Lanthanide-Doped Ceria Nanoparticles as Backside Coaters to Improve Silicon Solar Cell Efficiency
Abstract
:1. Introduction
2. Materials and Methods
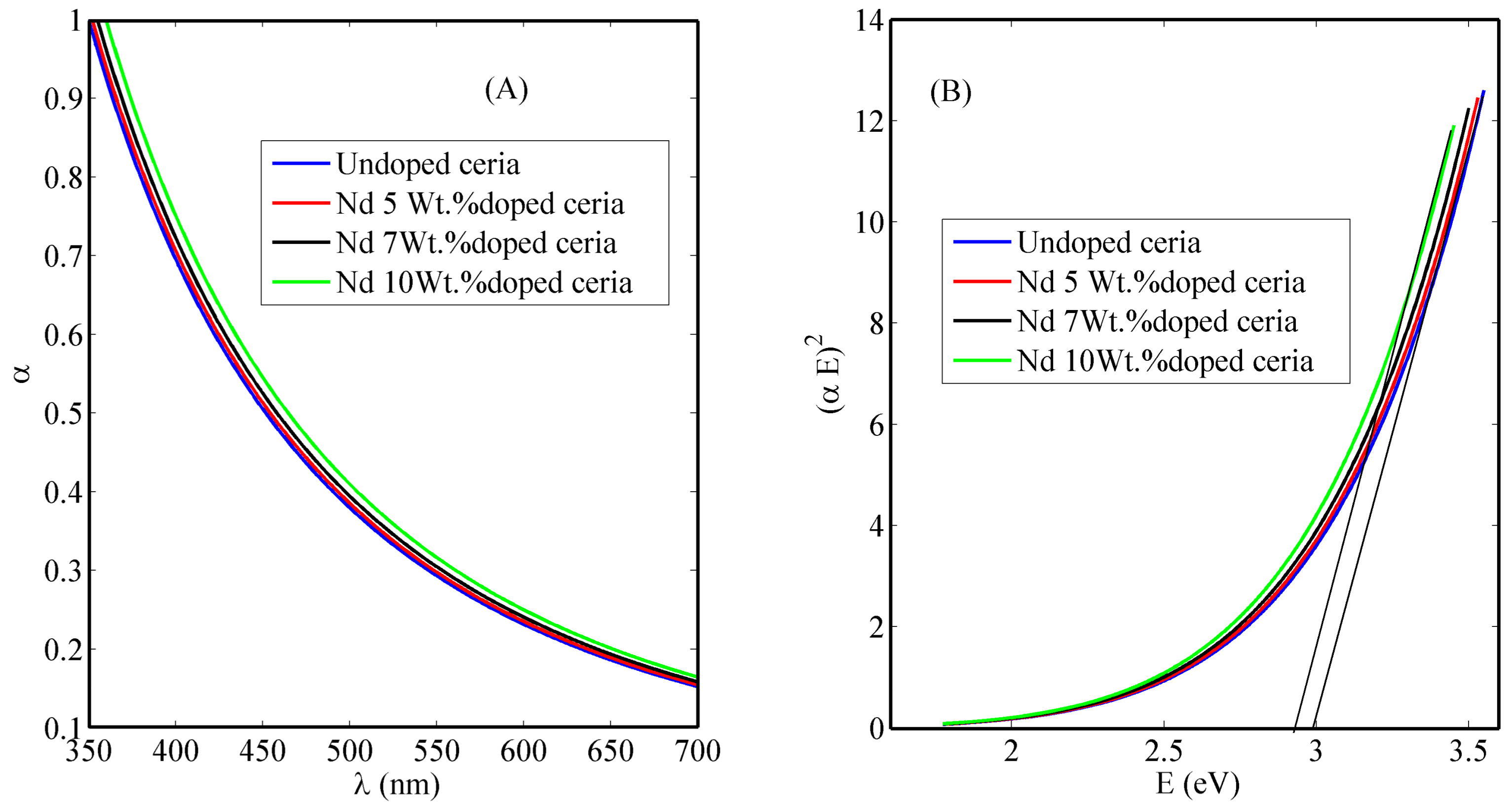
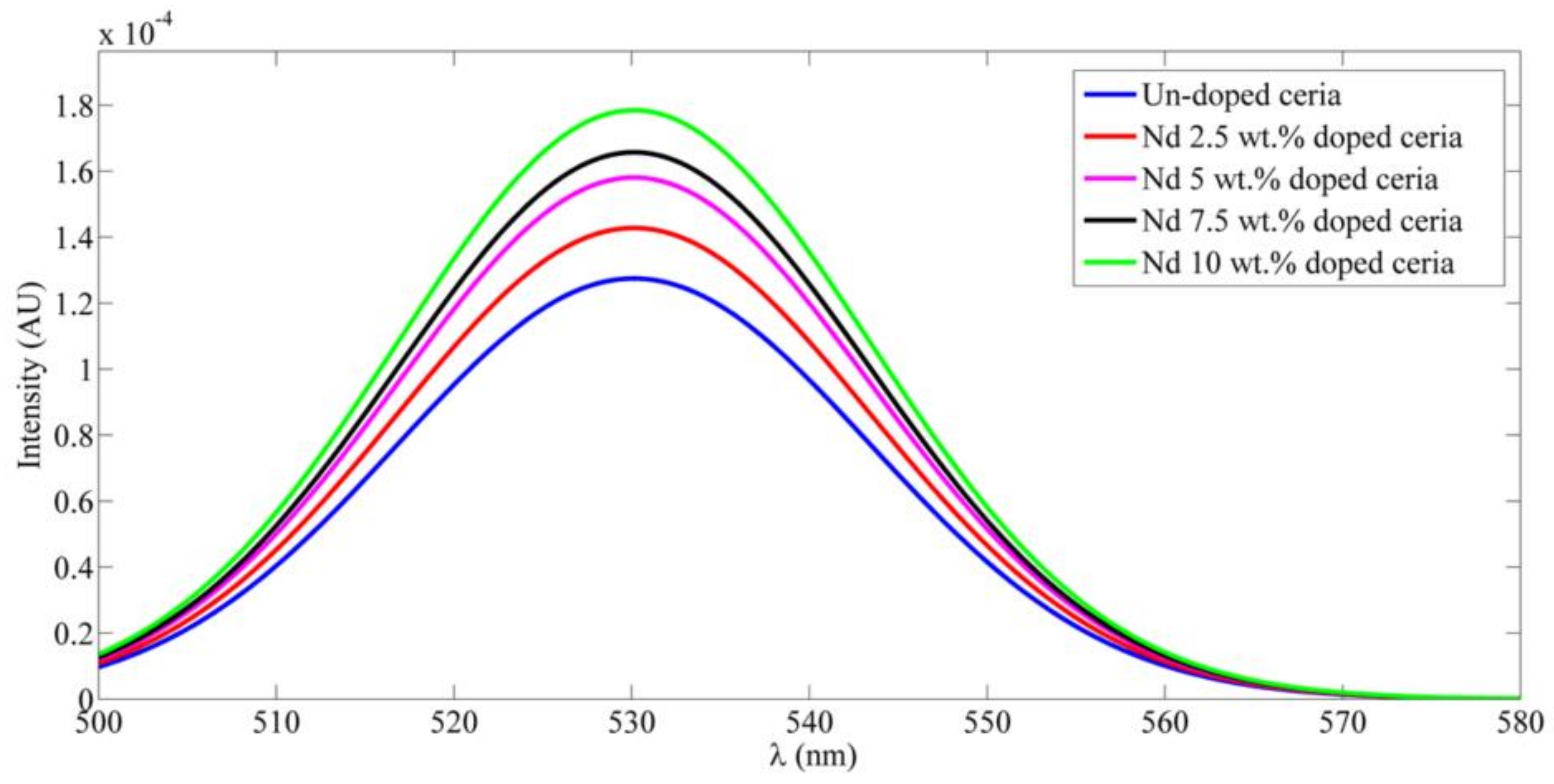
3. Results
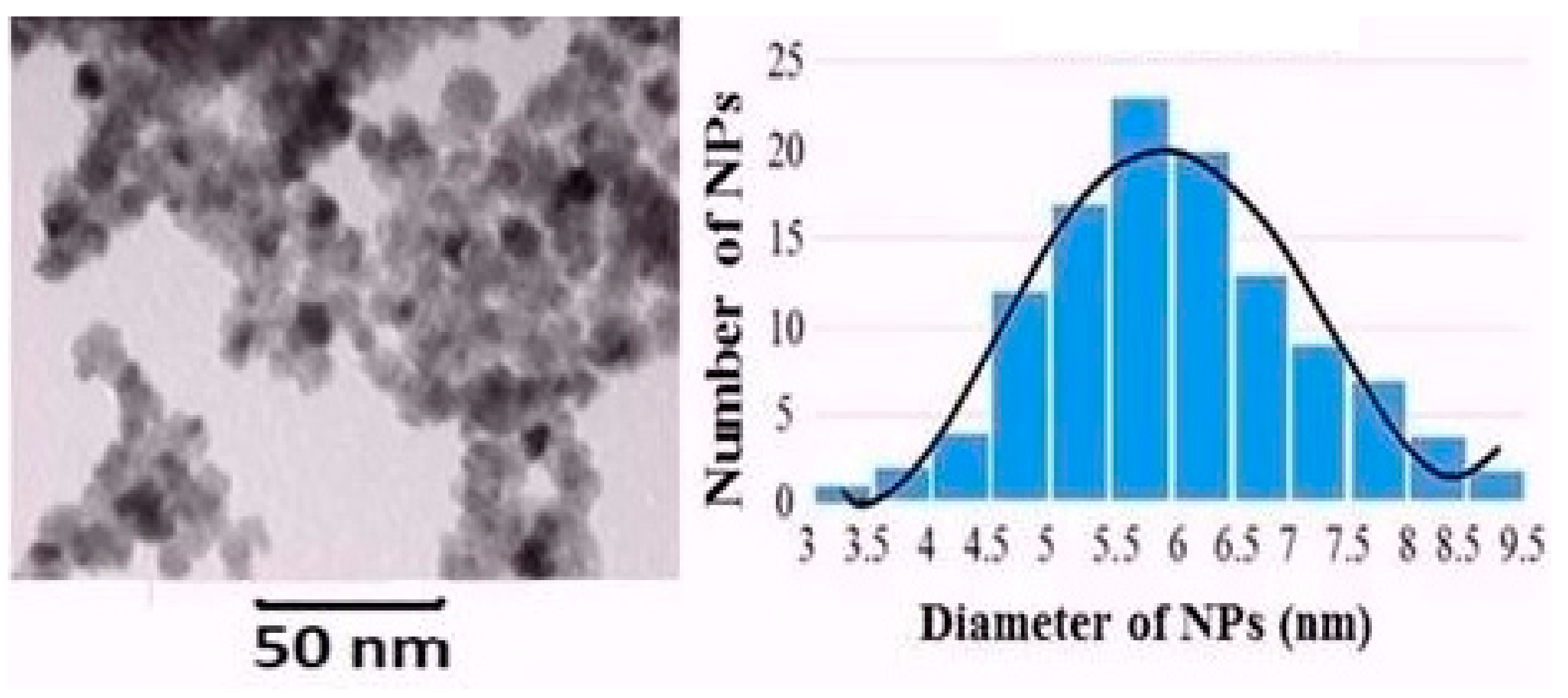
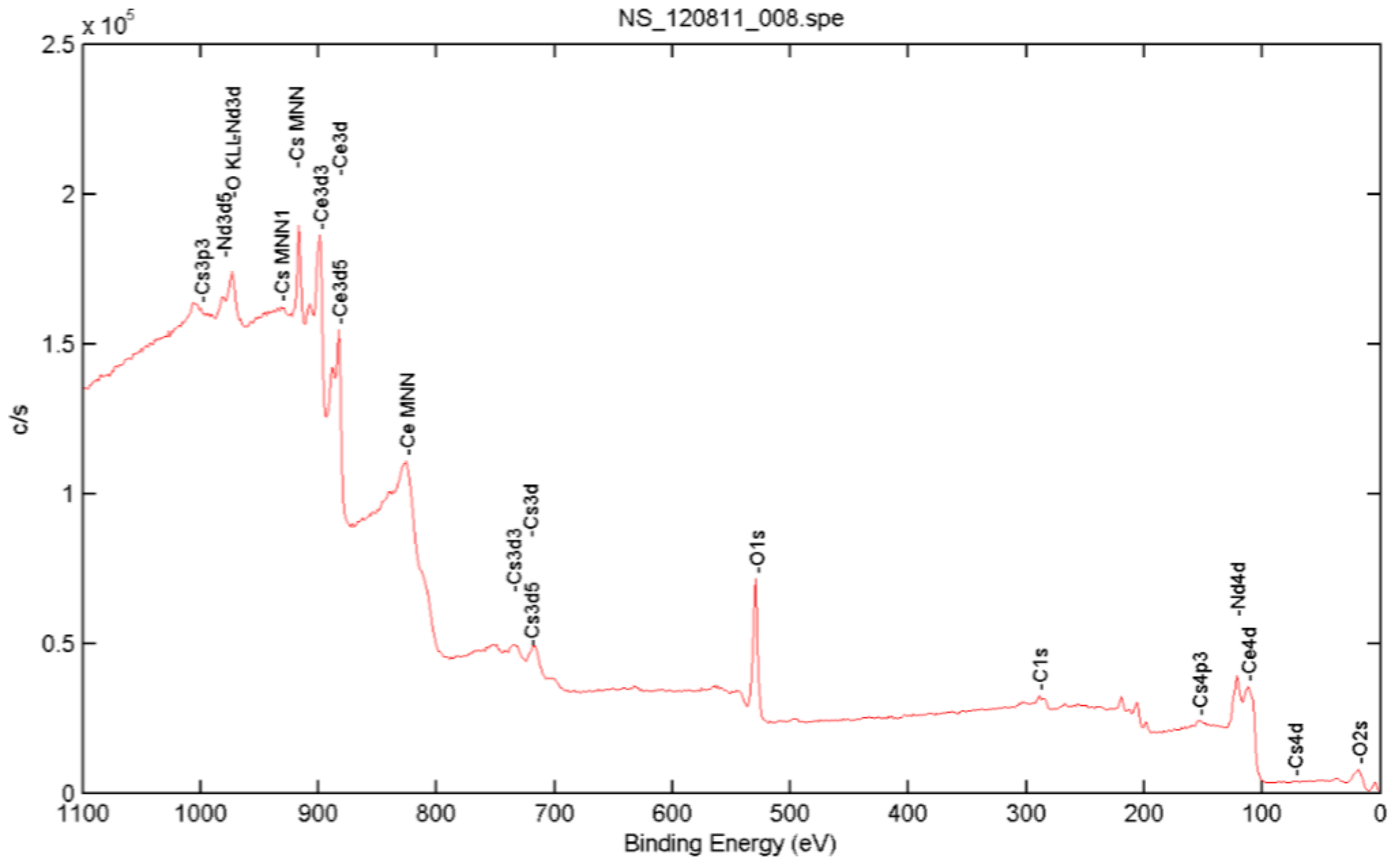
3.1. Synthesized Nanoparticle Characterization
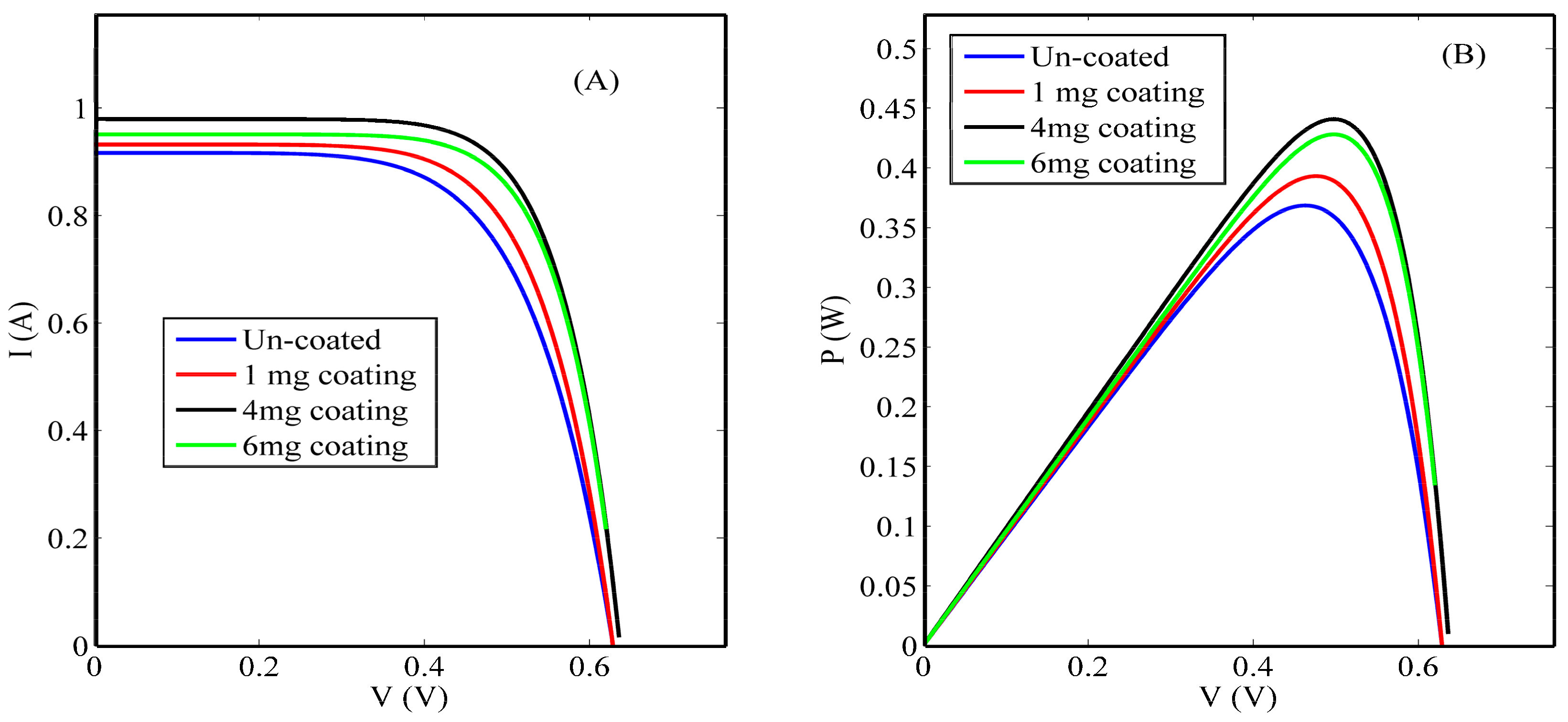
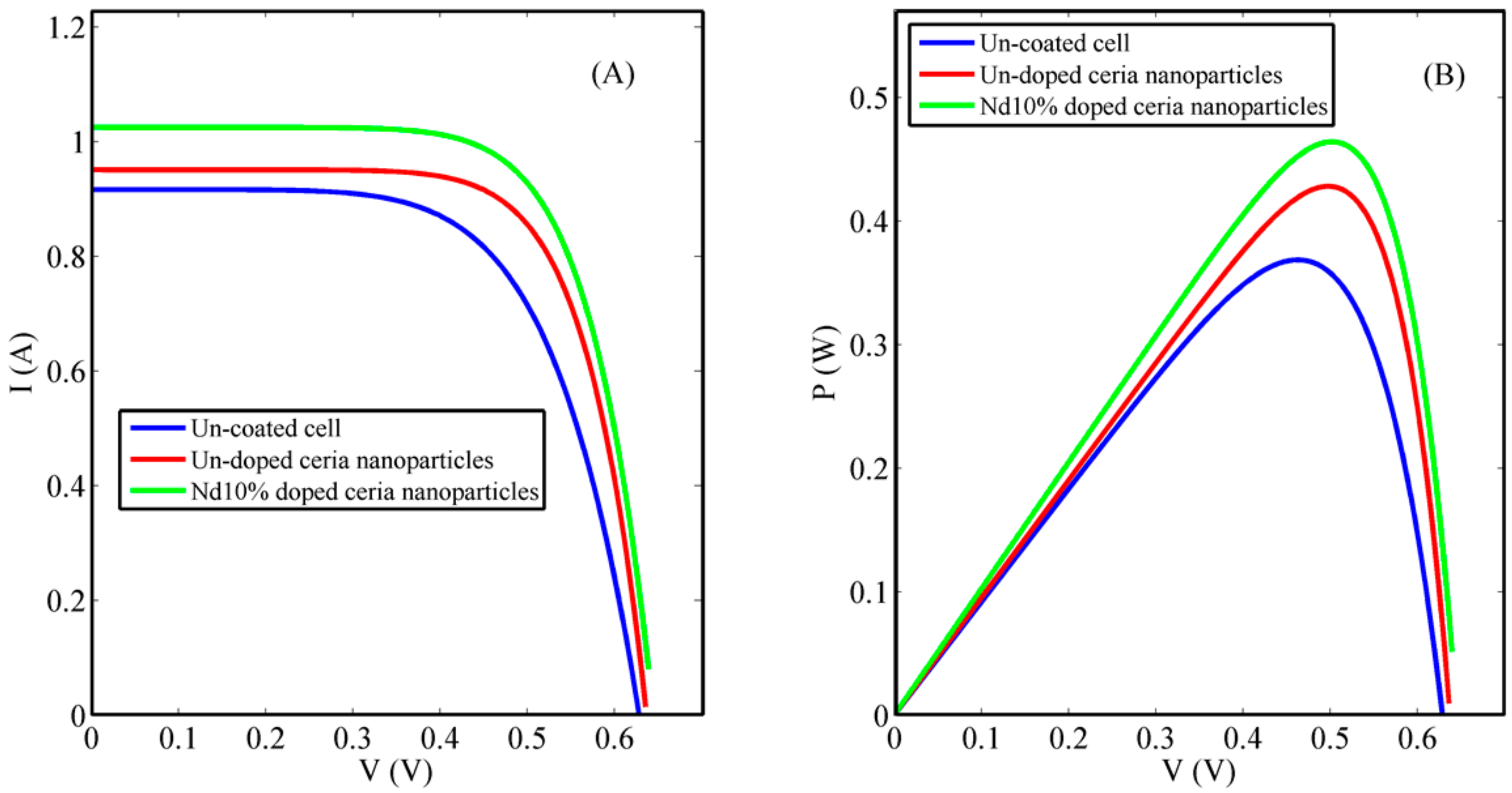
3.2. Solar Cell Characterization
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ivaturi, A.; Macdougall, S.K.; Martín-Rodríguez, R.; Quintanilla, M.; Marques-Hueso, J.; Krämer, K.W.; Richards, B.S. Optimizing infrared to near infrared upconversion quantum yield of β-NaYF4:Er3 in fluoropolymer matrix for photovoltaic devices. J. Appl. Phys. J. Appl. Phys. 2013, 114, 013505. [Google Scholar] [CrossRef]
- Rüdiger, M.; Fischer, S.; Frank, J.; Ivaturi, A.; Richards, B.S.; Krämer, K.W.; Goldschmidt, J.C. Bifacial n-type silicon solar cells for upconversion applications. Sol. Energy Mater. Sol. Cells 2014, 128, 57–68. [Google Scholar] [CrossRef]
- Fischer, S.; Favilla, E.; Tonelli, M.; Goldschmidt, J.C. Record efficient upconverter solar cell devices with optimized bifacial silicon solar cells and monocrystalline BaY2F8:30% Er3 upconverter. Sol. Energy Mater. Sol. Cells 2015, 136, 127–134. [Google Scholar] [CrossRef]
- Trupke, T.; Green, M.A.; Würfel, P. Improving solar cell efficiencies by up-conversion of sub-band-gap light. J. Appl. Phys. 2002, 92, 4117–4122. [Google Scholar] [CrossRef]
- Gibart, P.; Auzel, F.; Guillaume, J.; Zahraman, K. Below Band-Gap IR Response of Substrate-Free GaAs Solar Cells Using Two-Photon Up-Conversion. Jpn. J. Appl. Phys. 1996, 35, 4401–4402. [Google Scholar] [CrossRef]
- Strümpel, C.; Mccann, M.; Beaucarne, G.; Arkhipov, V.; Slaoui, A.; Švrček, V.; Tobias, I. Modifying the solar spectrum to enhance silicon solar cell efficiency—An overview of available materials. Sol. Energy Mater. Sol. Cells 2007, 91, 238–249. [Google Scholar] [CrossRef]
- Dyk, E.V.; Meyer, E. Analysis of the effect of parasitic resistances on the performance of photovoltaic modules. Renew. Energy 2004, 29, 333–344. [Google Scholar]
- Fischer, S.; Steinkemper, H.; Löper, P.; Hermle, M.; Goldschmidt, J.C. Modeling upconversion of erbium doped microcrystals based on experimentally determined Einstein coefficients. J. Appl. Phys. 2012, 111, 013109. [Google Scholar] [CrossRef]
- Shehata, N.; Clavel, M.; Meehan, K.; Samir, E.; Gaballah, S.; Salah, M. Enhanced erbium-doped ceria nanostructure coating to improve solar cell performance. Materials 2015, 8, 7663–7672. [Google Scholar] [CrossRef] [PubMed]
- Shehata, N.; Meehan, K.; Hudait, M.; Jain, N.; Shehata, N.; Meehan, K.; Jain, N. Control of oxygen vacancies and Ce+3 concentrations in doped ceria nanoparticles via the selection of lanthanide element. J. Nanopart. Res. 2012, 14, 1173–1183. [Google Scholar] [CrossRef]
- Chen, H.-I.; Chang, H.-Y. Homogeneous precipitation of cerium dioxide nanoparticles in alcohol/water mixed solvents. Colloids Surf. A Physicochem. Eng. Asp. 2004, 242, 61–69. [Google Scholar] [CrossRef]
- Shehata, N.; Samir, E.; Gaballah, S. New optical sensor for peroxides using neodymium-doped-ceria nanoparticles via fluorescence-quenching technique. Sens. Actuators B Chem. 2016, 231, 341–348. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Norrman, K.; Ghanbari-Siahkali, A.; Larsen, N.B. 6 Studies of spin-coated polymer films. Annu. Rep. Sect. C (Phys. Chem.) 2005, 101, 174–201. [Google Scholar] [CrossRef]
- Pankove, J. Optical Processes in Semiconductors, 1st ed.; Dover Publications Inc.: New York, NY, USA, 1971. [Google Scholar]
- Yin, L.; Wang, Y.; Pang, G.; Koltypin, Y.; Gedanken, A. Sonochemical synthesis of cerium oxide nanoparticles—Effect of additives and quantum size effect. J. Colloid Interface Sci. 2002, 246, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Shmyreva, A.N.; Borisov, A.V.; Maksimchuk, N.V. Electronic sensors built on nanostructured cerium oxide films. Nanotechnol. Russ. 2010, 5, 382–389. [Google Scholar] [CrossRef]
- Ivanov, V.K.; Sharikov, Y.F.; Polezhaeva, O.S.; Tret’yakov, Y.D.; Sharikov, F.Y. Formation of nanocrystalline ceria from cerium(III) nitrate solutions in aqueous alcohol. Dokl. Chem. 2006, 411, 223–225. [Google Scholar] [CrossRef]
- Shehata, N.; Samir, E.; Gaballah, S. Optical sensing of peroxide using ceria nanoparticles via fluorescence quenching technique. In Proceedings of the SPIE Photonics Europe, Brussels, Belgium, 3–7 April 2016. [Google Scholar]
- Shehata, N.; Samir, E.; Gaballah, S.; Salah, M. Optical sensing of peroxide using ceria nanoparticles via fluorescence quenching technique. J. Nanophotonics 2016, 3, 036002. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 1st ed.; Addison-Wesley Publishing Company Inc.: Boston, MA, USA, 1956. [Google Scholar]
- Gerling, L.; Mahato, S.; Voz, C.; Alcubilla, R.; Puigdollers, J. Characterization of Transition Metal Oxide/Silicon Heterojunctions for Solar Cell Applications. Appl. Sci. 2015, 5, 695–705. [Google Scholar] [CrossRef]
- Chava, R.K.; Kang, M. Improving the photovoltaic conversion efficiency of ZnO based dye sensitized solar cells by indium doping. J. Alloys Compd. 2016, 692, 67–76. [Google Scholar] [CrossRef]
- Favier, A.; Muñoz, D.; Nicolás, S.M.; Ribeyron, P.J. Boron-doped zinc oxide layers grown by metal-organic CVD for silicon heterojunction solar cells applications. Sol. Energy Mater. Sol. Cells 2011, 95, 1057–1061. [Google Scholar] [CrossRef]


| Condition | Conductivity (µS/cm) |
|---|---|
| Un-doped ceria nanoparticles | 232 |
| Nd 5 wt. % doped ceria nanoparticles | 260.7 |
| Nd 10 wt. % doped ceria nanoparticles | 270.9 |
| Condition | Concentration (mg/mL) | VOC (V) | ISC (A) | Efficiency (η%) |
|---|---|---|---|---|
| Un-coated solar cell | 0 | 0.6320 | 0.9165 | 14.74 |
| Ceria nanoparticle-coated solar cell | 1 | 0.6313 | 0.9321 | 15.72 |
| 4 | 0.6359 | 0.9195 | 17.64 | |
| 6 | 0.6199 | 0.9510 | 17.12 |
| Condition | VOC (V) | ISC (A) | Efficiency (η%) |
|---|---|---|---|
| Un-coated solar cell | 0.6320 | 0.9165 | 14.74 |
| Ceria nanoparticle coated cells | 0.6359 | 0.9195 | 17.64 |
| Nd 10% ceria nanoparticle coated cells | 0.6393 | 1.0249 | 18.56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajjiah, A.; Samir, E.; Shehata, N.; Salah, M. Lanthanide-Doped Ceria Nanoparticles as Backside Coaters to Improve Silicon Solar Cell Efficiency. Nanomaterials 2018, 8, 357. https://doi.org/10.3390/nano8060357
Hajjiah A, Samir E, Shehata N, Salah M. Lanthanide-Doped Ceria Nanoparticles as Backside Coaters to Improve Silicon Solar Cell Efficiency. Nanomaterials. 2018; 8(6):357. https://doi.org/10.3390/nano8060357
Chicago/Turabian StyleHajjiah, Ali, Effat Samir, Nader Shehata, and Mohamed Salah. 2018. "Lanthanide-Doped Ceria Nanoparticles as Backside Coaters to Improve Silicon Solar Cell Efficiency" Nanomaterials 8, no. 6: 357. https://doi.org/10.3390/nano8060357






