gas-Atomized Ti60Cu39Au1 Powders during Dealloying
Abstract
:1. Introduction
2. Materials and Experimental Procedure
3. Results and Discussion
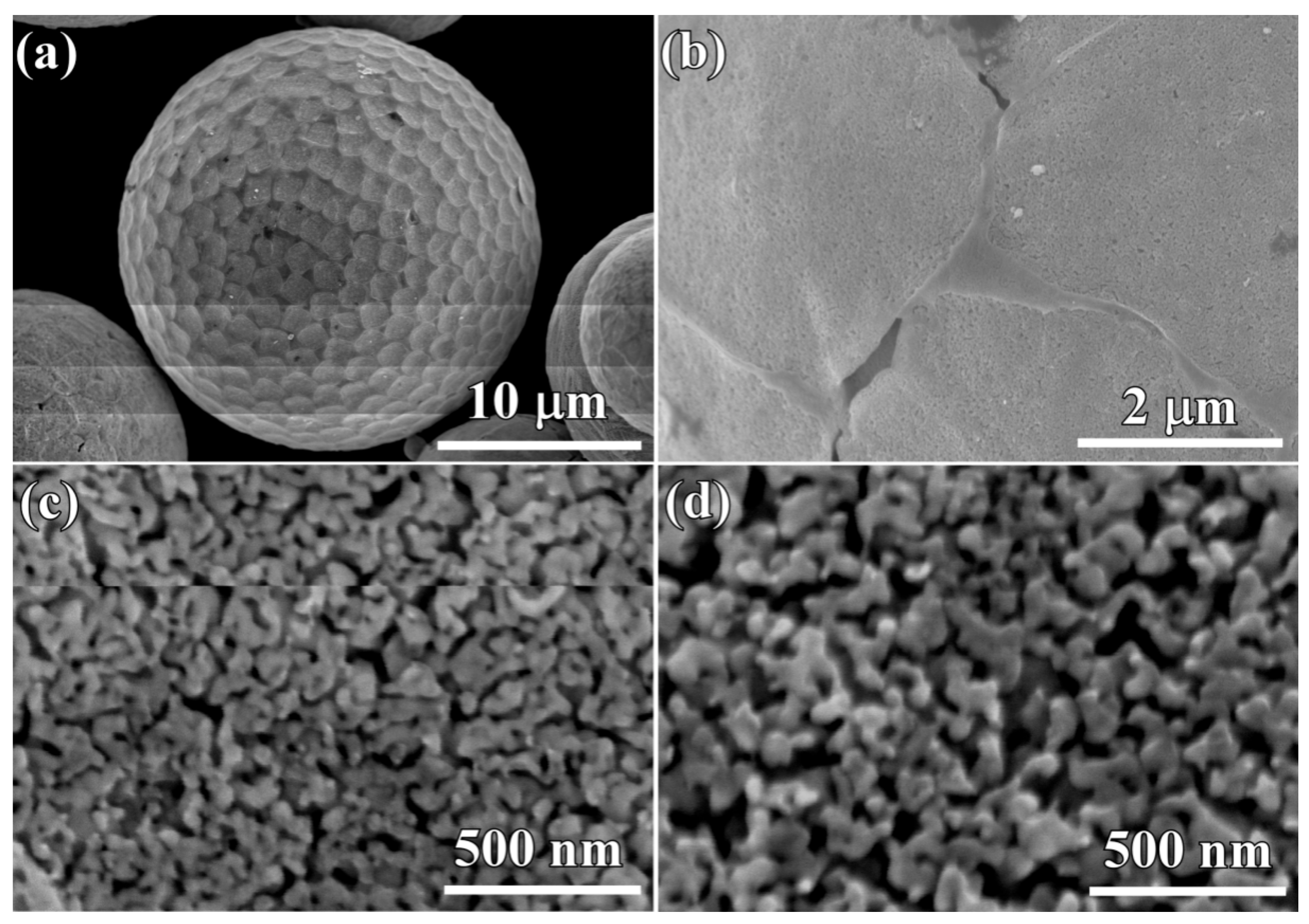
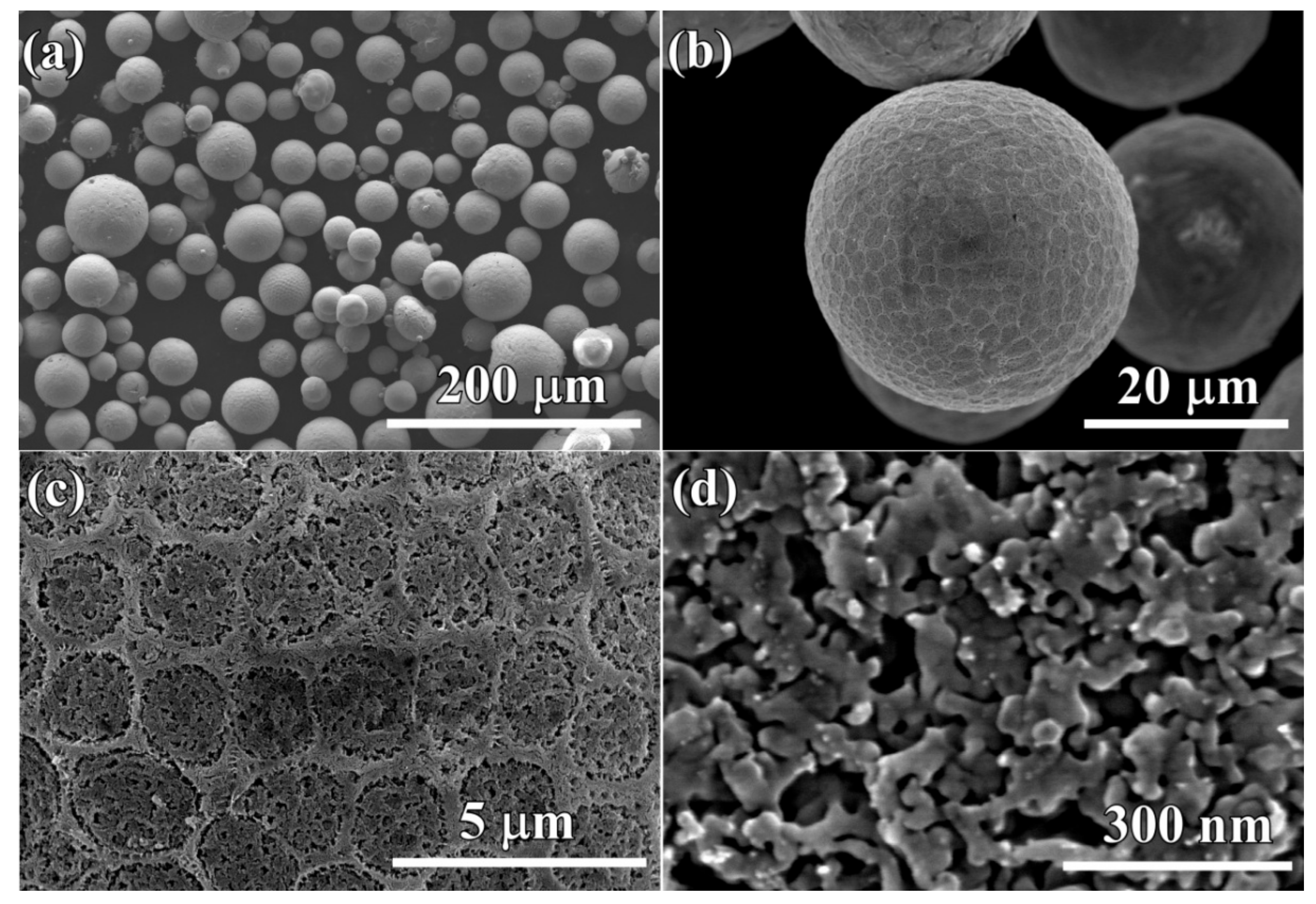
3.1. Characteristics of the Gas-Atomized Powders
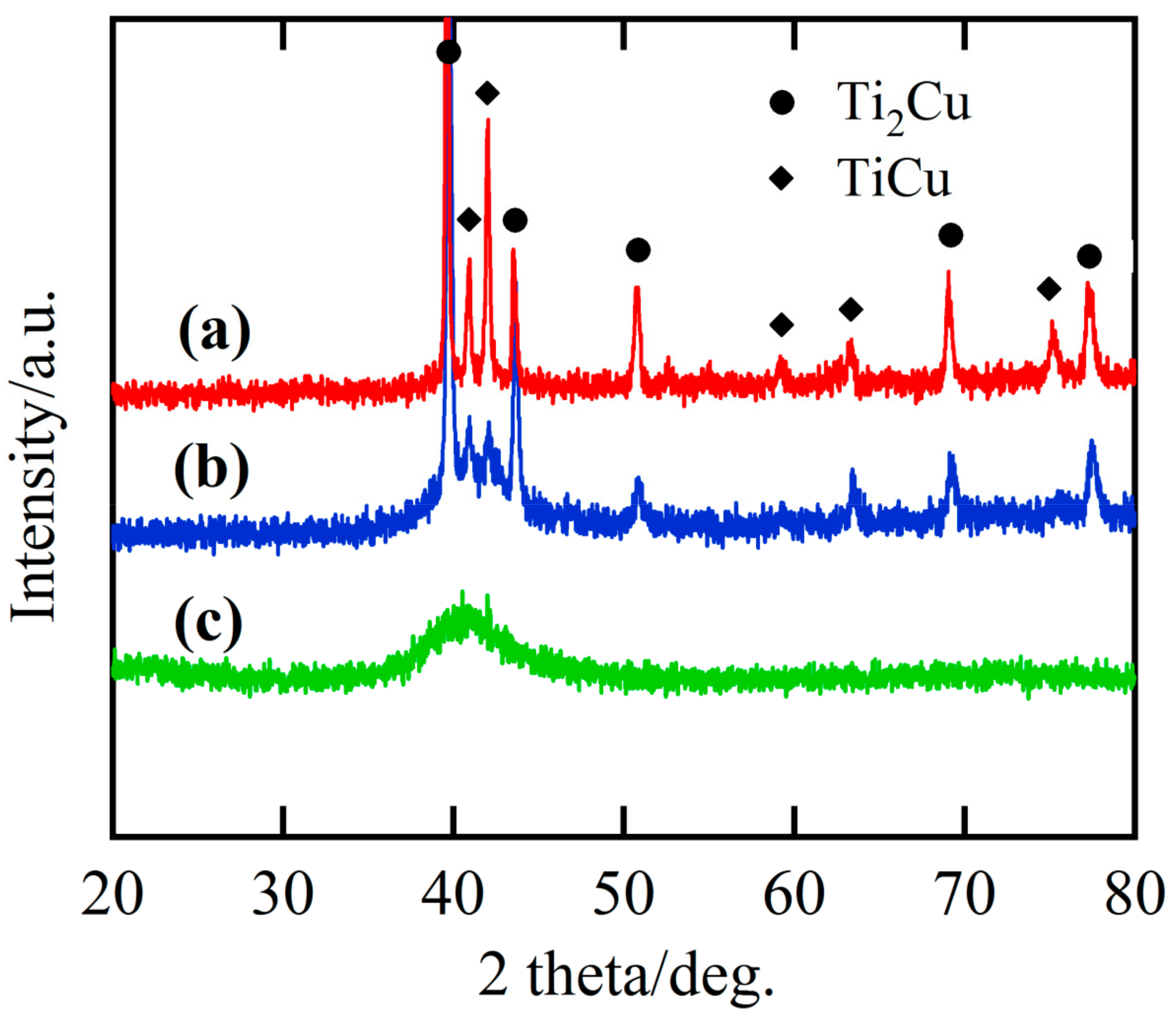
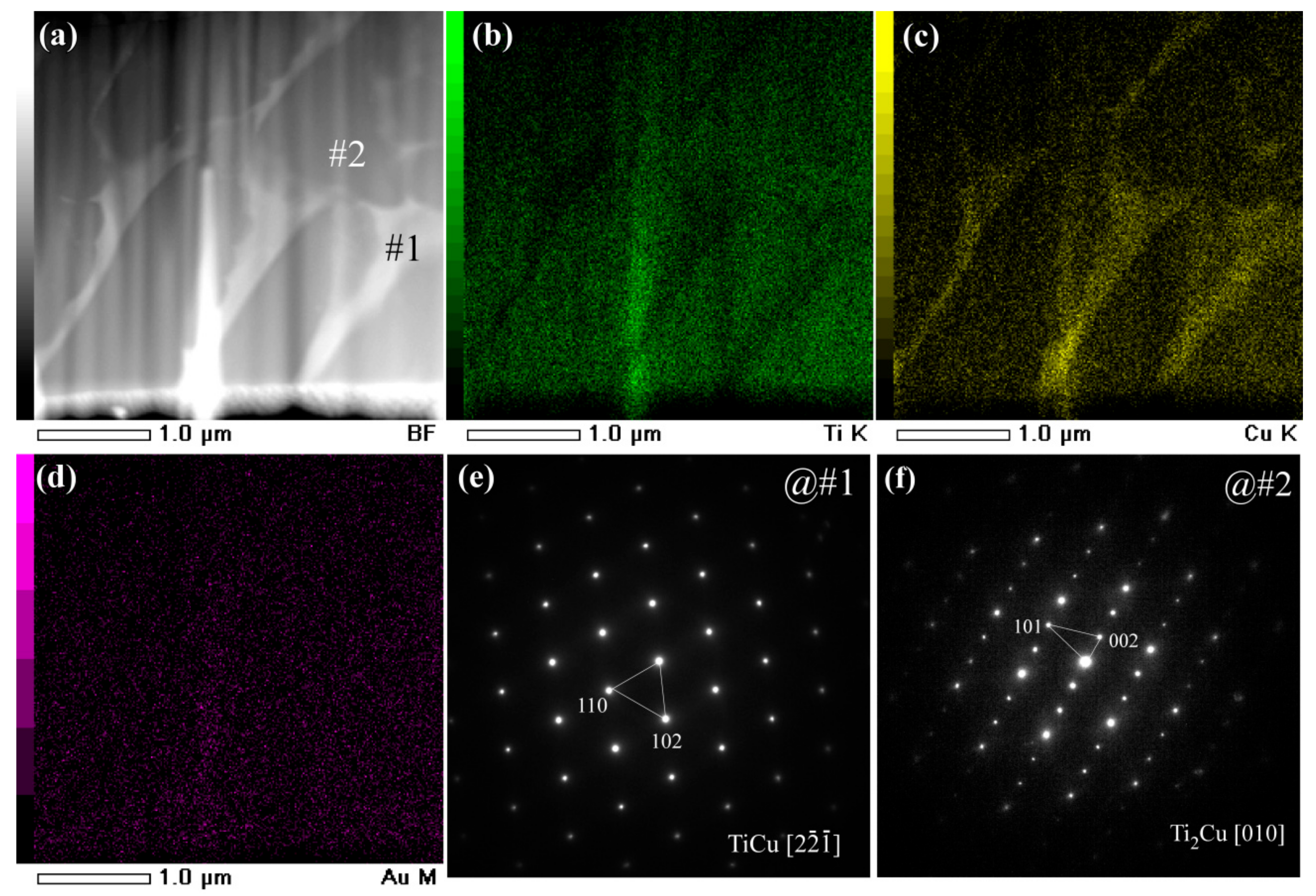
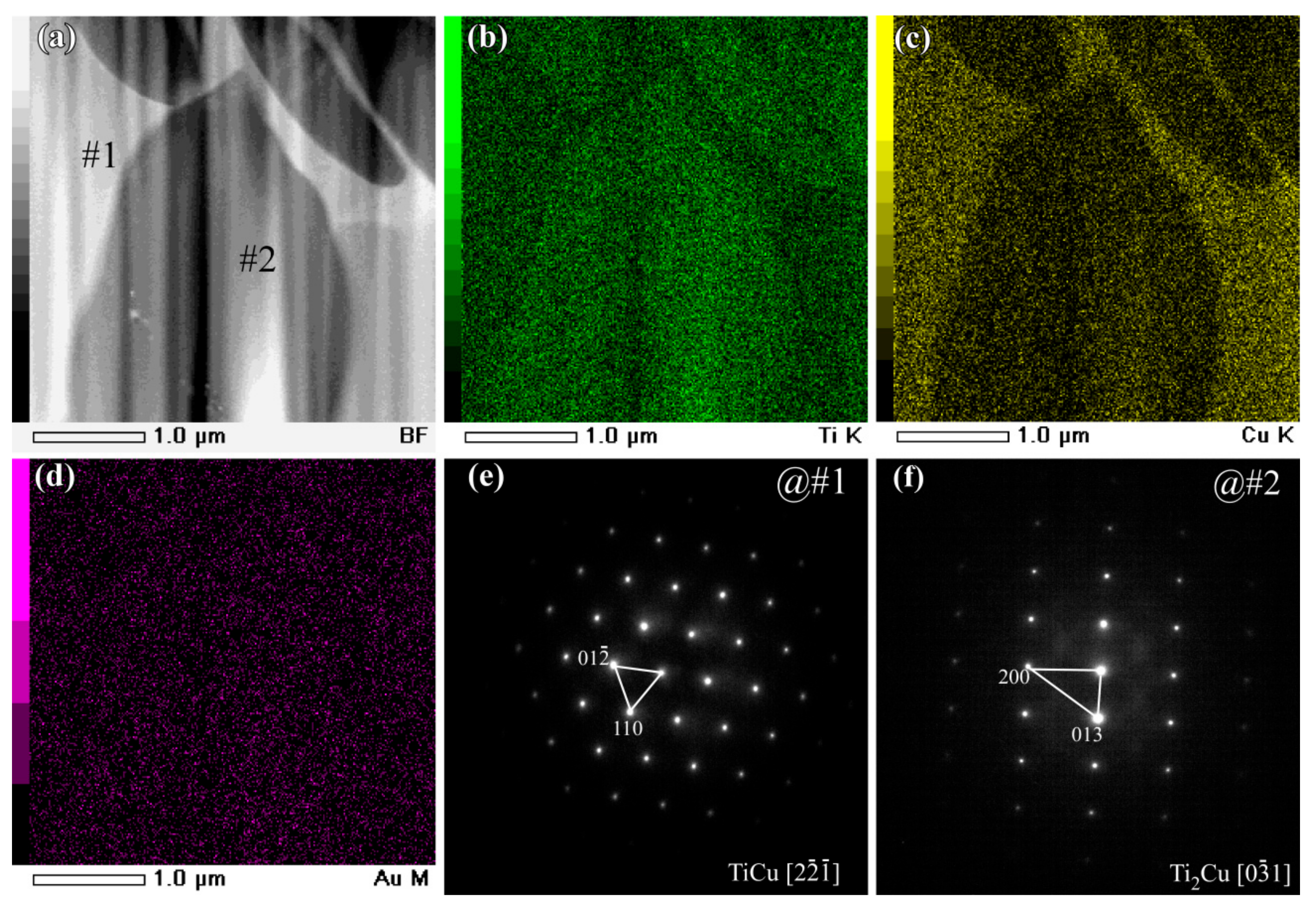
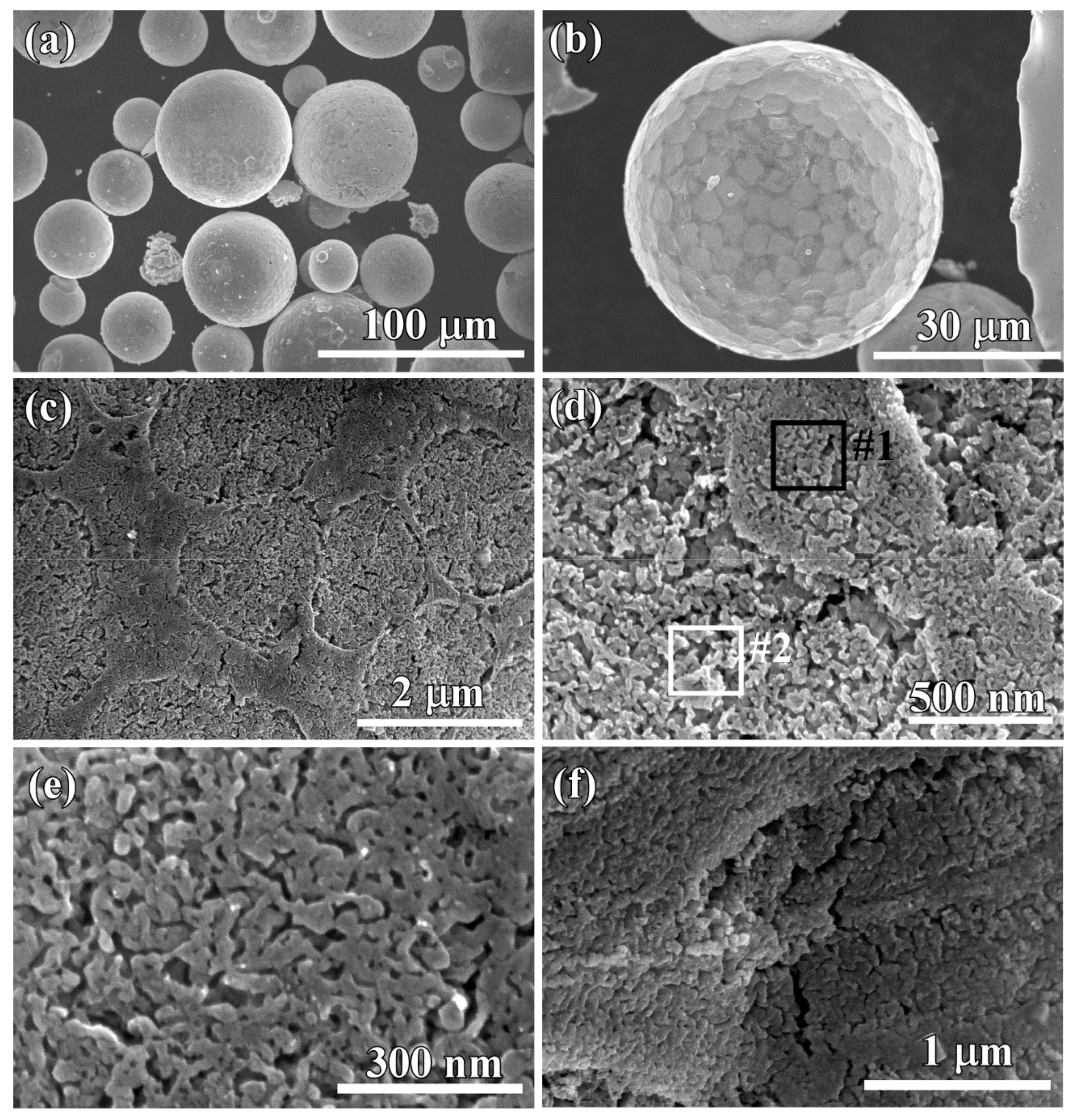
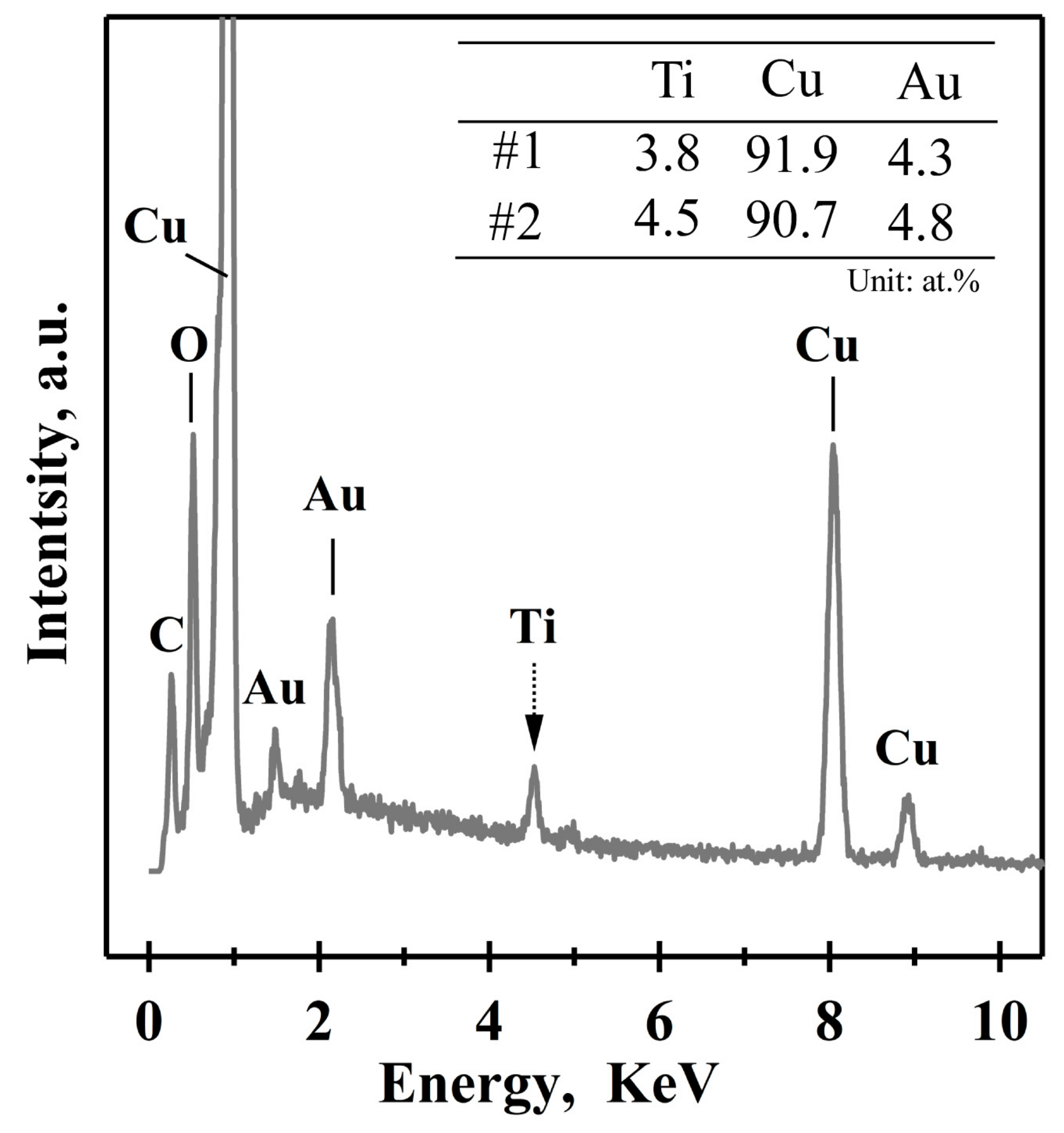
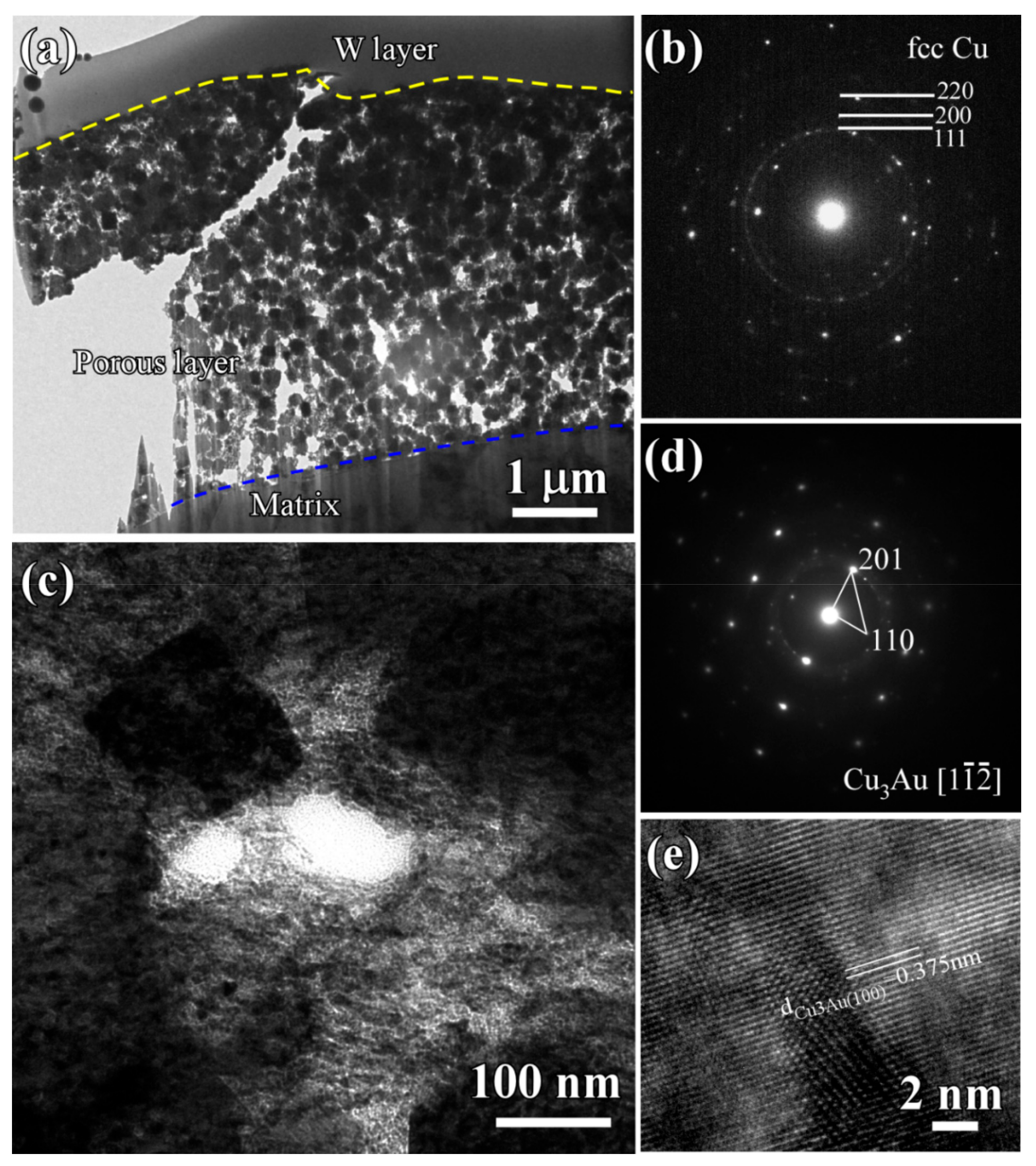
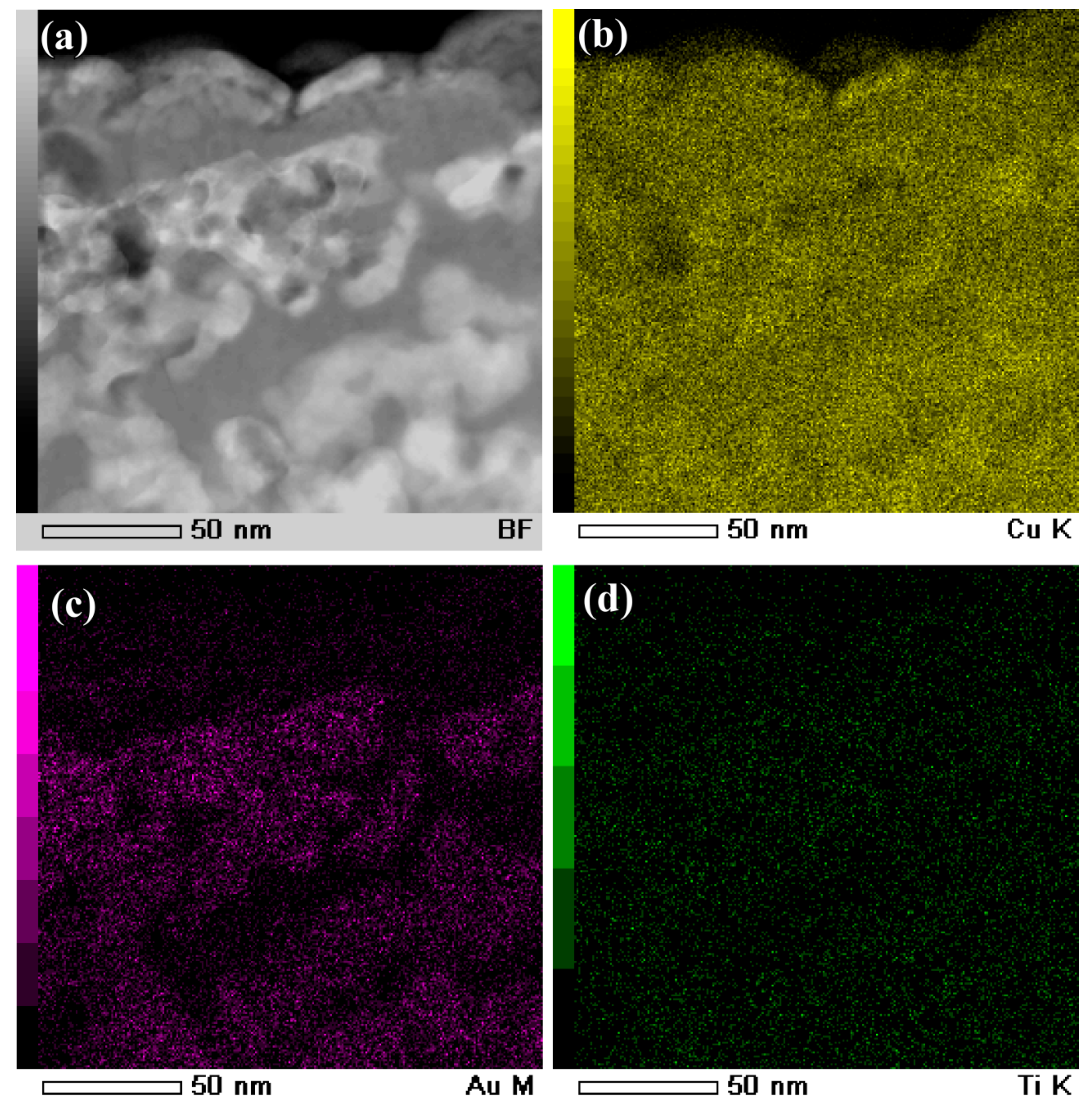
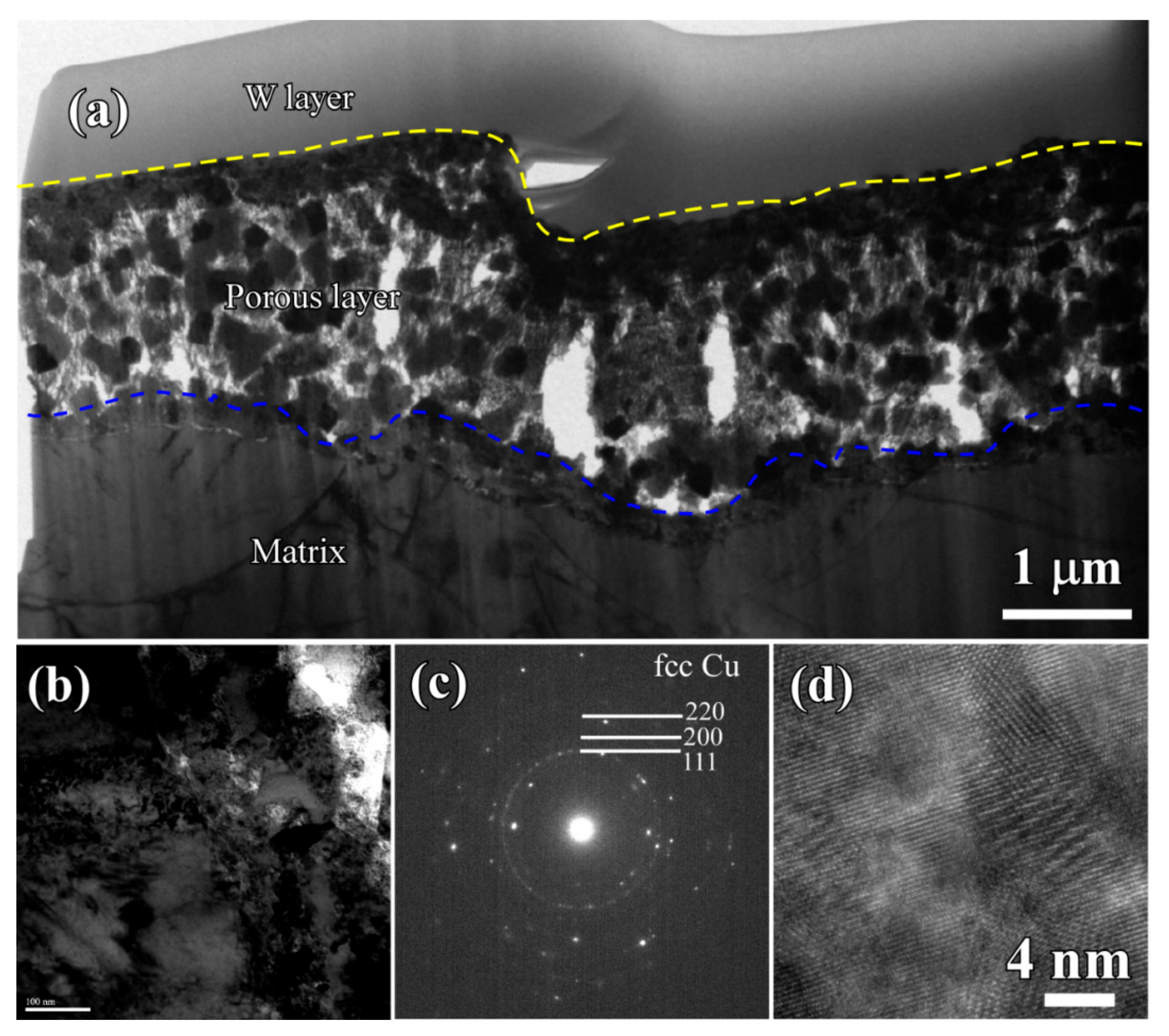
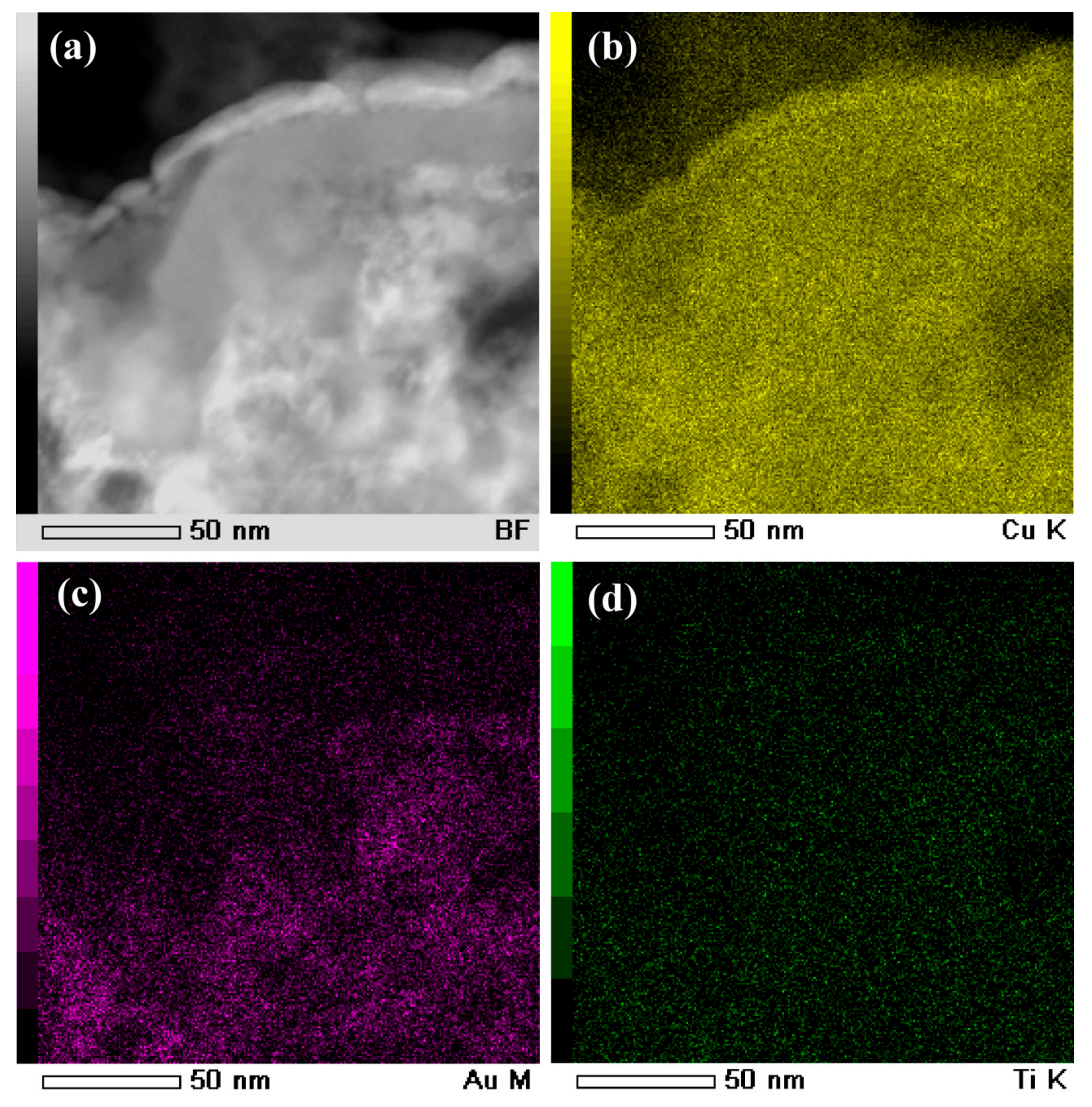
3.2. Evolution of Surface Nanoporous Layers on Gas-Atomized Ti60Cu39Au1 Powders during Dealloying
3.3. Discussion
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weissmüller, J.; Viswanath, R.N.; Kramer, D.; Zimmer, P.; Wurschum, R.; Gleiter, H. Charge-induced reversible strain in a metal. Science 2003, 300, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Hieda, M.; Garcia, R.; Dixon, M.; Daniel, T.; Allara, D.; Chan, M.H.W. Ultrasensitive quartz crystal microbalance with porous gold electrodes. Appl. Phys. Lett. 2004, 84, 628–630. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M.W.; Erlebacher, J. Metallic mesoporous nanocomposites for electrocatalysis. J. Am. Chem. Soc. 2004, 126, 6876–6877. [Google Scholar] [CrossRef] [PubMed]
- Ertenberg, R.W.; Andraka, B.; Takano, Y. Prospects of porous gold as a low-temperature heat exchanger for liquid and solid helium. Physica B 2000, 284, 2022–2023. [Google Scholar] [CrossRef]
- Lang, X.Y.; Hirata, A.; Fujita, T.; Chen, M.W. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat. Nanotechnol. 2011, 6, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Li, C.M. Single cell analysis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2061–2071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Li, C.M. Nanoporous metals: Fabrication strategies and advanced electrochemical applications in catalysis, sensing and energy systems. Chem. Soc. Rev. 2012, 41, 7016–7031. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.L.; Yu, Y.C.; Zhu, J.; Liu, S.F.; Muller, D.A.; Abruna, H.D. Morphology and activity tuning of Cu3Pt/C ordered intermetallic nanoparticles by selective electrochemical dealloying. Nano Lett. 2015, 15, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Kramer, D.; Volkert, C.A.; Rosner, H.; Erlebacher, J.; Weissmüller, J. Volume change during the formation of nanoporous gold by dealloying. Phys. Rev. Lett. 2009, 97, 035504. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Ding, Y.; Xu, C.X.; Inoue, A.; Sakurai, T.; Chen, M.W. Nanoporous metals by dealloying multicomponent metallic glasses. Chem. Mater. 2008, 20, 4548–4550. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B. Recent advances in porous Pt-based nanostructures: Synthesis and electrochemical applications. Chem. Soc. Rev. 2014, 43, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Romano, L.; Impellizzeri, G.; Bosco, L.; Ruffino, F.; Miritello, M.; Grimaldi, M.G. Nanoporosity induced by ion implantation in deposited amorphous Ge thin films. J. Appl. Phys. 2012, 111, 113515. [Google Scholar] [CrossRef]
- Mukherjee, N.; Paulose, M.; Varghese, O.K.; Mor, G.K.; Grimes, C.A. Fabrication of nanoporous tungsten oxide by galvanostatic anodization. J. Mater. Res. 2003, 18, 2296–2299. [Google Scholar] [CrossRef]
- Wada, T.; Ichitsubo, T.; Yubuta, K.; Segawa, H.; Yoshida, H.; Kato, H. Bulk-nanoporous-silicon negative electrode with extremely high cyclability for lithium-ion batteries prepared using a Top-down process. Nano Lett. 2014, 14, 4505–4510. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.S.; Leach, K.A.; Goldbach, J.T.; Kim, D.H.; Jho, J.Y.; Tuominen, M.; Hawker, C.J.; Russell, T.P. A simple route to metal nanodots and nanoporous metal films. Nano Lett. 2002, 2, 933–936. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlebacher, J. An atomistic description of dealloyingporosity evolution, the critical potential, and rate-limiting behavior. J. Electrochem. Soc. 2004, 151, C614–C626. [Google Scholar] [CrossRef]
- Sieradzki, K.; Newman, R.C. Micro-and Nano-Porous Metallic Structures. U.S. Patent Application No. 4,977,038, 11 December 1990. [Google Scholar]
- Snyder, J.; Livi, K.; Erlebacher, J. Dealloying silver/gold alloys in neutral silver nitrate solution: Porosity evolution, surface composition, and surface oxides. J. Electrochem. Soc. 2008, 155, C464–C473. [Google Scholar] [CrossRef]
- Snyder, J.; Asanithi, P.; Dalton, A.B.; Erlebacher, J. Stabilized nanoporous metals by dealloying ternary alloy precursors. Adv. Mater. 2008, 20, 4883–4886. [Google Scholar] [CrossRef]
- Jin, H.J.; Wang, X.L.; Parida, S.; Wang, K.; Seo, M.; Weissmuller, J. Nanoporous Au-Pt alloys as large strain electrochemical actuators. Nano Lett. 2009, 10, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.X.; Wang, R.Y.; Chen, M.W.; Zhang, Y.; Ding, Y. Dealloying to nanoporous Au/Pt alloys and their structure sensitive electrocatalytic properties. Phys. Chem. Chem. Phys. 2010, 12, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Guan, P.; McKenna, K.; Lang, X.; Hirata, A.; Zhang, L.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Tanaka, N.; et al. Atomic origins of the high catalytic activity of nanoporous gold. Nat. Mater. 2012, 11, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Dan, Z.H.; Qin, F.X.; Sugawara, Y.; Muto, I.; Hara, N. Fabrication of nanoporous copper by dealloying amorphous binary Ti-Cu alloys in hydrofluoric acid solutions. Intermetallics 2012, 29, 14–20. [Google Scholar] [CrossRef]
- Zhu, S.L.; He, J.L.; Yang, X.J.; Cui, Z.D.; Pi, L.L. Ti oxide nano-porous surface structure prepared by dealloying of Ti-Cu amorphous alloy. Electrochem. Commun. 2011, 13, 250–253. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Sugawara, Y.; Muto, I.; Hara, N. Effects of the initial microstructure of Ti-Cu alloys on final nanoporous copper via dealloying. J. Alloys Compd. 2013, 557, 166–171. [Google Scholar] [CrossRef]
- Chen, L.Y.; Yu, J.S.; Fujita, T.; Chen, M.W. Nanoporous copper with tunable nanoporosity for SERS applications. Adv. Funct. Mater. 2009, 19, 1221–1226. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, Y.; Zhen, Q. Generalized fabrication of nanoporous metals (Au, Pd, Pt, Ag, and Cu) through chemical dealloying. J. Phys. Chem. C 2009, 113, 12629. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Yamaura, S.; Xie, G.Q.; Makino, A.; Hara, N. Refinement of nanoporous copper by dealloying MgCuY amorphous alloys in sulfuric acids containing polyvinylpyrrolidone. J. Electrochem. Soc. 2014, 161, C120–C125. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Sugawara, Y.; Muto, I.; Makino, A.; Hara, N. Nickel-stabilized nanoporous copper fabricated from ternary TiCuNi amorphous alloys. Mater. Lett. 2013, 94, 128–131. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Makino, A.; Sugawara, Y.; Muto, I.; Hara, N. Fabrication of nanoporous copper by dealloying of amorphous Ti-Cu-Ag alloys. J. Alloys Compd. 2014, 586, S134–S138. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Sugawara, Y.; Muto, I.; Hara, N. Elaboration of nanoporous copper by modifying surface diffusivity by the minor addition of gold. Microporous. Mesoporous. Mater. 2013, 165, 257–264. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Akaki, T.; Koizumi, Y.; Minamino, Y.; Fujimoto, S. Selective pore growth on lamellar Ti-41at % Al alloy. Electrochem. Commun. 2013, 26, 117–120. [Google Scholar] [CrossRef]
- Mihailov, L.; Redzheb, M.; Spassov, T. Selective dissolution of amorphous and nanocrystalline Zr2Ni. Corros. Sci. 2013, 74, 308–313. [Google Scholar] [CrossRef]
- Rösler, J.; Näth, O.; Jäger, S.; Schmitz, F.; Mukherji, D. Fabrication of nanoporous Ni-based superalloy membranes. Acta Mater. 2005, 54, 1397–1406. [Google Scholar] [CrossRef]
- Dan, Z.H.; Qin, F.X.; Hara, N. Refinement of nanoporous copper: A summary of micro-alloying of Au-group and Pt-group elements. Mater. Trans. 2014, 55, 796–800. [Google Scholar] [CrossRef]
- Sun, J.S.; Wen, Z.; Han, L.P.; Chen, Z.W.; Lang, X.Y.; Jiang, Q. Nonprecious intermetallic Al7Cu4Ni nanocrystals seamlessly integrated in freestanding bimodal nanoporous copper for efficient hydrogen evolution catalysis. Adv. Funct. Mater. 2018, 28, 1706127. [Google Scholar] [CrossRef]
- Lu, X.; Balk, T.J.; Spolenak, R.; Arzta, E. Dealloying of Au-Ag thin films with a composition gradient: Influence on morphology of nanoporous Au. Thin Solid Film 2007, 515, 7122–7126. [Google Scholar] [CrossRef]
- Thorp, J.C.; Sieradzki, K.; Tang, L. Formation of nanoporous noble metal thin films by electrochemical dealloying of PtxSi1−xPtxSi1−x. Appl. Phys. Lett. 2006, 88, 033110. [Google Scholar] [CrossRef]
- Ruffino, F.; Torrisi, V.; Grillo, R.; Cacciato, G.; Zimbone, M.; Piccitto, G.; Grimaldi, M.G. Nanoporous Au structures by dealloying Au/Ag thermal- or laser-dewetted bilayers on surfaces. Superlattices Microstruct. 2017, 103, 28–47. [Google Scholar] [CrossRef]
- Srivastava, R.; Mani, P.; Hahn, N.; Strasser, P. Efficient oxygen reduction fuel cell electrocatalysis on voltammetrically dealloyed Pt-Cu-Co nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 8988–8991. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Core-shell compositional fine structures of dealloyed PtxNi1−x nanoparticles and their impact on oxygen reduction catalysis. Nano Lett. 2012, 12, 5423–5430. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Chen, Q.; McCue, I.; Snyder, J.; Crozier, P.; Erlebacher, J.; Sieradzki, K. Dealloying of noble-metal alloy nanoparticles. Nano Lett. 2014, 14, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Schaaf, P. Nanoporous gold nanoparticles. J. Mater. Chem. 2012, 22, 5344–5348. [Google Scholar] [CrossRef]
- Shui, J.L.; Chen, C.; Li, J.C.M. Evolution of nanoporous Pt-Fe alloy nanowires by dealloying and their catalytic property for oxygen reduction reaction. Adv. Funct. Mater. 2011, 21, 3357–3362. [Google Scholar] [CrossRef]
- Liu, L.F.; Pippel, E.; Scholz, R.; Gösele, U. Nanoporous Pt-Co alloy nanowires: Fabrication, characterization, and electrocatalytic properties. Nano Lett. 2009, 9, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, A.; Delacôte, C.; Molina-Luna, L.; Duerrschnabel, M.; Boujtita, M.; Thiry, D.; Du, K.; Ding, J.J.; Choi, C.H.; Tessier, P.; et al. Planar Arrays of Nanoporous Gold Nanowires: When Electrochemical Dealloying Meets Nanopatterning. ACS Appl. Mater. Interfaces 2016, 8, 6611–6620. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.X.; Searson, P.C. Synthesis and characterization of nanoporous gold nanowires. J. Phys. Chem. B 2003, 107, 4494–4499. [Google Scholar] [CrossRef]
- Liu, Z.; Searson, P.C. Single nanoporous gold nanowire sensors. J. Phys. Chem. B 2006, 110, 4318–4322. [Google Scholar] [CrossRef] [PubMed]
- Gargarella, P.; Pauly, S.; de Oliveira, M.F.; Kühn, U.; Eckert, J. Glass formation in the Ti-Cu system with and without Si additions. J. Alloys Compd. 2015, 618, 413–420. [Google Scholar] [CrossRef]
- Huston, E.L.; Cahn, J.W.; Hilliard, J.E. Spinodal decomposition during continuous cooling. Acta Metall. 1966, 14, 1053–1062. [Google Scholar] [CrossRef]
- Cohen, G.; Kuczynski, G.C. Coefficient of self-diffusion of copper. J. Appl. Phys. 1950, 21, 1339–1340. [Google Scholar] [CrossRef]
- Kaesche, H. Metallic Corrosion: Principles of Physical Chemistry and Current Problems; NACE: Houston, TX, USA, 1985. [Google Scholar]
- Pickering, H.W.; Wagner, C. Electrolytic Dissolution of binary alloys containing a noble metal. J. Electrochem. Soc. 1967, 114, 698–706. [Google Scholar] [CrossRef]
- Sieradzki, K.; Dimitrova, N.; Movrina, D.; McCalla, C.; Vasiljevica, N.; Erlebacher, J. The dealloying critical potential. J. Electrochem. Soc. 2002, 149, B370–B377. [Google Scholar] [CrossRef]
- Sieradzki, K. Curvature effects in alloy dissolution. J. Electrochem. Soc. 1993, 140, 2868–2872. [Google Scholar] [CrossRef]
- Sieradzki, K.; Corderman, R.R.; Shukla, K.; Newman, R.C. Computer simulations of corrosion: Selective dissolution of binary alloys. Philos. Mag. A. 1989, 59, 713–746. [Google Scholar] [CrossRef]
- Chen, Q.; Sieradzki, K. Mechanisms and morphology evolution in dealloying. J. Electrochem. Soc. 2013, 160, C226–C231. [Google Scholar] [CrossRef]
- Chen, Q.; Sieradzki, K. Spontaneous evolution of bicontinuous nanostructures in dealloyed Li-based systems. Nat. Mater. 2013, 12, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- McCue, I.; Snyder, J.; Li, X.; Chen, Q.; Sieradzki, K.; Erlebacher, J. Apparent inverse Gibbs-Thomson effect in dealloyed nanoporous nanoparticles. Phys. Rev. Lett. 2012, 108, 225503. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Han, B.; Persson, K.; Friesen, C.; He, T.; Sieradzki, K.; Ceder, G. Electrochemical stability of nanometer-scale Pt particles in acidic environments. J. Am. Chem. Soc. 2009, 132, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Buffat, Ph.; Borel, J. Size effect on the melting temperature of gold particles. Phys. Rev. A 1976, 13, 2287–2298. [Google Scholar] [CrossRef]
- Dick, K.; Dhanasekaran, T.; Zhang, Z.; Meisel, D. Size-dependent melting of Silica-encapsulated gold nanoparticles. J. Am. Chem. Soc. 2002, 124, 2312–2317. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dan, Z.; Qu, J.; Yang, Y.; Qin, F.; Chang, H. Evolution of Nanoporous Surface Layers on Gas-Atomized Ti60Cu39Au1 Powders during Dealloying. Nanomaterials 2018, 8, 581. https://doi.org/10.3390/nano8080581
Dan Z, Qu J, Yang Y, Qin F, Chang H. Evolution of Nanoporous Surface Layers on Gas-Atomized Ti60Cu39Au1 Powders during Dealloying. Nanomaterials. 2018; 8(8):581. https://doi.org/10.3390/nano8080581
Chicago/Turabian StyleDan, Zhenhua, Jiahui Qu, Yulin Yang, Fengxiang Qin, and Hui Chang. 2018. "Evolution of Nanoporous Surface Layers on Gas-Atomized Ti60Cu39Au1 Powders during Dealloying" Nanomaterials 8, no. 8: 581. https://doi.org/10.3390/nano8080581





