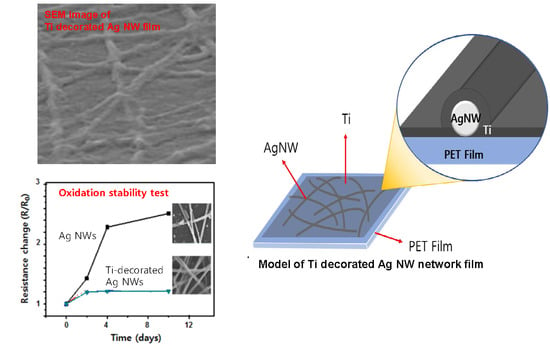
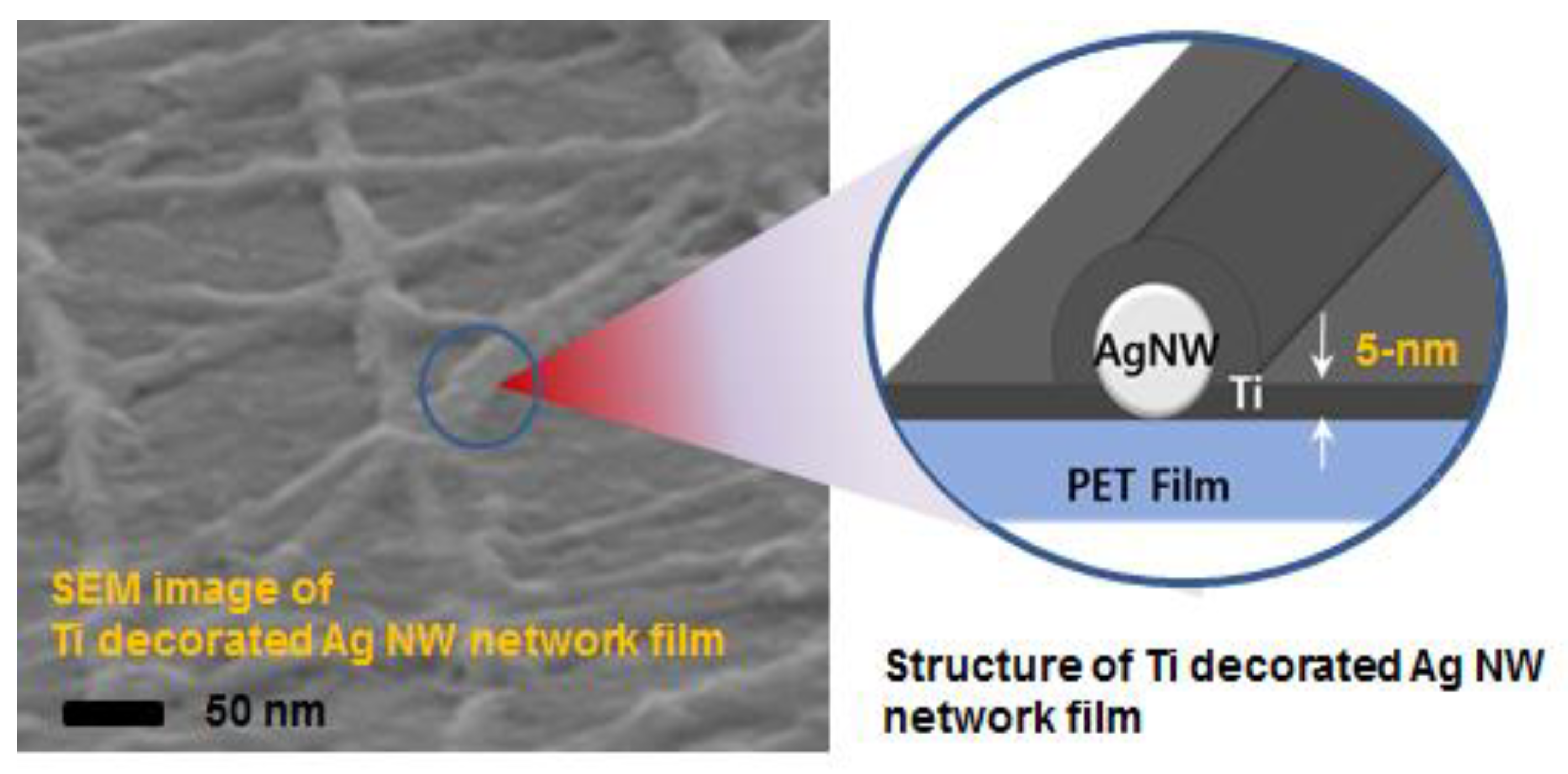
Ambient-Stable and Durable Conductive Ag-Nanowire-Network 2-D Films Decorated with a Ti Layer
Abstract
:1. Introduction
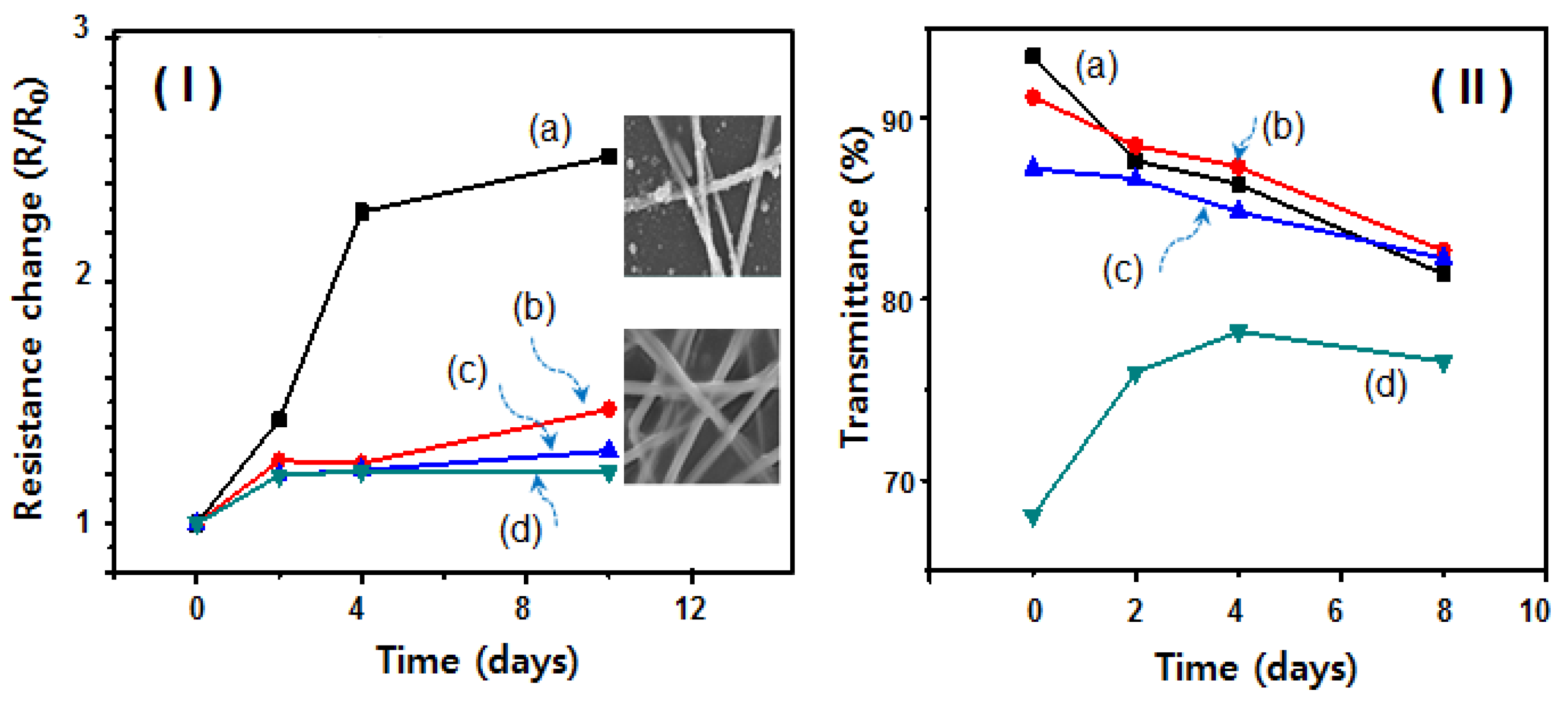
2. Results and Discussion
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Y.; Xiang, J.; Yang, C.; Lu, W.; Lieber, C.M. Single-crystal metallic nanowires andmetal/semiconductor nanowire heterostructures. Nature 2004, 430, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Gates, B.; Mayers, B.; Xia, Y. Crystalline Silver Nanowires by Soft Solution Processing. Nano Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Sun, Y.J.; Yin, Y.D.; Mayers, B.; Herricks, T.; Xia, Y. Uniform Silver Nanowires Synthesis by Reducing AgNO3 with Ethylene Glycol in the Presence of Seeds and Poly(Vinyl Pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- De Silva, R.R.; Yang, M.; Choi, S.I.; Chi, M.; Luo, M.; Zhang, C.; Li, Z.Y.; Camargo, P.H.C.; Ribeiro, S.J.L.; Xia, Y. Facile Synthesis of Sub-20 nm Silver Nanowires through a Bromide-Mediated Polyol Method. ACS Nano 2016, 10, 7892–7900. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.; Sun, Y.; Chen, J.; Chang, H.; Li, Z.; Li, X.; Xia, Y. Shape-Controlled Synthesis of Silver and Gold Nanostructures. MRS Bull. 2005, 30, 356–361. [Google Scholar] [CrossRef]
- Lee, E.J.; Chang, M.H.; Kim, Y.S.; Kim, J.Y. High-pressure polyol synthesis of ultrathin silver nanowires: Electrical and optical properties. APL Mater. 2013, 1, 042118. [Google Scholar] [CrossRef]
- Li, B.; Ye, S.; Stewart, I.E.; Alvarez, S.; Wily, B.J. Synthesis and Purification of Silver Nanowires to Make Conducting Films with a Transmittance of 99%. Nano Lett. 2015, 15, 6722–6726. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, Y.H.; Hwang, D.K.; Choi, W.K.; Kim, J.Y. Synthesis and optoelectronic characteristics of 20 nm diameter silver nanowires for highly transparent electrode films. RSC Adv. 2016, 6, 11702–11710. [Google Scholar] [CrossRef]
- Ahn, Y.; Jeong, Y.; Lee, Y. Improved Thermal Oxidation Stability of Solution-Processable Silver Nanowire Transparent Electrode by Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2012, 4, 6410–6414. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.; Xu, Y.; Han, X.; Munday, J.N.; Hu, L. Optical haze of transparent and conductive silver nanowire films. Nano Res. 2013, 6, 461–468. [Google Scholar] [CrossRef]
- Hsu, P.C.; Wu, H.; Carney, T.J.; McDowell, M.T.; Yang, Y.; Garnett, E.C.; Li, M.; Hu, L.; Cui, Y. Passivation Coating on Electrospun Copper Nanofibers for Stable Transparent Electrodes. ACS Nano 2012, 6, 5150–5156. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, P.; Seo, D.M.; Kim, S.H.; Kim, J.J. Effects of TiO2 shells on optical and thermal properties of silver nanowires. Mater. Chem. 2012, 22, 11651. [Google Scholar] [CrossRef]
- Zilberberg, K.; Gasse, F.; Pagui, R.; Polywka, A.; Behrendt, A.; Trost, S.; Heiderhoff, R.; Gӧrrn, P.; Riedl, T. Highly Robust Indium-Free Transparent Conductive Electrodes Based on Composites of Silver Nanowires and Conductive Metal Oxides. Adv. Funct. Mater. 2014, 24, 1671–1678. [Google Scholar] [CrossRef]
- Morgenstern, F.S.F.; Kabra, D.; Massip, S.; Brenner, T.J.K.; Lyons, P.E.; Coleman, J.N.; Friend, R.H. Ag-nanowire films coated with ZnO nanoparticles as a transparent electrode for solar cells. Appl. Phys. Lett. 2011, 99, 183307. [Google Scholar] [CrossRef]
- Song, T.B.; Rim, Y.S.; Liu, F.; Bob, B.; Ye, S.; Hsieh, Y.T.; Yang, Y. Highly Robust Silver Nanowire Network for Transparent Electrode. ACS Appl. Mater. Interfaces 2015, 7, 24601–24607. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Song, L.; Tao, Z.; Shao, X.; Hung, Y.; Cui, Q.; Guo, X. Neutral-pH PEDOT: PSS as over-coating layer for stable silver nanowire flexible transparent conductive films. Org. Electron. 2014, 15, 3654–3659. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, B.; He, Z.; Zhao, X.; Wang, H.; Yang, H.; Wu, Y.; Cao, Y. High-efficiency ITO-free polymer solar cells using highly conductive PEDOT:PSS surfactant bilayer transparent anodes. Energy Environ. Sci. 2013, 6, 1956–1964. [Google Scholar]
- Peng, L.; Peng, X.; Liu, B.; Wu, C.Z.; Xie, Y.; Yu, G. Ultrathin two-dimensional MnO2/graphene hybrid nanostructures for high-performance, flexible planar supercapacitors. Nano Lett. 2013, 13, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Koumoto, K.; Wang, Y.; Zhang, R.; Kosuga, A.; Funahashi, R. Oxide Thermoelectric Materials: A Nanostructuring Approach. Annu. Rev. Mater. Res. 2010, 40, 363–394. [Google Scholar] [CrossRef]
- Ohta, H.; Kim, S.W.; Mune, Y.; Mizoguchi, T.; Nomura, K.; Ohta, S.; Nomura, T.; Nakanishi, Y.; Ikuhara, Y.; Hirano, M.; et al. Giant thermoelectric Seebeck coefficient of atwo-dimensional electron gas in SrTiO3. Nat. Mater. 2007, 6, 129–134. [Google Scholar]
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Shoesmith, D.W.; Ikeda, B.M.; LeNeveu, D.M. Modeling the Failure of Nuclear Waste Containers. Corrosion (Houston) 1997, 53, 820–829. [Google Scholar] [CrossRef]
- Fujitsuka, N.; Sakata, J.; Miyachi, Y.; Mizuno, K.; Ohtsuka, K.; Taga, Y.; Tabata, O. Monolithic pyroelectric infrared image sensor using PVDF thin film. Sens. Actuators A Phys. 1998, 66, 237–243. [Google Scholar] [CrossRef]
- Ramakrishna, M.V.S.; Karunasiri, G.; Neuzil, P.; Sridhar, U.; Zeng, W.J. Highly sensitive infrared temperature sensor using self-heating compensated microbolometers. Sens. Actuators A Phys. 2000, 79, 122–127. [Google Scholar] [CrossRef]
- Yu, B.; Miyamoto, Y.; Sugino, O.; Sasaki, T.; Ohno, T. Favorable formation of the C49-TiSi2 phase on Si(001) determined by first-principles calculations. Appl. Phys. Lett. 1998, 72, 1176. [Google Scholar] [CrossRef]
- Jo, H.A.; Jang, H.W.; Hwang, B.Y.; Kim, Y.H.; Kim, J.Y. Synthesis of small diameter silver nanowires via a magnetic-ionic-liquid-assisted polyol process. RSC Adv. 2016, 6, 104273–104279. [Google Scholar] [CrossRef]
- Sun, Y.; Herricks, T.; Mayers, B.; Xia, Y. Polyol Synthesis of Uniform Silver Nanowires: A Plausible Growth Mechanism and the Supporting Evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Karim, S.; Toimil-Molares, M.E.; Balogh, A.G.; Ensinger, W.; Cornelius, T.W.; Klan, E.U.; Neumann, R. Morphological evolution of Au nanowires controlled by Rayleigh instability. Nanotechnology 2006, 17, 5954–5959. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-M.; Hwang, B.-Y.; Lee, K.-W.; Kim, J.-Y. Ambient-Stable and Durable Conductive Ag-Nanowire-Network 2-D Films Decorated with a Ti Layer. Nanomaterials 2018, 8, 321. https://doi.org/10.3390/nano8050321
Kim Y-M, Hwang B-Y, Lee K-W, Kim J-Y. Ambient-Stable and Durable Conductive Ag-Nanowire-Network 2-D Films Decorated with a Ti Layer. Nanomaterials. 2018; 8(5):321. https://doi.org/10.3390/nano8050321
Chicago/Turabian StyleKim, Yoon-Mi, Bu-Yeon Hwang, Ki-Wook Lee, and Jin-Yeol Kim. 2018. "Ambient-Stable and Durable Conductive Ag-Nanowire-Network 2-D Films Decorated with a Ti Layer" Nanomaterials 8, no. 5: 321. https://doi.org/10.3390/nano8050321