In Vitro Cytotoxicity and Morphological Assessments of GO-ZnO against the MCF-7 Cells: Determination of Singlet Oxygen by Chemical Trapping
Abstract
1. Introduction
2. Material and Methods
2.1. Graphene Oxide (GO)-ZnO Nanocomposites’ Preparation
2.2. Cell Culturing and Labeling (MCF-7, Breast Cancer Cell Line)
2.3. In Vitro Cellular Cytotoxicity methyl-thiazole-tetrazolium (MTT) Assay
2.4. Membrane Integrity
2.5. Reactive Oxygen Species Fluorescence
2.6. Cell’s Morphological Analysis
2.7. Cell Mortality Assay
2.8. Apoptosis Detection Assay
2.9. Exposure of Singlet Oxygen by Chemical Trapping
2.10. Characterization
2.11. Statistical Analysis
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Analysis
3.2. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Analysis (EDAX) of Graphene Oxide (GO)-ZnO
3.3. Raman Spectroscopy and Ultraviolet-Visible (UV-Vis) Analysis
3.4. Cellular Uptake and Cytotoxicity of GOZnO towards MCF-7 Cells
3.5. GO-ZnO Effect on Membrane Integrity
3.6. Morphological Variations and Cell Mortality on MCF-7 Cancerous Cells by GO-ZnO
3.7. Reactive Oxygen Species (ROS) Generation
3.8. Apoptotic Detection Assay
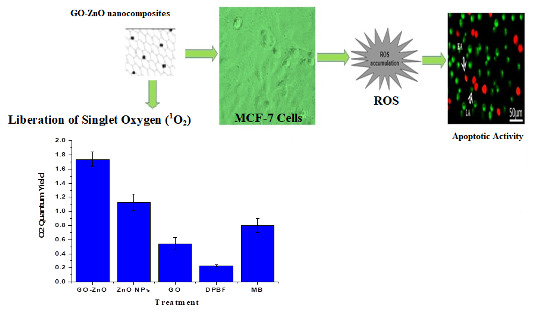
3.9. Liberation of Singlet Oxygen (1O2)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mousa, S.A.; Bharali, D.J. Nanotechnology-based detection and targeted therapy in cancer: Nano-bio paradigms and applications. Cancers 2011, 3, 2888–2903. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T. Targeted nanoparticles for cancer therapy: Promises and challenges. J. Nanomed. Nanotechnol. 2011, 2, 103e. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene-based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Shi, X.; Gong, H.; Li, Y.; Wang, C.; Cheng, L.; Liu, Z. Graphene-based magnetic plasmonic nanocomposite for dual bioimaging and photothermal therapy. Biomaterials 2013, 34, 4786–4793. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.S.; An, S.S.A. Oxidative stress and antibacterial properties of a graphene oxide-cystamine nanohybrid. Int. J. Nanomed. 2015, 10, 549–556. [Google Scholar]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Cui, L.; Dusan, L. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, Y.; Liu, Y.; Li, J. DNA-Directed Self-Assembly of Graphene Oxide with Applications to Ultrasensitive Oligonucleotide Assay. ACS Nano 2011, 5, 3817–3822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, Z. Morphology, crystallization behavior, and dynamic mechanical properties of biodegradable poly(ε-caprolactone)/thermally reduced graphene nanocomposites. Ind. Eng. Chem. Res. 2011, 50, 13885–13891. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, L. Insight into the Reaction Mechanism of Graphene Oxide with Oxidative Free Radical. Chem. Res. Chin. Univ. 2017, 33, 689–694. [Google Scholar] [CrossRef]
- Shi, S.; Yang, K.; Hong, H.; Chen, F.; Valdovinos, H.F.; Goel, S.; Barnhart, T.E.; Liu, Z.; Cai, W. VEGFR targeting leads to significantly enhanced tumor uptake of nanographene oxide in vivo. Biomaterials 2015, 39, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.M.P.; Goulart, V.A.M.; Gomes, K.N.; Ladeira, M.S.; Santos, A.K.; Lorencon, E. Graphene-based nanomaterials: Biological and medical applications and toxicity. Nanomedicine 2015, 10, 2423–2450. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Paula, A.J.; Lima, R.; Alves, O.L.; Duran, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Horva, L.; Magrez, A.; Burghard, M.; Kern, K.; Forro, L.; Schwaller, B. Evaluation of the toxicity of graphene derivatives on cells of the lung luminal surface. Carbon 2013, 64, 45–60. [Google Scholar] [CrossRef]
- Qin, X.C.; Guo, Z.Y.; Liu, Z.M.; Zhang, W.; Wan, M.M.; Yang, B.W. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J. Photochem. Photobiol. B Biol. 2013, 120, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, K.H. Synthesis of ZnO nanostructures composed of nanosheets with controllable morphologies. Cryst. Growth Des. 2009, 10, 1289. [Google Scholar] [CrossRef]
- Soares, J.W.; Steeves, D.M.; Ziegler, D.; DeCristofano, B.S. Surface Modification of Nanocrystalline Zinc Oxide for Bio-Sensing Applications. Proc. SPIE 2006, 6370, 637011. [Google Scholar]
- Shen, C.; James, S.A.; de Jonge, M.D.; Turney, T.W.; Wright, P.F.A.; Feltis, B.N. Relating cytotoxicity, zinc ions, and reactive oxygen in ZnO nanoparticle-exposed human immune cells. Toxicol. Sci. 2013, 136, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Dayem, A.A.; Kim, J.H. Biocompatibility effects of biologically synthesized graphene in primary mouse embryonic fibroblast cells. Nanoscale Res. Lett. 2013, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Chen, Y.W.; Hung, W.T.; Chen, I.W.; Chen, S.Y. Quantum-Dot-Tagged Reduced Graphene Oxide Nanocomposites for Bright Fluorescence Bioimaging and Photothermal Therapy Monitored In Situ. Adv. Mater. 2012, 24, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Muller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D.D. Mechanically Strong, Electrically Conductive, and Biocompatible Graphene Paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Su, W.C.; Ku, B.K.; Kulkarni, P.; Cheng, Y.S. Deposition of graphene nanomaterial aerosols in human upper airways. J. Occup. Environ. Hyg. 2015, 13, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, R.; Molander, S.; Sandén, B.A. Review of potential environmental and health risks of the nanomaterial graphene. Hum. Ecol. Risk Assess. 2013, 19, 873–887. [Google Scholar] [CrossRef]
- Wang, Y.W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.H.; Liu, Y.; Wang, H. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; An, S.S.; Hulme, J. Current applications of graphene oxide in nanomedicine. Int. J. Nanomed. 2015, 10, 9–24. [Google Scholar]
- Ali-Boucetta, H.; Bitounis, D.; Raveendran-Nair, R.; Servant, A.; Van Den Bossche, J.; Kostarelos, K. Purified graphene oxide dispersions lack in vitro cytotoxicity and in vivo pathogenicity. Adv. Healthc. Mater. 2013, 2, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Y.; Yu, J.; Wang, S.; Ge, S.; Song, X. Application of ZnO/graphene and S6 aptamers for sensitive photoelectrochemical detection of SK-BR-3 breast cancer cells based on a disposable indium tin oxide device. Biosens. Bioelectron. 2014, 51, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, M.K.; John, H.; Gopinath, P.; Philip, R. Synthesis of reduced graphene oxide–ZnO hybrid with enhanced optical limiting properties. J. Mater. Chem. C 2013, 1, 3669–3676. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.H. Cytotoxicity of biologically synthesized silver nanoparticles in mda-mb-231 human breast cancer cells. Biomed. Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef] [PubMed]
- Fakhar-e-Alam, M.; Kishwar, S.; Khan, Y.; Siddique, M.; Atif, M.; Nur, O. Tumoricidal effects of nanomaterials in HeLa cell line. Laser Phys. 2011, 21, 1978–1988. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Quadri, F.; Prox, J.D.; Guo, L. Cytotoxicity of ZnO Nanowire Arrays on Excitable Cells. Nanomaterials 2017, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, K.L. Interactions of Graphene Oxide with Model Cell Membranes: Probing Nanoparticle Attachment and Lipid Bilayer Disruption. Langmuir 2015, 31, 12076–12086. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Fakhar-E.-Alam, M.; Fatima, M.; Shaheen, F.; Iqbal, S.; Atif, M. Photodynamic Effect of Ni Nanotubes on an HeLa Cell Line. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.T.; Liu, J.H. In vitro toxicity evaluation of grapheme oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Arooj, S.; Nazir, S.; Nadhman, A.; Ahmad, N.; Muhammad, B.; Ahmad, I.; Mazhar, K.; Abbasi, R. Novel ZnO:Ag nanocomposites induce significant oxidative stress in human fibroblast malignant melanoma (Ht144) cells. Beilstein J. Nanotechnol. 2015, 6, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Gu, L.; Howell, S.B.; Sailor, M.J. Porous Silicon Nanoparticle Photosensitizers for Singlet Oxygen and Their Phototoxicity against Cancer Cells. ACS Nano 2011, 5, 3651–3659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, N.; Jiu, H.; Qi, G.; Huang, Y. ZnO-reduced graphene oxide nanocomposites as efficient photocatalysts for photocatalytic reduction of CO2. Ceram. Int. 2015, 41, 6256–6262. [Google Scholar] [CrossRef]
- Zhan, Z.; Zheng, L.; Pan, Y.; Sun, G.; Li, L. Self-powered, visible-light photodetector based on thermally reduced graphene oxide–ZnO (RGO–ZnO) hybrid nanostructure. J. Mater. Chem. 2012, 22, 2589–2595. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Babaei, S. Graphene Oxide/Zinc Oxide (GO/ZnO) Nanocomposite as a Superior Photocatalyst for Degradation of Methylene Blue (MB)-Process Modeling by Response Surface Methodology (RSM). J. Braz. Chem. Soc. 2017, 28. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, T.; Zhao, X.; Wang, X.; Zhou, L.; Yang, Y.; Liao, F.; Ju, Y. Poly(lactic acid)/graphene oxide–ZnO nanocomposite films with good mechanical, dynamic mechanical, anti-UV and antibacterial properties. Chem. Technol. Biotechnol. 2015, 90, 1677–1684. [Google Scholar] [CrossRef]
- Kavitha, M.K.; Gopinath, P.; John, H. Reduced graphene oxide–ZnO self-assembled films: Tailoring the visible light photoconductivity by the intrinsic defect states in ZnO. Phys. Chem. Chem. Phys. 2015, 17, 14647–14655. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yun, K. Graphene oxide-modified ZnO particles: Synthesis, characterization, and antibacterial properties. Int. J. Nanomed. 2015, 10, 79–92. [Google Scholar]
- Fatima, M.; Fakhar-E.-Alam, M.; Atif, M.; Zaidi, S.S.Z.; Suleman, R.; Shakoor, M.N.; Aziz, M.H. Apoptotic effect of α-Fe2O3 and SiO2 nanoparticles in human rhabdomyosarcoma cell line. Laser Phys. 2014, 24. [Google Scholar] [CrossRef]
- Ramesha, G.; Kumara, A.V.; Muralidhara, H.; Sampath, S. Graphene and Graphene Oxide as Effective Adsorbents toward Anionic and Cationic Dyes. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.S.; Pandey, A.P.; Patil, P.O.; Tekade, A.R.; Bari, S.B. Deshmukh PK.Graphene oxide based magnetic nanocomposites for efficient treatment of breast cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aburto, R.; Narayanan, T.N.; Nagaoka, Y.; Hasumura, T.; Mitcham, T.M.; Fukuda, T.; Cox, P.J.; Bouchard, R.R.; Maekawa, T.; Sakthi Kumar, D. Fluorinated graphene oxide; a new multimodal material for biological applications. Adv. Mater. 2013, 25, 5632–5637. [Google Scholar] [CrossRef] [PubMed]
- Vallabani, N.V.; Mittal, S.; Shukla, R.K.; Pandey, A.K.; Dhakate, S.R.; Pasricha, R.; Dhawan, A. Toxicity of graphene in normal human lung cells (BEAS-2B). J. Biomed. Nanotechnol. 2011, 7, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Lammel, T.; Boisseaux, P.; Fernández-Cruz, M.L.; Navas, J.M. Internalization and cytotoxicity of graphene oxide and carboxyl graphenenanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Fibre Toxicol. 2013, 10, 743–8977. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, R.; Gurumallesh Prabu, H. Synthesis Characterization, Antimicrobial, Antioxidant, and Cytotoxic Activities of ZnO Nanorods on Reduced Graphene Oxide. J. Inorg. Organometall. Polym. Mater. 2017, 28. [Google Scholar] [CrossRef]
- Hu, Z.; Li, J.; Li, C.; Zhao, S.; Li, N.; Wang, Y.; Wei, F.; Chen, L.; Huang, Y. Folic acid-conjugated graphene–ZnO nanohybrid for targeting photodynamic therapy under visible light irradiation. J. Mater. Chem. B 2013, 1, 5003–5013. [Google Scholar] [CrossRef]
- De Marzi, L.; Ottaviano, L.; Perrozzi, F.; Nardone, M.; Santucci, S.; De Lapuente, J.; Borras, M.; Treossi, E.; Palermo, V.; Poma, A. Flake size-dependent cyto and genotoxic evaluation of graphene oxide on in vitro A549, CaCo2 and vero cell lines. J. Biol. Regul. Homeost. Agents 2014, 28, 281–289. [Google Scholar] [PubMed]
- Kang, S.M.; Kim, T.H.; Choi, J.W. Cell chip to detect effects of graphene oxide nanopellet on human neural stem cell. J. Nanosci. Nanotechnol. 2012, 12, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, F.; Hammad Aziz, M.; Fakhar-e-Alam, M.; Atif, M.; Fatima, M.; Ahmad, R.; Hanif, A.; Anwar, S.; Zafar, F.; Abbas, G.; et al. An In Vitro Study of the Photodynamic Effectiveness of GO-Ag Nanocomposites against Human Breast Cancer Cells. Nanomaterials 2017, 7, 401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Dorn, M.; Vogt, J.; Spemann, D.; Yu, W.; Mao, Z.W. A quantitative study of the intracellular concentration of graphene/noble metal nanoparticle composites and their cytotoxicity. Nanoscale 2014, 6, 8535–8542. [Google Scholar] [CrossRef] [PubMed]
- Mullick Chowdhury, S.; Lalwani, G.; Zhang, K.; Yang, J.Y.; Neville, K.; Sitharaman, B. Cell Specific Cytotoxicity and Uptake of Graphene Nanoribbons. Biomaterials 2013, 34, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, S.; Sawosz, E.; Grodzik, M.; Winnicka, A.; Prasek, M.; Wierzbicki, M.; Chwalibog, A. In vitro evaluation of the effects of graphene platelets on glioblastoma multiforme cells. Int. J. Nanomed. 2013, 8, 413–420. [Google Scholar]
- Cheng, C.; Nie, S.; Li, S. Biopolymer functionalized reduced grapheme oxide with enhanced biocompatibility via mussel inspired coatings/anchors. J. Mater. Chem. B 2013, 1, 265–275. [Google Scholar] [CrossRef]
- Ajdari, Z.; Rahman, H.; Shameli, K.; Abdullah, R.; Ghani, M.A.; Yeap, S.; Abbasiliasi, S.; Ajdari, D.; Ariff, A. Novel Gold Nanoparticles Reduced by Sargassum glaucescens: Preparation, Characterization and Anticancer Activity. Molecules 2016, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yotnda, P. Production and Detection of Reactive Oxygen Species (ROS) in Cancers. J. Vis. Exp. 2011, 57, 3357. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ouyang, S.; Mu, L.; An, J.; Zhou, Q. Effects of graphene oxide and oxidized carbon nanotubes on the cellular division, microstructure, uptake, oxidative stress, and metabolic profilesEnviron. Sci. Technol. 2015, 49, 10825–10833. [Google Scholar] [CrossRef] [PubMed]













© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaheen, F.; Aziz, M.H.; Fatima, M.; Khan, M.A.; Ahmed, F.; Ahmad, R.; Ahmad, M.A.; Alkhuraiji, T.S.; Akram, M.W.; Raza, R.; et al. In Vitro Cytotoxicity and Morphological Assessments of GO-ZnO against the MCF-7 Cells: Determination of Singlet Oxygen by Chemical Trapping. Nanomaterials 2018, 8, 539. https://doi.org/10.3390/nano8070539
Shaheen F, Aziz MH, Fatima M, Khan MA, Ahmed F, Ahmad R, Ahmad MA, Alkhuraiji TS, Akram MW, Raza R, et al. In Vitro Cytotoxicity and Morphological Assessments of GO-ZnO against the MCF-7 Cells: Determination of Singlet Oxygen by Chemical Trapping. Nanomaterials. 2018; 8(7):539. https://doi.org/10.3390/nano8070539
Chicago/Turabian StyleShaheen, Fozia, Muhammad Hammad Aziz, Mahvish Fatima, Muhammad Ajmal Khan, Faisal Ahmed, Riaz Ahmad, Muhammad Ashfaq Ahmad, Turki S. Alkhuraiji, Muhammad Waseem Akram, Rizwan Raza, and et al. 2018. "In Vitro Cytotoxicity and Morphological Assessments of GO-ZnO against the MCF-7 Cells: Determination of Singlet Oxygen by Chemical Trapping" Nanomaterials 8, no. 7: 539. https://doi.org/10.3390/nano8070539
APA StyleShaheen, F., Aziz, M. H., Fatima, M., Khan, M. A., Ahmed, F., Ahmad, R., Ahmad, M. A., Alkhuraiji, T. S., Akram, M. W., Raza, R., & Ali, S. M. (2018). In Vitro Cytotoxicity and Morphological Assessments of GO-ZnO against the MCF-7 Cells: Determination of Singlet Oxygen by Chemical Trapping. Nanomaterials, 8(7), 539. https://doi.org/10.3390/nano8070539