Solution-Grown Dendritic Pt-Based Ternary Nanostructures for Enhanced Oxygen Reduction Reaction Functionality
Abstract
:1. Introduction
2. Experimental Methods
2.1. Synthesis of Binary PtNi, PtCo and PtFe Nanostructures
2.2. Synthesis of Ternary Pt(NiCo), Pt(FeNi) and Pt(FeCo) Nanostructures
2.3. Nanostructure Characterization Techniques
2.4. Electrochemical Measurements
3. Results and Discussion
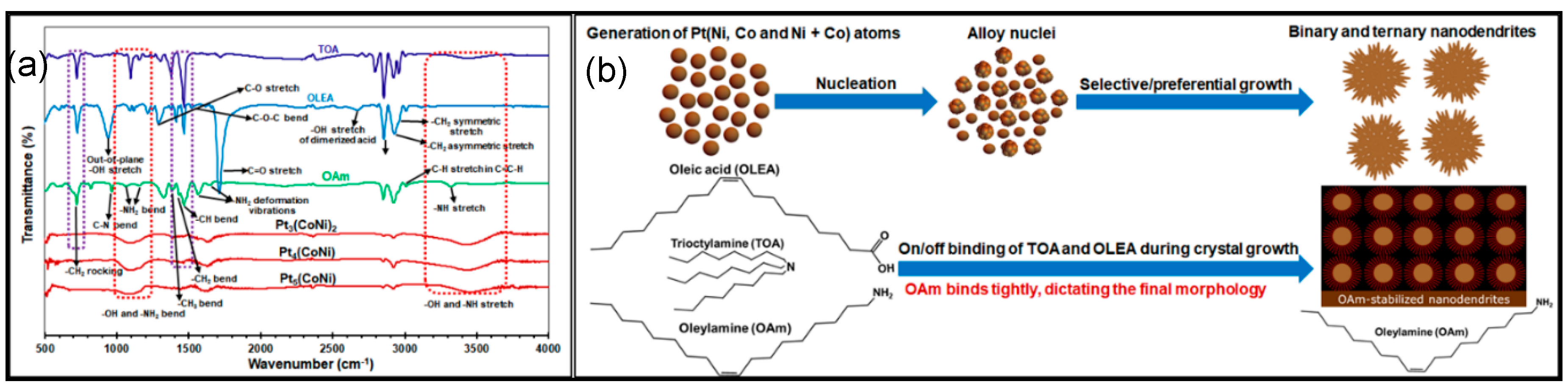
3.1. Influence of Different Metal Precursors on Nucleation and Growth Mechanism of Pt 3D Transition Metals (Ni, Co, Fe)
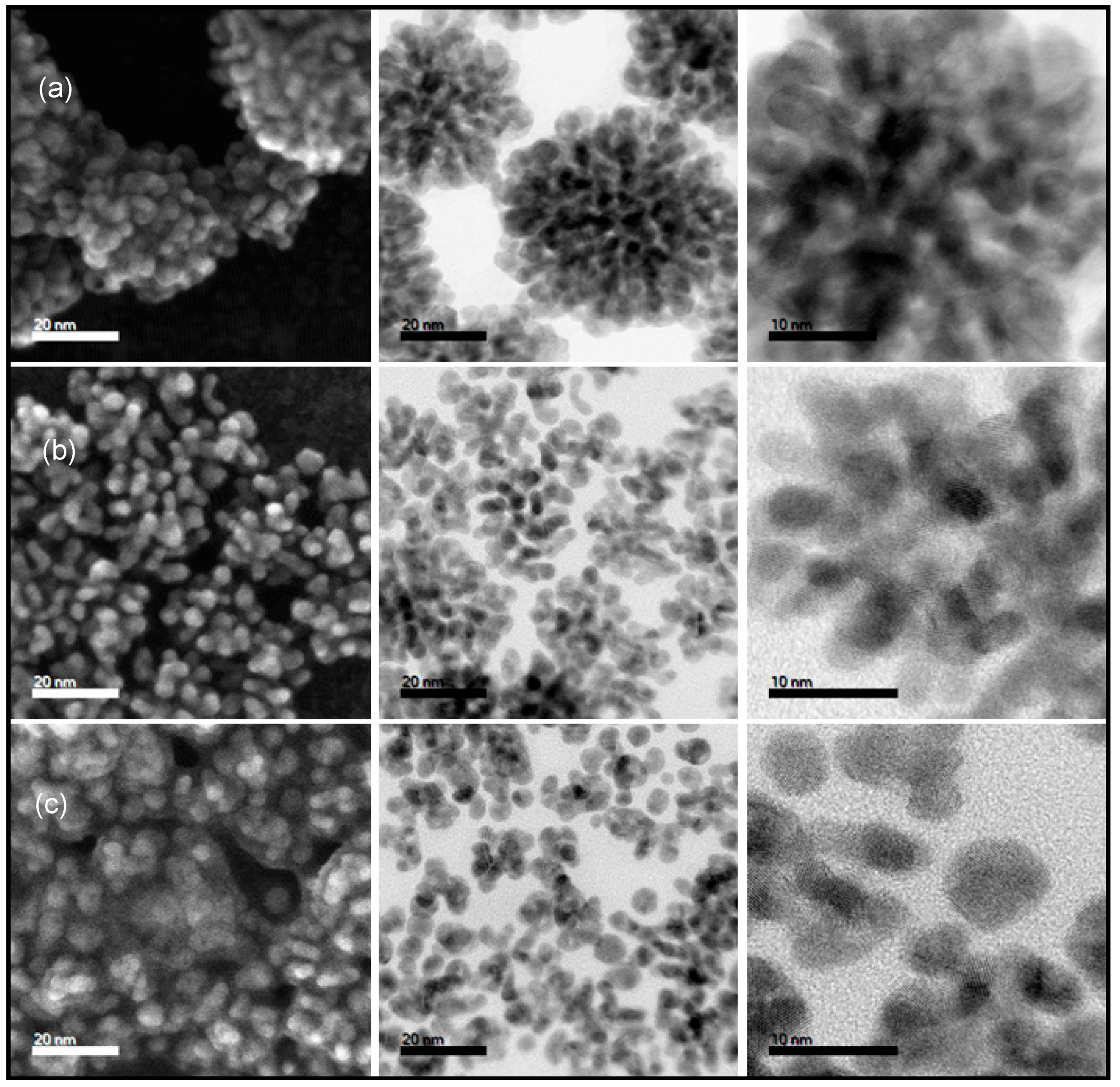
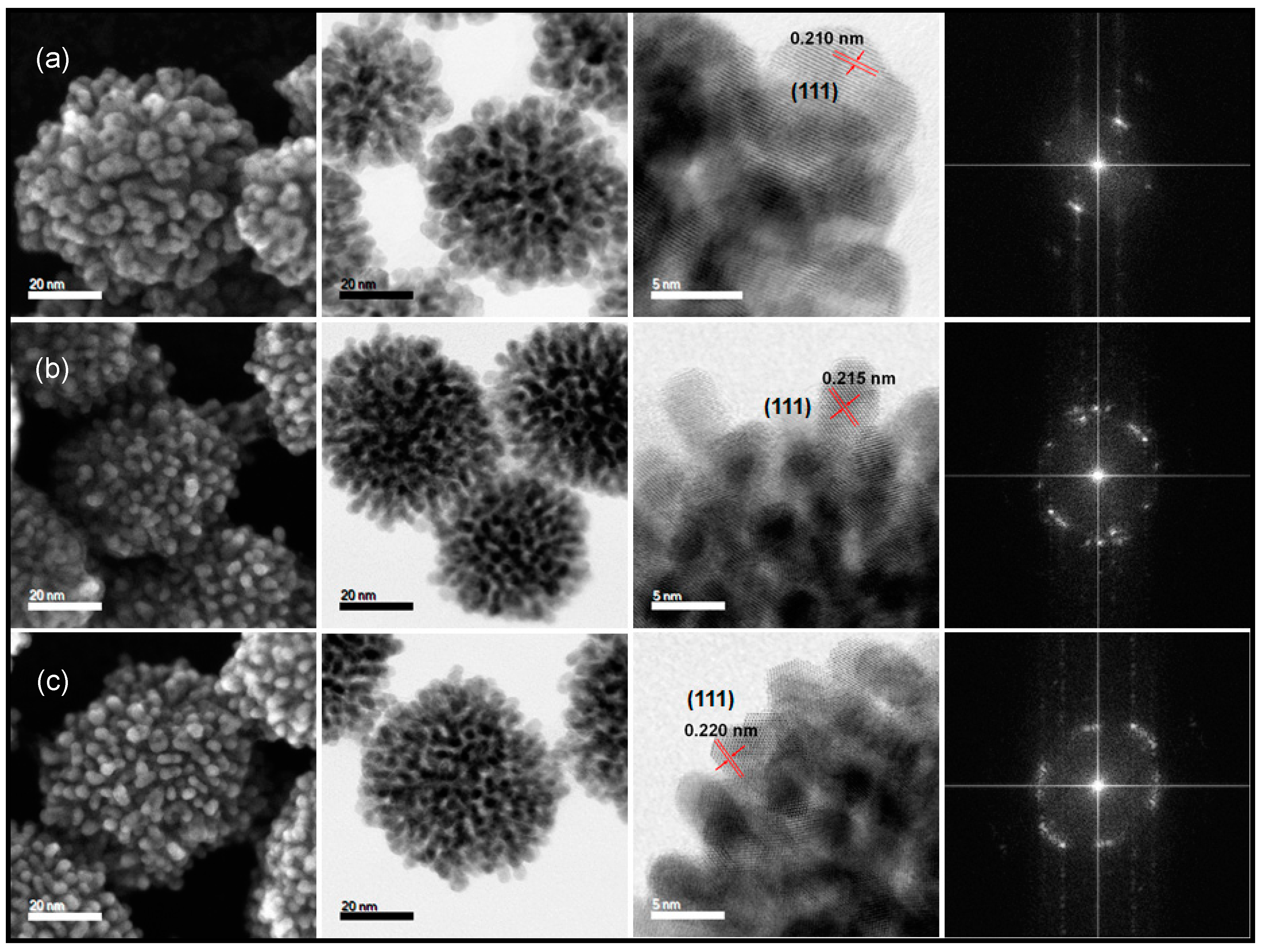
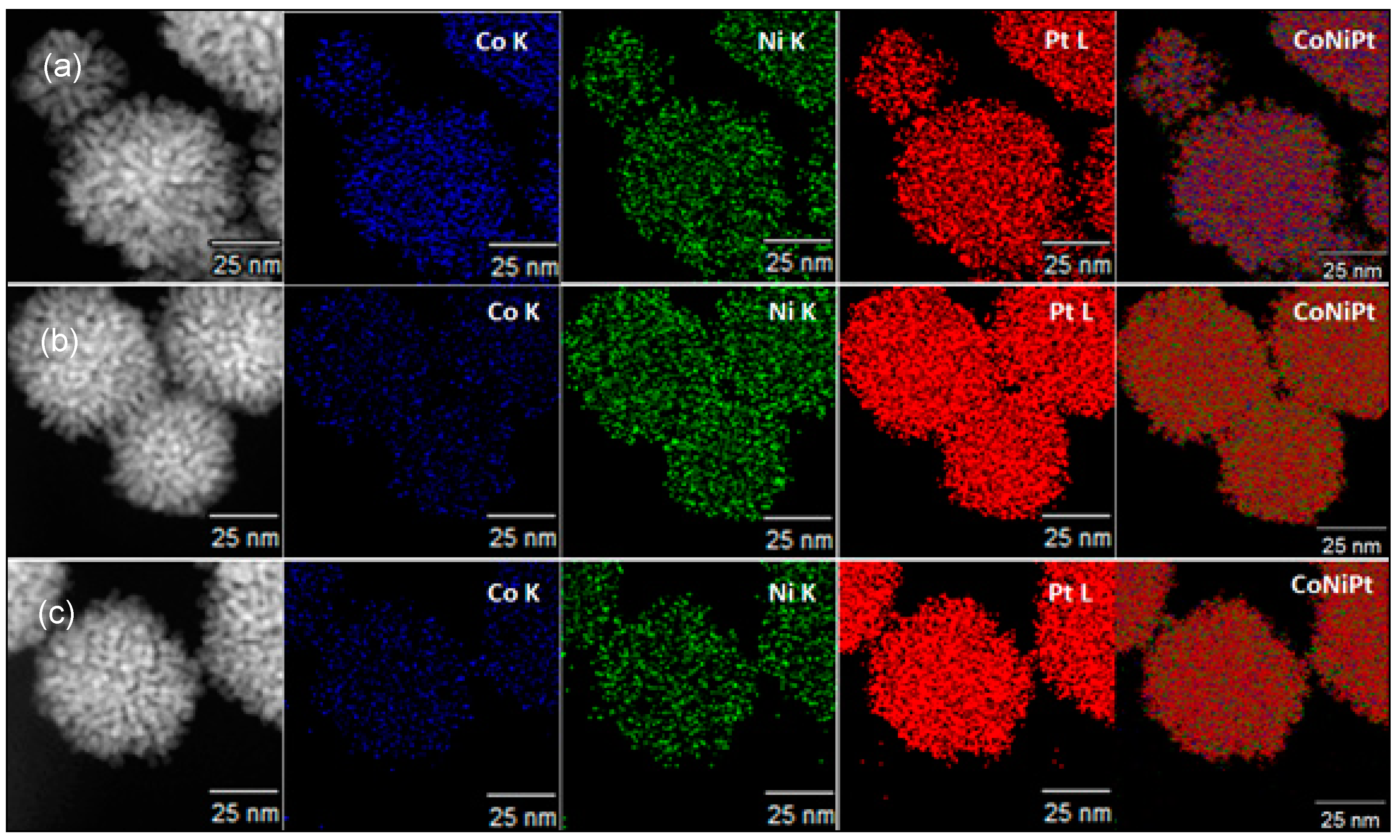
3.2. The Influence of Alloying Feed Ratios on the Morphology of Pt(NiCo) Alloys
3.3. The Effect of Synthesis Chemistry on Nanoalloys’ Morphological Evolution
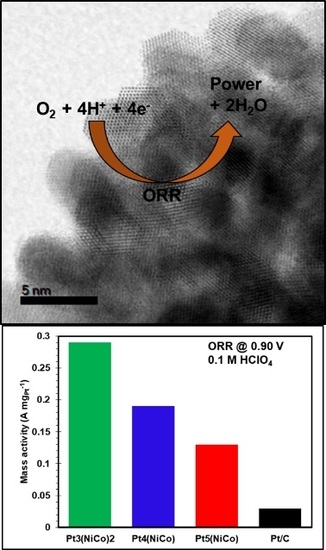
3.4. Catalyst Activity Measurements towards ORR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, M.; Wu, D.; Cao, R. Recent Advances in the Stabilization of Platinum Electrocatalysts for Fuel-Cell Reactions. ChemCatChem 2014, 6, 26–45. [Google Scholar] [CrossRef]
- Greeley, J.; Mavrikakis, M. Alloy catalysts designed from first principles. Nat. Mater. 2004, 3, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Toda, T. Enhancement of the Electroreduction of Oxygen on Pt Alloys with Fe, Ni, and Co. J. Electrochem. Soc. 1999, 146, 3750–3756. [Google Scholar] [CrossRef]
- Peng, Z.; Yang, H. Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 2009, 4, 143–164. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt Bimetallic Nanodendrites with High Activity for Oxygen Reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt Nanoparticles and Ferromagnetic FePt Nanocrystal Superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Xia, Y. Metal Nanocrystals with Highly Branched Morphologies. Angew. Chem. Int. Ed. 2011, 50, 76–85. [Google Scholar] [CrossRef]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly Crystalline Multimetallic Nanoframes with Three-Dimensional Electrocatalytic Surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Feng, M.; Zhou, J.; Liu, Z.; Li, M.; Fan, Z.; Tsen, O.; Miao, J.; Duan, X.; Huang, Y. Composition tunable ternary Pt-Ni-Co octahedra for optimized oxygen reduction activity. Chem. Commun. 2016, 52, 11215–11218. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Chen, M.; Du, C.; Sun, Y.; Tan, Q.; Du, L.; Chen, G.; Yin, G. Facile synthesis of Pt3Ni alloy nanourchins by temperature modulation and their enhanced electrocatalytic properties. J. Alloys Compd. 2015, 645, 309–316. [Google Scholar] [CrossRef]
- Porter, N.S.; Wu, H.; Quan, Z.; Fang, J. Shape-Control and Electrocatalytic Activity-Enhancement of Pt-Based Bimetallic Nanocrystals. Acc. Chem. Res. 2013, 46, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Du, X.X.; He, Y.; Wang, X.X.; Wang, J.N. Fine-grained and fully ordered intermetallic PtFe catalysts with largely enhanced catalytic activity and durability. Energy Environ. Sci. 2016, 9, 2623–2632. [Google Scholar] [CrossRef]
- Wang, L.; Nemoto, Y.; Yamauchi, Y. Direct Synthesis of Spatially-Controlled Pt-on-Pd Bimetallic Nanodendrites with Superior Electrocatalytic Activity. J. Am. Chem. Soc. 2011, 133, 9674–9677. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yang, H. Synthesis and Oxygen Reduction Electrocatalytic Property of Pt-on-Pd Bimetallic Heteronanostructures. J. Am. Chem. Soc. 2009, 131, 7542–7543. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yamauchi, Y. Strategic Synthesis of Trimetallic Au@Pd@Pt Core−Shell Nanoparticles from Poly(vinylpyrrolidone)-Based Aqueous Solution toward Highly Active Electrocatalysts. Chem. Mater. 2011, 23, 2457–2465. [Google Scholar] [CrossRef]
- Graham, L.; Collins, G.; Holmes, J.D.; Tilley, R.D. Synthesis and catalytic properties of highly branched palladium nanostructures using seeded growth. Nanoscale 2016, 8, 2867–2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, H.; Nemoto, Y.; Yamauchi, Y. Rapid and Efficient Synthesis of Platinum Nanodendrites with High Surface Area by Chemical Reduction with Formic Acid. Chem. Mater. 2010, 22, 2835–2841. [Google Scholar] [CrossRef]
- Lim, S.I.; Ojea-Jiménez, I.; Varon, M.; Casals, E.; Arbiol, J.; Puntes, V. Synthesis of Platinum Cubes, Polypods, Cuboctahedrons, and Raspberries Assisted by Cobalt Nanocrystals. Nano Lett. 2010, 10, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Ralph, T.R.; Hards, G.A.; Keating, J.E.; Campbell, S.A.; Wilkinson, D.P.; Davis, M.; St-Pierre, J.; Johnson, M.C. Low Cost Electrodes for Proton Exchange Membrane Fuel Cells: Performance in Single Cells and Ballard Stacks. J. Electrochem. Soc. 1997, 144, 3845–3857. [Google Scholar] [CrossRef]
- Shui, J.I.; Chen, C.; Li, J.C.M. Evolution of Nanoporous Pt-Fe Alloy Nanowires by Dealloying and their Catalytic Property for Oxygen Reduction Reaction. Adv. Funct. Mater. 2011, 21, 3357–3362. [Google Scholar] [CrossRef]
- Sugimoto, W.; Aoyama, K.; Kawaguchi, T.; Murakami, Y.; Takasu, Y. Kinetics of CH3OH oxidation on PtRu/C studied by impedance and CO stripping voltammetry. J. Electroanal. Chem. 2005, 576, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Yin, Y.; Alivisatos, A.P. Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 2004, 437, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Watt, J.; Young, N.; Haigh, S.; Kirkland, A.; Tilley, R.D. Synthesis and Structural Characterization of Branched Palladium Nanostructures. Adv. Mater. 2009, 21, 2288–2293. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Ding, J.; Chow, G.-M. Morphological Control of Synthesis and Anomalous Magnetic Properties of 3-D Branched Pt Nanoparticles. Langmuir 2008, 24, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, S.; Srinivasan, S.; Soriaga, M.P.; McBreen, J. Effect of Preparation Conditions of Pt Alloys on Their Electronic, Structural, and Electrocatalytic Activities for Oxygen Reduction—XRD, XAS, and Electrochemical Studies. J. Phys. Chem. 1995, 99, 4577–4589. [Google Scholar] [CrossRef]
- Ma, Y.; Balbuena, P.B. Pt surface segregation in bimetallic Pt3M alloys: A density functional theory study. Surf. Sci. 2008, 602, 107–113. [Google Scholar] [CrossRef]
- Watt, J.; Cheong, S.; Toney, M.F.; Ingham, B.; Cookson, J.; Bishop, P.T.; Tilley, R.D. Ultrafast Growth of Highly Branched Palladium Nanostructures for Catalysis. ACS Nano 2010, 4, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Daimon, H.; Lee, Y.; Kim, J.; Sun, S. Synthesis of Monodisperse Pt Nanocubes and Their Enhanced Catalysis for Oxygen Reduction. J. Am. Chem. Soc. 2007, 129, 6974–6975. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Sun, S.; Gaschler, W.; Doyle, H.; Betley, T.A.; Kagan, C.R. Colloidal synthesis of nanocrystals and nanocrystal superlattices. IBM J. Res. Dev. 2001, 45, 47–56. [Google Scholar] [CrossRef]
- Yang, H.T.; Su, Y.K.; Shen, C.M.; Yang, T.Z.; Gao, H.J. Synthesis and magnetic properties of ε-cobalt nanoparticles. Surf. Interface Anal. 2004, 36, 155–160. [Google Scholar] [CrossRef]
- Erley, W.; Hemminger, J.C. Spectroscopic identification of an HCNH species on Pt(111). Surf. Sci. 1994, 316, 1025–1030. [Google Scholar] [CrossRef]
- Lee, D.H.; Condrate, R.A.; Lacourse, W.C. FTIR spectral characterization of thin film coatings of oleic acid on glasses Part II Coatings on glass from different media such as water, alcohol, benzene and air. J. Mater. Sci. 2000, 35, 4961–4970. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Marković, N.M. Improved Oxygen Reduction Activity on Pt3Ni(111) via Increased Surface Site Availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serov, A.; Kwak, C. Review of non-platinum anode catalysts for DMFC and PEMFC application. Appl. Catal. B Environ. 2009, 90, 313–320. [Google Scholar] [CrossRef]
- Maillard, F.; Schreier, S.; Hanzlik, M.; Savinova, E.R.; Weinkauf, S.; Stimming, U. Influence of particle agglomeration on the catalytic activity of carbon-supported Pt nanoparticles in CO monolayer oxidation. Phys. Chem. Chem. Phys. 2005, 7, 385–393. [Google Scholar] [CrossRef]
- Solla-Gullon, J.; Vidal-Iglesias, F.J.; Herrero, E.; Feliu, J.M.; Aldaz, A. CO monolayer oxidation on semi-spherical and preferentially oriented (100) and (111) platinum nanoparticles. Electrochem. Commun. 2006, 8, 189–194. [Google Scholar] [CrossRef]
- Maillard, F.; Eikerling, M.; Cherstiouk, O.V.; Schreier, S.; Savinova, E.; Stimming, U. Size effects on reactivity of Pt nanoparticles in CO monolayer oxidation: The role of surface mobility. Faraday Discuss. 2004, 125, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, K.J.J.; Strmcnik, D.; Blizanac, B.B.; Stamenkovic, V.; Arenz, M.; Markovic, N.M. Measurement of oxygen reduction activities via the rotating disc electrode method: From Pt model surfaces to carbon-supported high surface area catalysts. Electrochim. Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Paulus, U.A.; Schmidt, T.J.; Gasteiger, H.A.; Behm, R.J. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: A thin-film rotating ring-disk electrode study. J. Electroanal. Chem. 2001, 495, 134–145. [Google Scholar] [CrossRef]
- Nilekar, A.U.; Alayoglu, S.; Eichhorn, B.; Mavrikakis, M. Preferential CO Oxidation in Hydrogen: Reactivity of Core−Shell Nanoparticles. J. Am. Chem. Soc. 2010, 132, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhou, Z.; Zhuang, J.; Wang, X. Pd-Pt random alloy nanocubes with tunable compositions and their enhanced electrocatalytic activities. Chem. Commun. 2010, 46, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kumar, D.; Yi, C.-W.; Goodman, D.W. The Promotional Effect of Gold in Catalysis by Palladium-Gold. Science 2005, 310, 291–293. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leteba, G.M.; Mitchell, D.R.G.; Levecque, P.B.J.; Lang, C.I. Solution-Grown Dendritic Pt-Based Ternary Nanostructures for Enhanced Oxygen Reduction Reaction Functionality. Nanomaterials 2018, 8, 462. https://doi.org/10.3390/nano8070462
Leteba GM, Mitchell DRG, Levecque PBJ, Lang CI. Solution-Grown Dendritic Pt-Based Ternary Nanostructures for Enhanced Oxygen Reduction Reaction Functionality. Nanomaterials. 2018; 8(7):462. https://doi.org/10.3390/nano8070462
Chicago/Turabian StyleLeteba, Gerard M., David R. G. Mitchell, Pieter B. J. Levecque, and Candace I. Lang. 2018. "Solution-Grown Dendritic Pt-Based Ternary Nanostructures for Enhanced Oxygen Reduction Reaction Functionality" Nanomaterials 8, no. 7: 462. https://doi.org/10.3390/nano8070462