Novel Sulfur/Ethylenediamine-Functionalized Reduced Graphene Oxide Composite as Cathode Material for High-performance Lithium-Sulfur Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Material Preparation
2.2. Characterization
2.3. Electrochemical Measurements
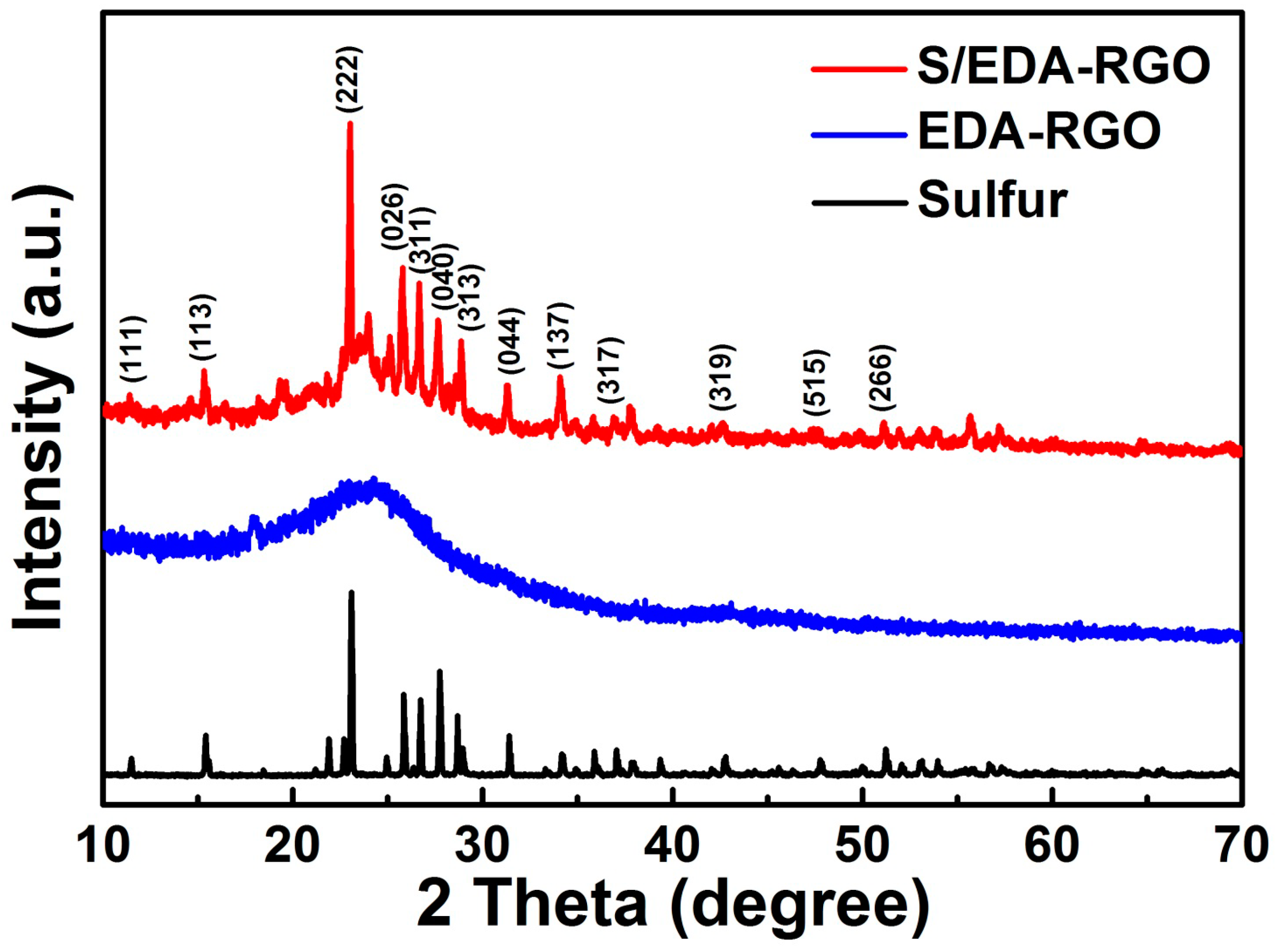
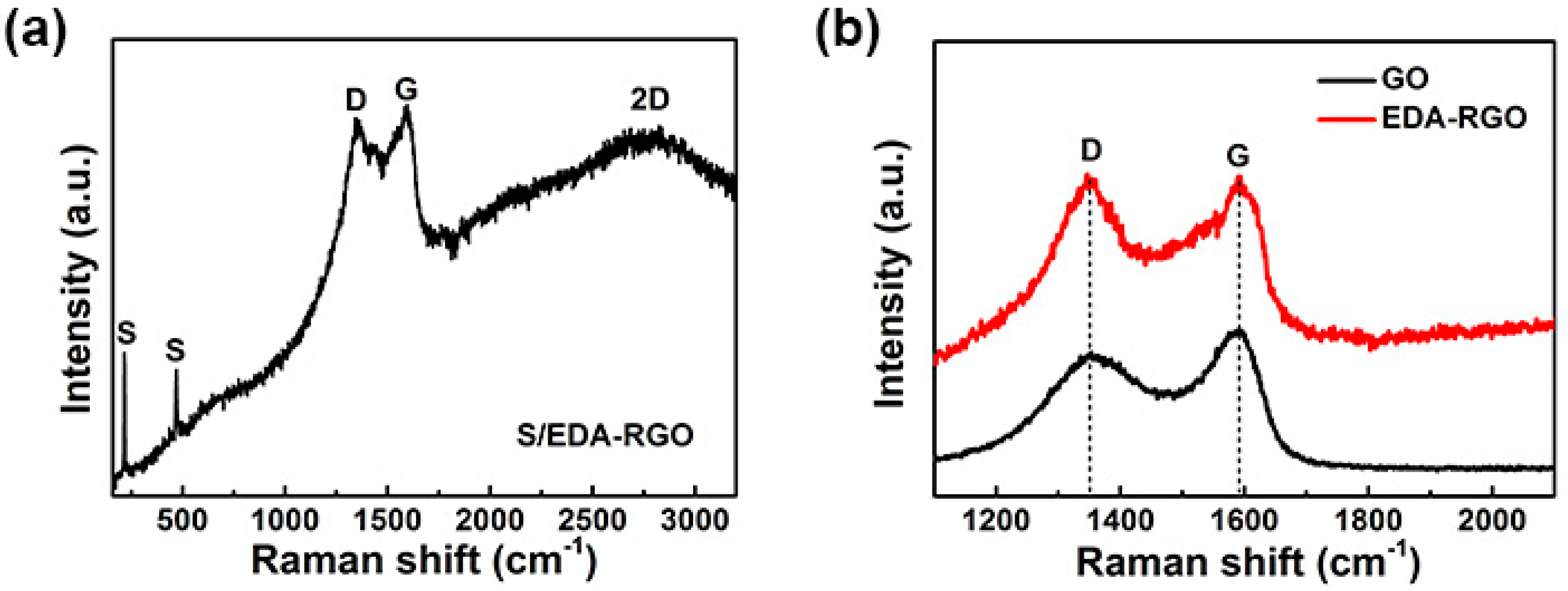
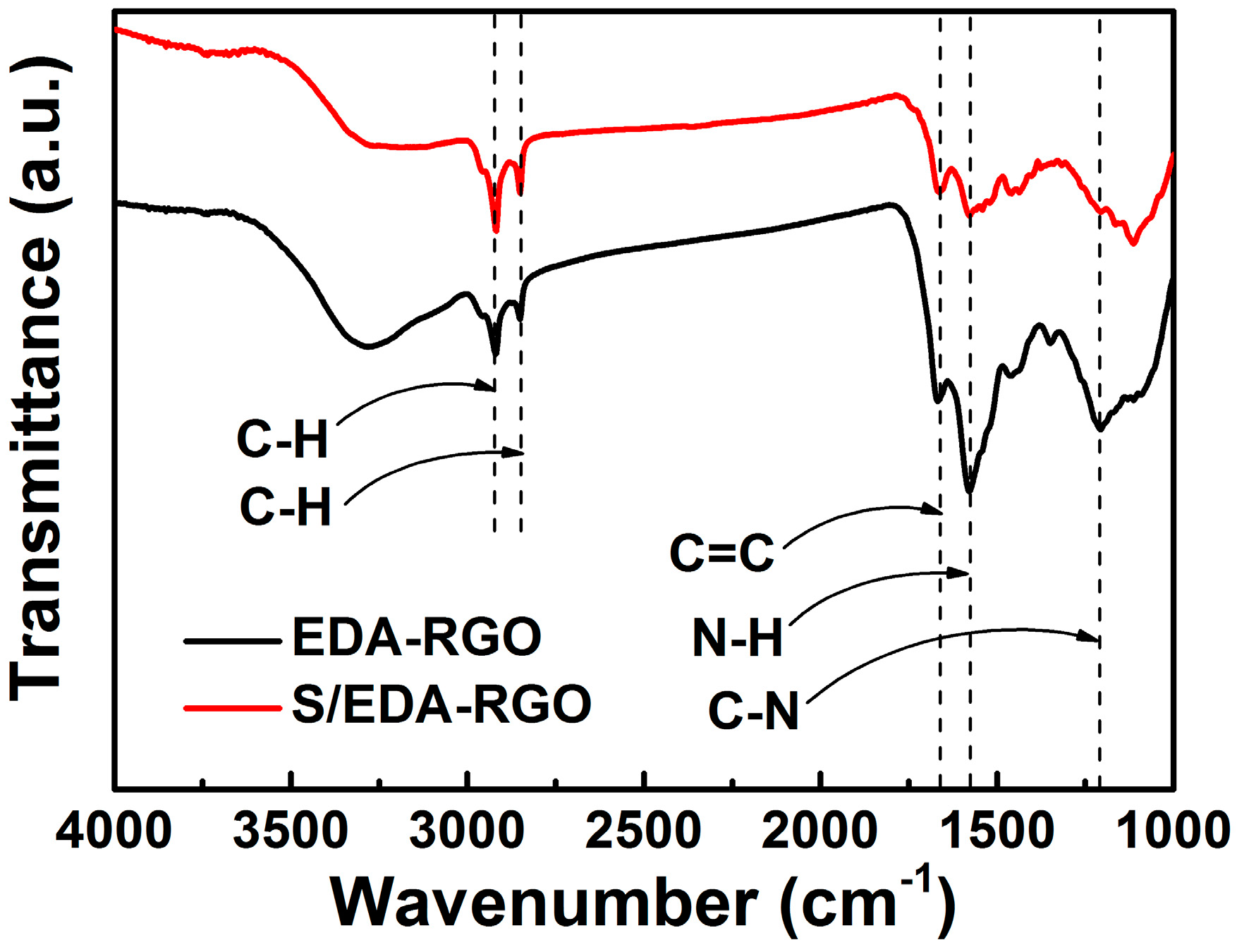
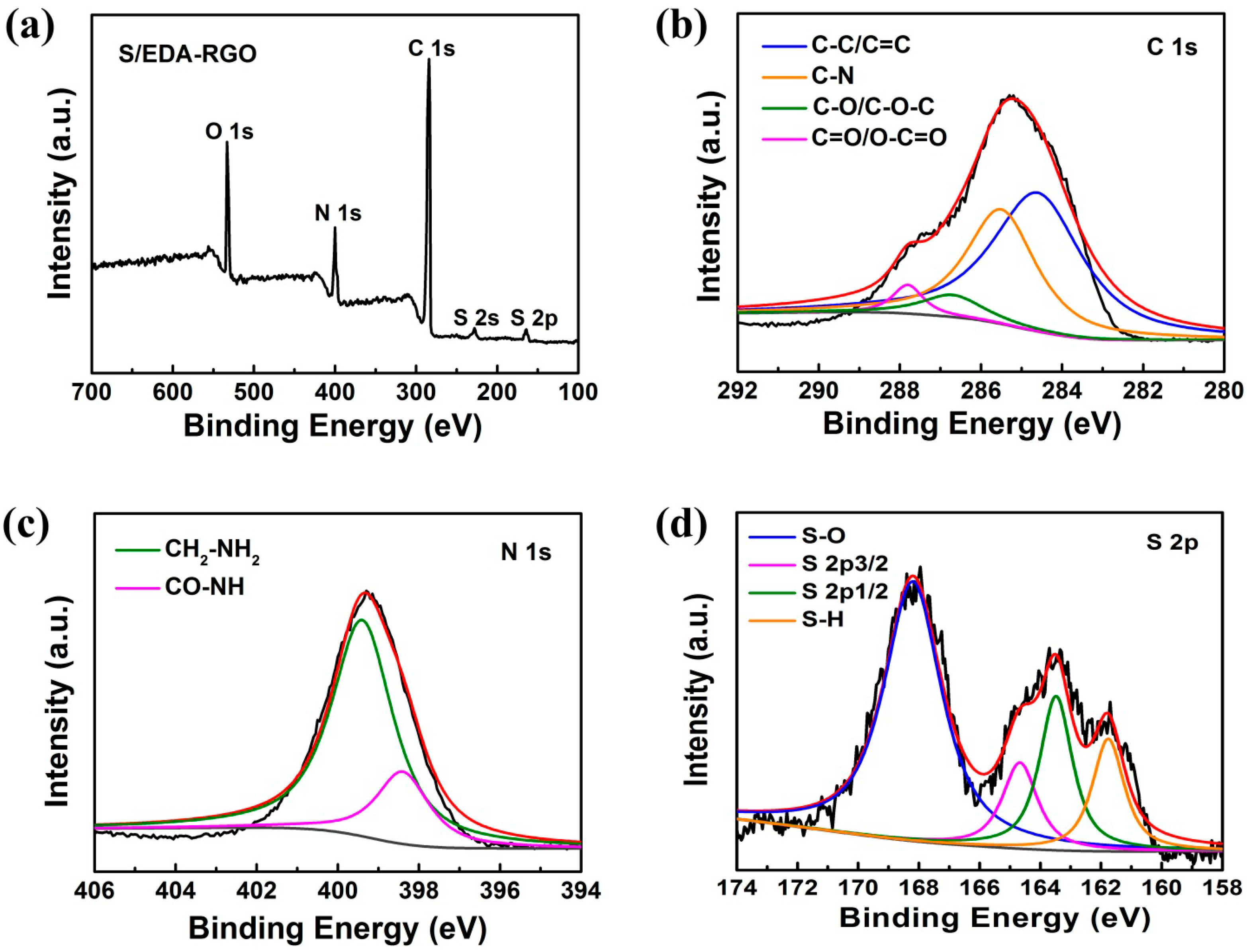
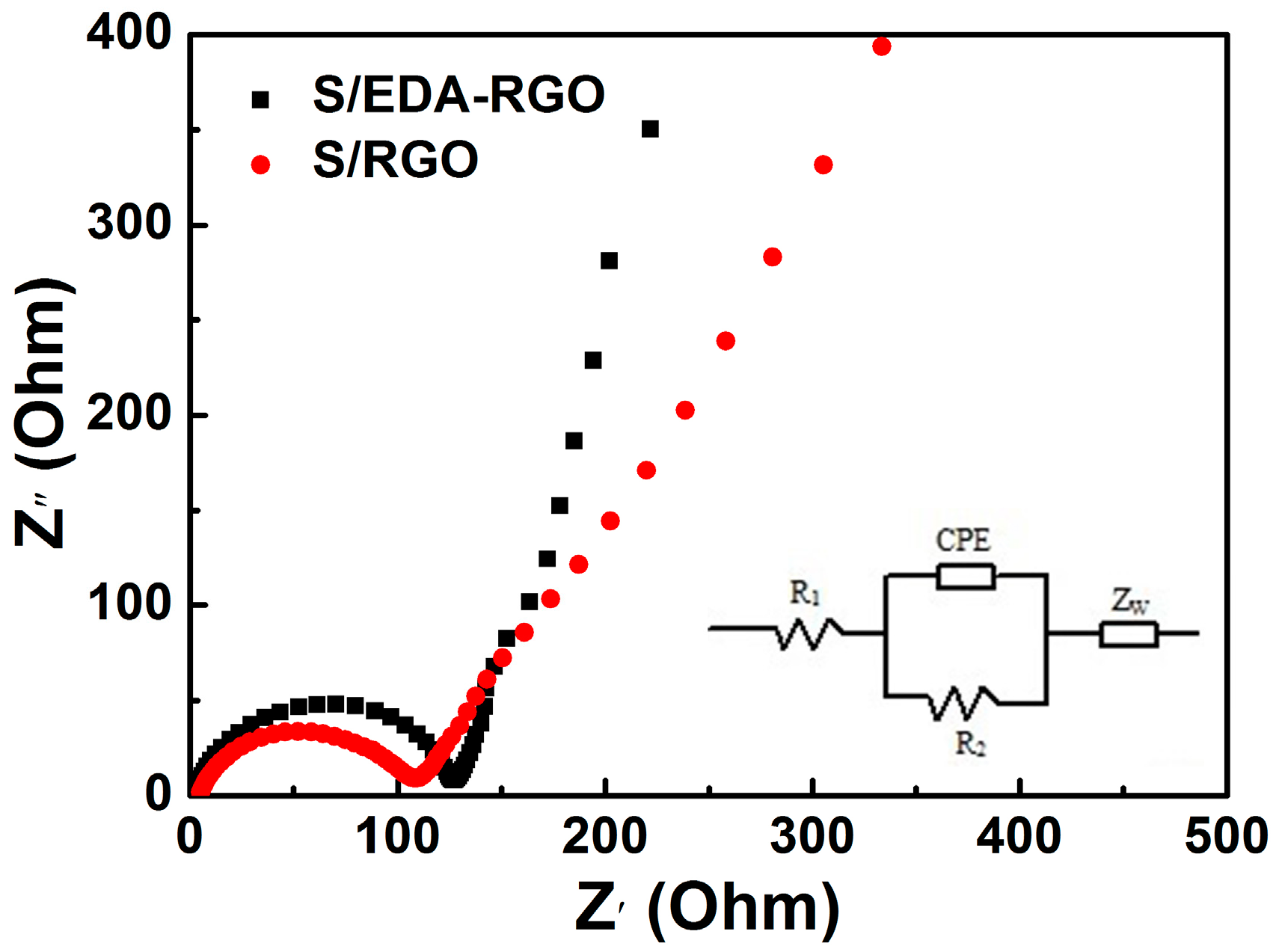
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosenman, A.; Markevich, E.; Salitra, G.; Aurbach, D.; Garsuch, A.; Chesneau, F.F. Review on Li-Sulfur battery systems: An integral perspective. Adv. Energy Mater. 2015, 5, 1500212. [Google Scholar] [CrossRef]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium-sulfur batteries: Electrochemistry materials, and prospects. Angew Chem. Int. Ed. Engl. 2013, 52, 13186–13200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Lai, Y.Q.; Zhang, Z.; Zhang, K.; Li, J. Al2O3-coated porous separator for enhanced electrochemical performance of lithium sulfur batteries. Electrochim. Acta 2014, 129, 55–61. [Google Scholar] [CrossRef]
- Hassoun, J.; Kim, J.; Lee, D.J.; Jung, H.G.; Lee, S.M.; Sun, Y.K.; Scrosat, B. A contribution to the progress of high energy batteries: A metal-free, lithium-ion, silicon-sulfur battery. J. Power Sources 2012, 202, 308–313. [Google Scholar] [CrossRef]
- Yim, T.; Park, M.S.; Yu, J.S.; Kim, K.J.; Im, K.Y.; Kim, J.H.; Jeong, G.; Jo, Y.N.; Woo, S.G.; Kang, K.S.; et al. Effect of chemical reactivity of polysulfide toward carbonate-based electrolyte on the electrochemical performance of Li-S batteries. Electrochim. Acta 2013, 107, 454–460. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, J.Z.; Chen, H.H.; Vijayakumar, M.; Zheng, J.M.; Pa, H.J.; Walter, E.D.; Hu, M.Y.; Deng, X.H.; Feng, J.; et al. Following the transient reactions in lithium-sulfur batteries using an in situ nuclear magnetic resonance technique. Nano Lett. 2015, 15, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Tran, D.T. New insight into liquid electrolyte of rechargeable lithium/sulfur battery. Electrochim. Acta 2013, 114, 296–302. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Li, H.; Tan, T.; Li, J. Porous three-dimensional reduced graphene oxide for high performance lithium-sulfur batteries. J. Alloy. Compd. 2018, 739, 290–297. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. Graphene-enveloped sulfur in a one pot reaction: A cathode with good coulombic efficiency and high practical sulfur content. Chem. Commun. 2012, 48, 1233–1235. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Sun, Z.; Zhang, Y.; Wang, X.; Bakenov, Z.; Yin, F. Micro-spherical sulfur/graphene oxide composite via spray drying for high performance lithium sulfur batteries. Nanomaterials 2018, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Jung, Y.H.; Jung, W.K.; Jung, D.S.; Choi, J.W.; Kim, D.K. Encapsulated monoclinic sulfur for stable cycling of Li-S rechargeable batteries. Adv. Mater. 2013, 25, 6547–6553. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.C.; Xu, Y.H.; Wang, C.S. Sulfur-impregnated disordered carbon nanotubes cathode for lithium-sulfur batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Rao, M.; Aloni, S.; Wang, L.; Cairns, E.J.; Zhang, Y. Porous carbon nanofiber-sulfur composite electrodes for lithium/sulfur cells. Energy Environ. Sci. 2011, 4, 5053. [Google Scholar] [CrossRef]
- Zheng, G.Y.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow carbon nanofiber-encapsulated sulfur cathode for high specific capacity rechargeable lithium batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous hollow carbon@sulfur composites for high-power lithium/sulfur batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode lithium/sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Zhai, Y.P.; Xu, G.L.; Jiang, Y.X.; Zhao, D.Y.; Li, J.T.; Huang, L.; Sun, S.G. Ordered mesoporous carbon/sulfur nanocomposite of high performances as cathode for lithium-sulfur battery. Electrochim. Acta 2011, 56, 9549–9555. [Google Scholar] [CrossRef]
- Li, H.; Wei, Y.; Zhang, Y.; Zhang, C.; Wang, G.; Zhao, Y.; Yin, F.; Bakenov, Z. In situ sol-gel synthesis of ultrafine ZnO nanocrystals anchored on graphene as anode material for lithium-ion batteries. Ceram. Int. 2016, 42, 12371–12377. [Google Scholar] [CrossRef]
- Shi, W.H.; Zhu, J.X.; Sim, D.H.; Tay, Y.Y.; Lu, Z.Y.; Zhang, X.J.; Sharma, Y.; Srinivasan, M.; Zhang, H.; Hng, H.H.; et al. Achieving high specific charge capacitances in Fe3O4/reduced graphene oxide nanocomposites. J. Mater. Chem. 2011, 21, 3422–3427. [Google Scholar] [CrossRef]
- Huang, X.; Sun, B.; Li, K.; Chen, S.; Wang, G. Mesoporous graphene paper immobilized sulfur as a flexible electrode for lithium-sulfur batteries. J. Mater. Chem. A 2013, 1, 13484–13489. [Google Scholar] [CrossRef]
- Wang, Z.G.; Niu, X.Y.; Xiao, J.; Wang, C.M.; Liu, J.; Gao, F. First principle prediction of nitrogen-doped carbon nanotubes as a high-performance cathode for Li-S batteries. RSC Adv. 2013, 3, 16775–16780. [Google Scholar] [CrossRef]
- Zu, C.X.; Manthiram, A. Hydroxylated graphene-sulfur nanocomposites for high-rate lithium-sulfur batteries. Adv. Energy Mater. 2013, 3, 1008–1012. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, L.; Glans, P.A.; Zhang, Y.; Zhu, J.; Guo, J. Electronic structure and chemical bonding of a graphene oxide-sulfur nanocomposite for use in superior performance lithium-sulfur cells. Phys. Chem. Chem. Phys. 2012, 14, 13670–13675. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, O.Y.; Duan, W. Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Xu, Y.X.; Zhao, L.; Bai, H.; Hong, W.J.; Li, C.; Shi, G.Q. Chemically converted graphene induced molecular flattening of 5, 10, 15, 20-tetrakis (1-methyl-4-pyridinio) porphyrin and its application for optical detection of cadmium ions. J. Am. Chem. Soc. 2009, 131, 13490–13497. [Google Scholar] [CrossRef] [PubMed]
- Tursun, A.; Aminam, U.; Jamal, R.; Adalet, R. Solid-state synthesis of polyaniline/single-walled carbon nanotubes: A comparative study with polyaniline/multi-walled carbon nanotubes. Materials 2012, 5, 1219–1231. [Google Scholar]
- Chen, R.; Zhao, T.; Lu, J.; Wu, F.; Li, L.; Chen, J.; Tan, G.; Ye, Y.; Amine, K. Graphene-based three-dimensional hierarchical sandwich-type architecture for high-performance Li/S batteries. Nano Lett. 2013, 13, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chen, Y.; Xu, Y.; Yi, F.; Zhu, Y.; Liu, Y.; Yang, J.; Wang, C. In situ formed lithium sulfide/microporous carbon cathodes for lithium-ion batteries. ACS Nano 2013, 7, 10995–11003. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Soares, J.; Oliveros, M.E.; Garin, G. Structural analysis of polycrystalline graphene systems by Raman spectroscopy. Carbon 2015, 95, 646–652. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 2003, 53, 1126–1130. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, Y.; Konarov, A.; Gosselink, D.; Soboleski, H.G.; Chen, P. A novel sulfur/polypyrrole/multi-walled carbon nanotube nanocomposite cathode with core-shell tubular structure for lithium rechargeable batteries. Solid State Ionics 2013, 238, 30–35. [Google Scholar] [CrossRef]
- Yun, Q.; Qin, X.; He, Y.B.; Lv, W.; Kaneti, Y.V.; Li, B.; Yang, Q.H.; Kang, F. Micronsized spherical Si/C hybrids assembled via water/oil system for high-performance lithium ion battery. Electrochim. Acta 2016, 211, 982–988. [Google Scholar] [CrossRef]
- Wang, Y.X.; Huang, L.; Sun, L.C.; Xie, S.Y.; Xu, G.L.; Chen, S.R.; Xu, Y.F.; Li, J.T.; Chou, S.L.; Dou, S.X.; et al. Facile synthesis of a interleaved expanded graphite-embedded sulphur nanocomposite as cathode of Li-S batteries with excellent lithium storage performance. J. Mater. Chem. 2012, 22, 4744–4750. [Google Scholar] [CrossRef]
- Wu, T.; Cai, X.; Tan, S.; Li, H.; Liu, J.; Yang, W. Adsorption characteristics of acrylonitrile p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions. Chem. Eng. J. 2011, 173, 144–149. [Google Scholar] [CrossRef]
- Vuković, G.D.; Marinković, A.D.; Čolić, M.; Ristić, M.D.; Aleksić, R.; Perić-Grujić, A.A.; Uskoković, P.S. Removal of cadmium from aqueous solutions by oxided and ethylenediamine-functionalized multi-walled carbon nanotubes. Chem. Eng. J. 2010, 157, 238–248. [Google Scholar] [CrossRef]
- Veličković, Z.S.; Marinković, A.D.; Bajić, Z.J.; Marković, J.M.; Perić-Grujić, A.A.; Uskoković, P.S.; Ristić, M.D. Oxidized and ethylenediamine-functionalized multi-walled carbon nanotubes for the separation of the Low concentration arsenate from water. Sep. Sci. Technol. 2013, 48, 2047–2058. [Google Scholar] [CrossRef]
- Ramanathan, T.; Fisher, F.T.; Ruoff, R.S.; Brinson, L.C. Amino-functionalized carbon nanotubes for binding to polymers and biological systems. Chem. Mater. 2005, 17, 1290–1295. [Google Scholar] [CrossRef]
- Cao, L.; Sun, Q.Q.; Gao, Y.H.; Liu, L.T.; Shi, H.F. Novel acid-base hybrid membrane based on amine-functionalized reduced graphene oxide and sulfonated polyimide for vanadium redox flow battery. Electrochim. Acta 2015, 158, 24–34. [Google Scholar] [CrossRef]
- Xu, L.Q.; Yang, W.J.; Neoh, K.G.; Kang, E.T.; Fu, G.D. Dopamine-induced reduction and functionalization of graphene oxide nanosheets. Macromolecules 2010, 43, 8336–8339. [Google Scholar] [CrossRef]
- Zhang, S.L.; Song, Y.H.; Si, D.Y. Performance of reduced graphene oxide-sulfur composite prepared by hydrothermal method. Battery Bimon. 2016, 46, 129–132. [Google Scholar]
- Huang, T.; Zhang, L.; Chen, H.; Gao, C. Sol-gel fabrication of a non-laminated graphene oxide membrane for oil/water separation. J. Mater. Chem. A 2015, 3, 19517–19524. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhass, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Deng, Z.F.; Zhang, Z.A.; Lai, Y.Q.; Liu, J.; Li, J.; Liu, Y.X. Electrochemical impedance spectroscopy study of a lithium/sulfur battery: Modeling and analysis of capacity fading. J. Electrochem. Soc. 2013, 160, A553–A558. [Google Scholar] [CrossRef]
- Canas, N.A.; Hirose, K.; Pascucci, B.; Wagner, N.; Friedrich, K.A.; Hiesgen, R. Investigation of lithium-sulfur batteries using electrochemical impedance spectroscopy. Electrochim. Acta 2013, 97, 42–51. [Google Scholar] [CrossRef]
- Ahad, S.A.; Kumae, P.R.; Kim, J.H.; Kim, D.J.; Ragupathy, P. Catecholamine-functionalized reduced graphene oxide: A scalable carbon host for stable cycling in lithium-sulfur batteries. Electrochim. Acta 2017, 246, 451–458. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Bakenov, Z.; Zhao, Y.; Konarov, A.; Dian, T.N.L.; Nalik, M.; Paron, T.; Chen, P. One-step synthesis of branched sulfur/polypyrrole nanocomposite cathode for lithium rechargeable batteries. J. Power Sources 2012, 208, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Bakenov, Z. A simple approach to synthesize nanosized sulfur/graphene oxide materials for high-performance lithium/sulfur batteries. Ionics 2015, 20, 1047–1050. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Zhang, Y.; Bakenov, Z.; Yin, F. Synthesis of Multiwalled Carbon Nanotube Aqueous Suspension with Surfactant Sodium Dodecylbenzene Sulfonate for Lithium/Sulfur Rechargeable Batteries. Electrochemistry 2016, 84, 7–11. [Google Scholar] [CrossRef]
- Lu, L.Q.; Lu, L.J.; Wang, Y. Sulfur film-coated reduced graphene oxide composite for lithium-sulfur batteries. J. Mater. Chem. A 2013, 1, 9173–9181. [Google Scholar] [CrossRef]

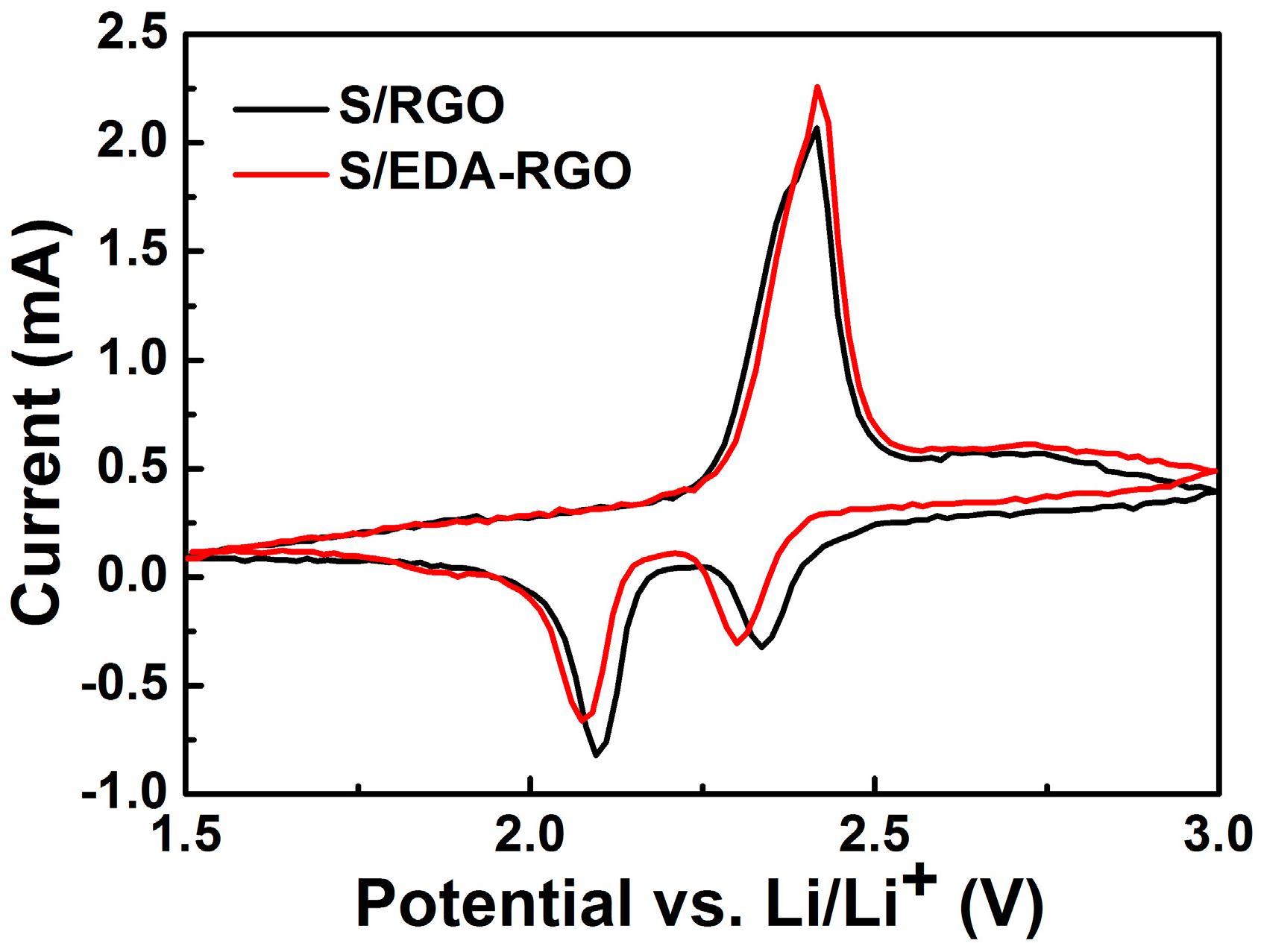
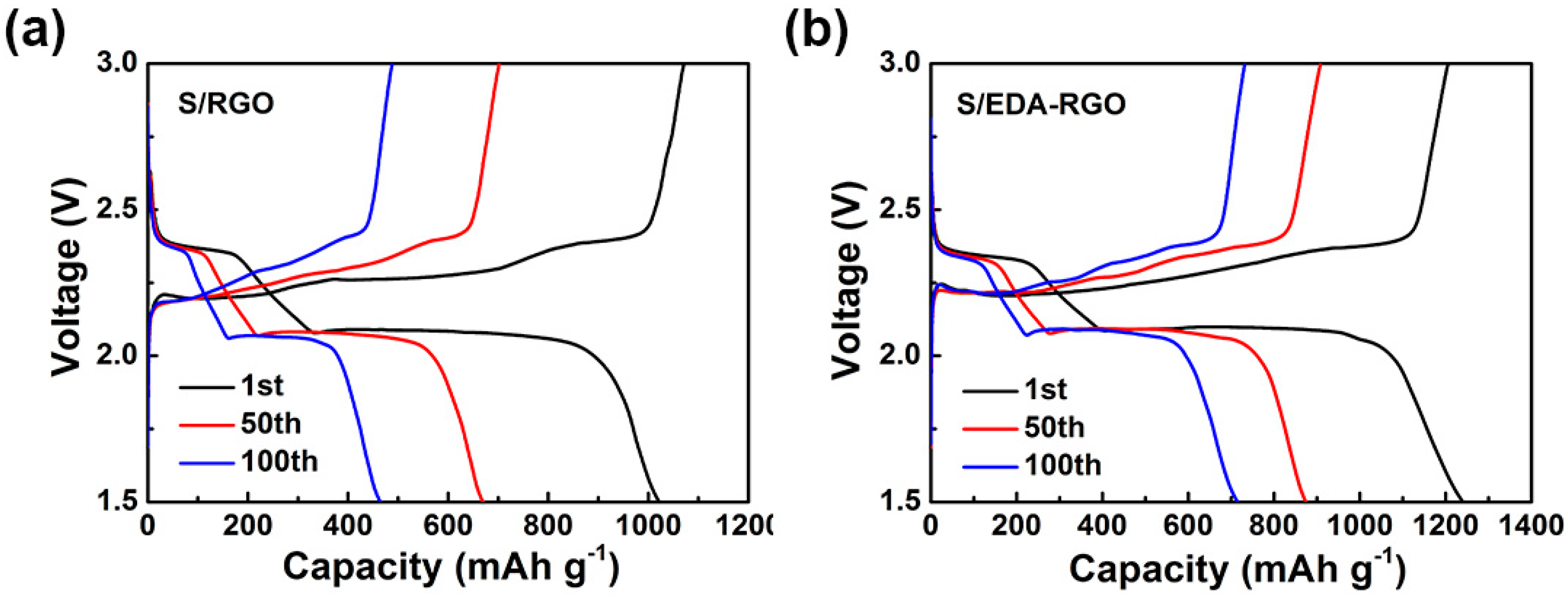
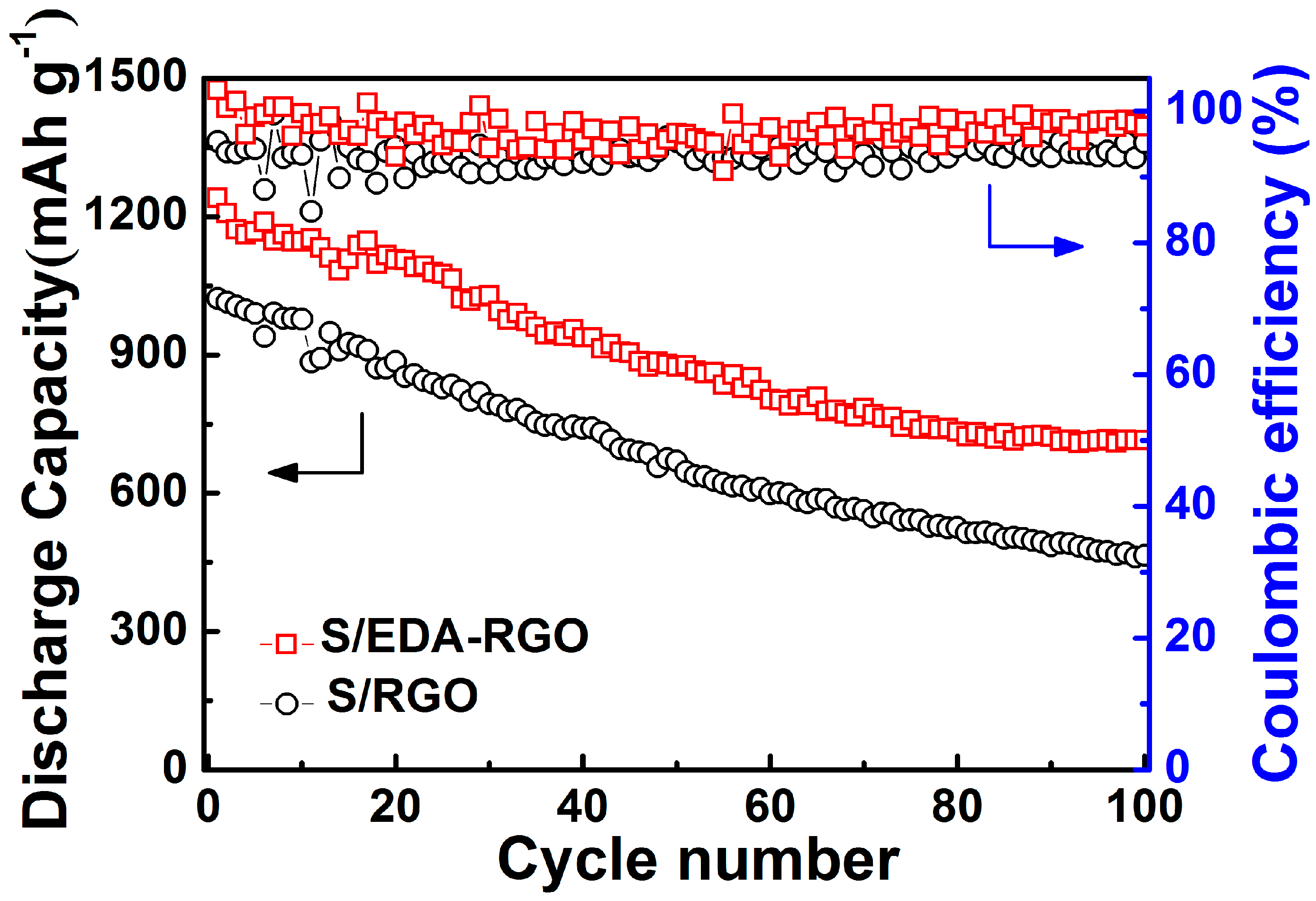
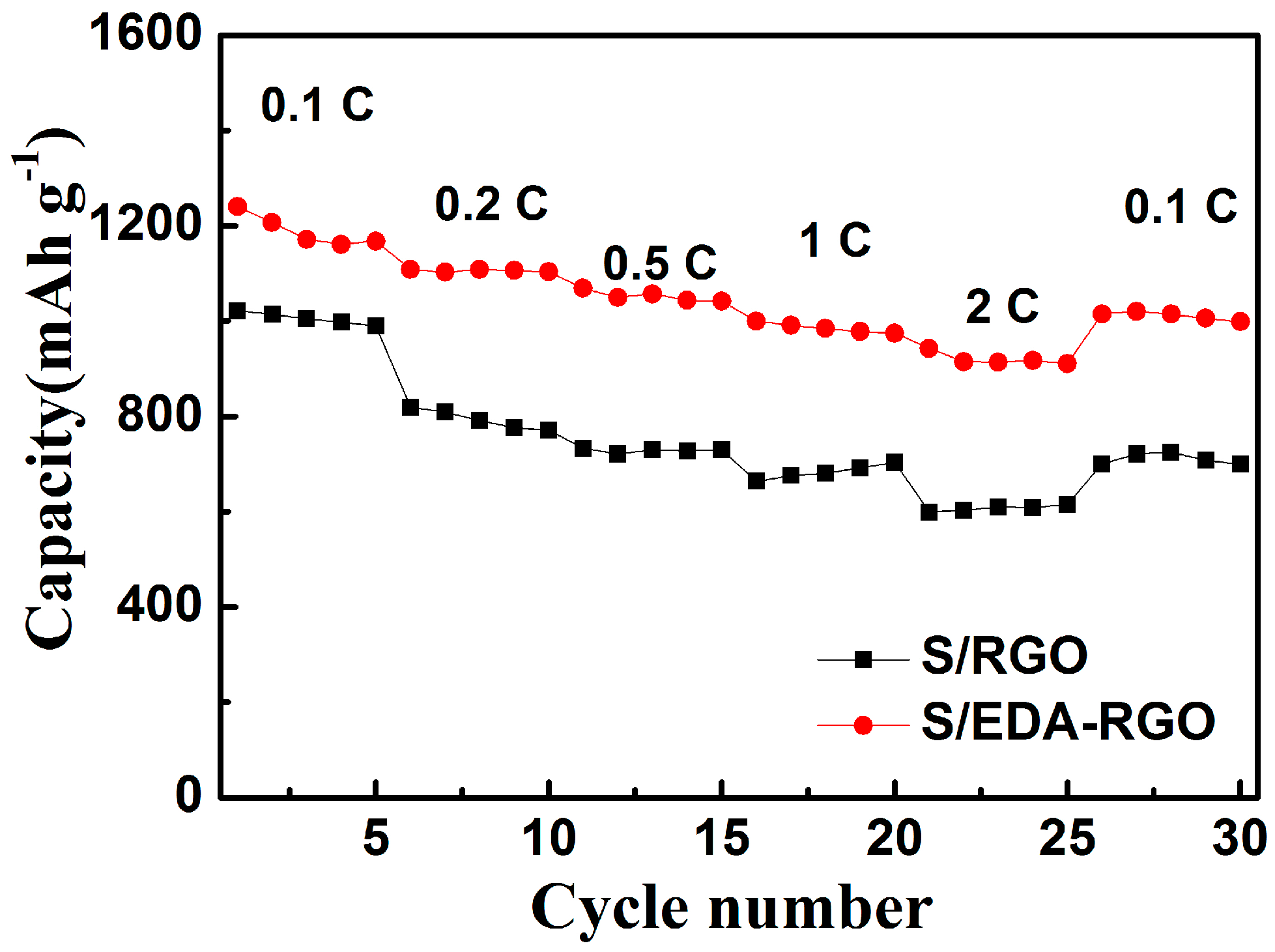
| Material | Initial Discharge Capacity (mAh g−1) | Discharge Capacity (mAh g−1) (after 100 Cycles) | Capacity Decay (%) | Current Density | Reference |
|---|---|---|---|---|---|
| S/GO | 1053 | 591 | 43.8 | 0.1c | [48] |
| S/MWNT | 1394 | 700 | 49.7 | 0.1c | [49] |
| S/RGO | 1316 | 476 | 63.8 | 0.1c | [50] |
| S/EDA-RGO | 1240 | 714 | 42.4 | 0.1c | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Sun, Z.; Zhang, Y.; Tan, T.; Tian, Y.; Chen, Z. Novel Sulfur/Ethylenediamine-Functionalized Reduced Graphene Oxide Composite as Cathode Material for High-performance Lithium-Sulfur Batteries. Nanomaterials 2018, 8, 303. https://doi.org/10.3390/nano8050303
Chen Z, Sun Z, Zhang Y, Tan T, Tian Y, Chen Z. Novel Sulfur/Ethylenediamine-Functionalized Reduced Graphene Oxide Composite as Cathode Material for High-performance Lithium-Sulfur Batteries. Nanomaterials. 2018; 8(5):303. https://doi.org/10.3390/nano8050303
Chicago/Turabian StyleChen, Zhuo, Zhenghao Sun, Yongguang Zhang, Taizhe Tan, Yuan Tian, and Zhihong Chen. 2018. "Novel Sulfur/Ethylenediamine-Functionalized Reduced Graphene Oxide Composite as Cathode Material for High-performance Lithium-Sulfur Batteries" Nanomaterials 8, no. 5: 303. https://doi.org/10.3390/nano8050303




