The Enhanced Red Emission and Improved Thermal Stability of CaAlSiN3:Eu2+ Phosphors by Using Nano-EuB6 as Raw Material
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Measurements and Characterization
3. Results and Discussion
3.1. Structure Characterization
3.2. XPS Analysis
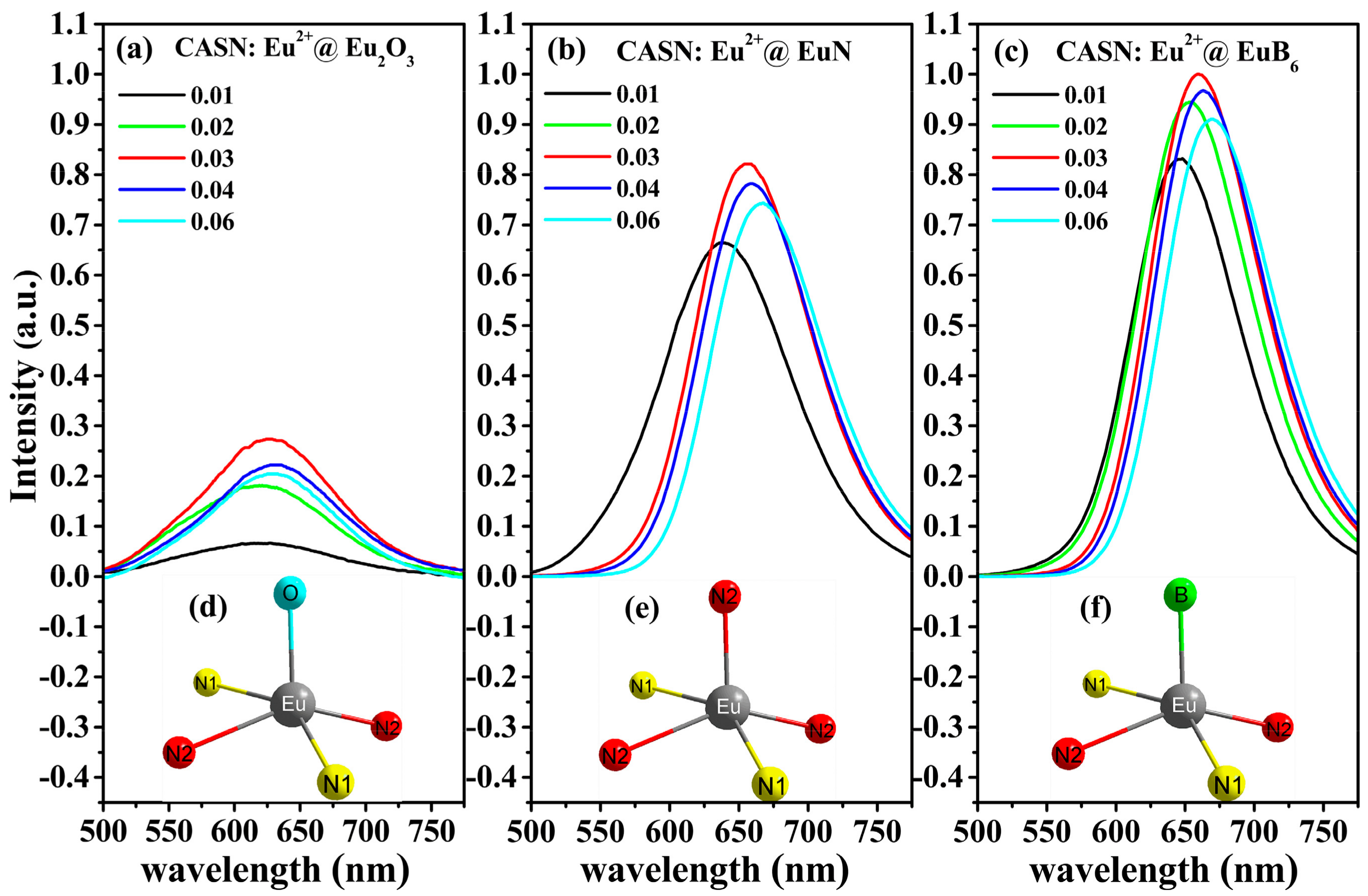
3.3. Photoluminescence Properties
3.4. Thermal Stability Analysis
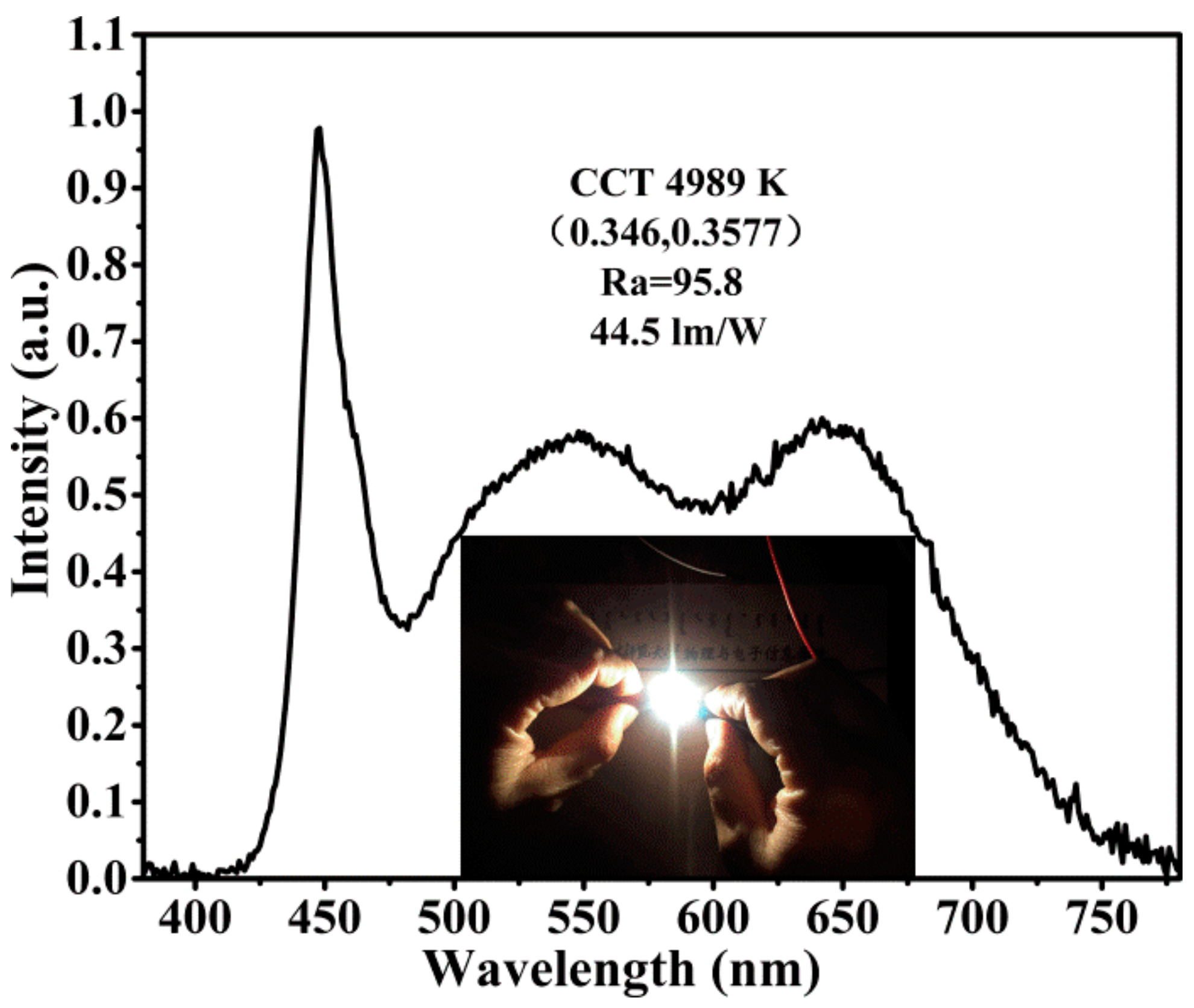
3.5. The Fabrication of W-LEDs Device
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, Y.H.; Arunkumar, P.; Kim, B.Y.; Unithrattil, S.; Kim, E.; Moon, S.H.; Hyun, J.Y.; Kim, K.H.; Lee, D.; Lee, J.S.; et al. A zero-thermal-quenching phosphor. Nat. Mater. 2017, 16, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Stanish, P.C.; Radovanovic, P.V. Energy Transfer between Conjugated Colloidal Ga2O3 and CdSe/CdS Core/Shell Nanocrystals for White Light Emitting Applications. Nanomaterials 2016, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhao, W.; Zhuang, W.; Du, F.; Zhou, Y.; Yu, Y.; Wang, L. Selective coordination of N3− and tuning of luminescence in garnet (Y1−x,Lax)3(Al,Si)5(O,N)12:Ce3+ phosphors. J. Alloys Compd. 2017, 726, 658–663. [Google Scholar] [CrossRef]
- Wang, L.; Xie, R.-J.; Li, Y.; Wang, X.; Ma, C.-G.; Luo, D.; Takeda, T.; Tsai, Y.-T.; Liu, R.-S.; Hirosaki, N. Ca1−xLixAl1−xSi1+xN3:Eu2+ solid solutions as broadband, color-tunable and thermally robust red phosphors for superior color rendition white light-emitting diodes. Light Sci. Appl. 2016, 5, e16155. [Google Scholar] [CrossRef]
- Pan, F.; Zhou, M.; Zhang, J.; Zhang, X.; Wang, J.; Huang, L.; Kuang, X.; Wu, M. Double substitution induced tunable luminescent properties of Ca3−xYxSc2−xMgxSi3O12:Ce3+ phosphors for white LEDs. J. Mater. Chem. C 2016, 4, 5671–5678. [Google Scholar] [CrossRef]
- Li, X.; Budai, J.D.; Liu, F.; Howe, J.Y.; Zhang, J.; Wang, X.-J.; Gu, Z.; Sun, C.; Meltzer, R.S.; Pan, Z. New yellow Ba0.93Eu0.07Al2O4 phosphor for warm-white light-emitting diodes through single-emitting-center conversion. Light Sci. Appl. 2013, 2, e50. [Google Scholar] [CrossRef]
- Xie, R.J.; Hirosaki, N.; Li, Y.; Takeda, T. Rare-Earth Activated Nitride Phosphors: Synthesis, Luminescence and Applications. Materials 2010, 3, 3777–3793. [Google Scholar] [CrossRef]
- Zhang, W.T.; Wang, Y.L.; Gao, Y.; Long, J.P.; Li, J.F. Sol-gel assisted synthesis and photoluminescence property of Sr2Si5N8:Eu2+, Dy3+red phosphor for white light emitting diodes. J. Alloys Compd. 2016, 667, 341–345. [Google Scholar] [CrossRef]
- Suehiro, T.; Xie, R.J.; Hirosaki, N. Facile Synthesis of (Sr,Ca)2Si5N8:Eu2+-Based Red-Emitting Phosphor for Solid-State Lighting. Ind. Eng. Chem. Res. 2013, 52, 7453–7456. [Google Scholar] [CrossRef]
- Li, H.L.; Xie, R.J.; Hirosaki, N.; Takeda, T.; Zhou, G.H. Synthesis and Luminescence Properties of Orange-Red-Emitting M2Si5N8:Eu2+(M=Ca, Sr, Ba) Light-Emitting Diode Conversion Phosphors by a Simple Nitridation of MSi2. Int. J. Appl. Ceram. Technol. 2009, 6, 459–464. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, W.J.; Rainwater, B.; Liu, L.X.; Zhang, H.L.; Liu, Y.X.; Guo, X.S.; Zhou, J.Y.; Xie, E.Q. M2Si5N8:Eu2+-based (M=Ca, Sr) red-emitting phosphors fabricated by nitrate reduction process. Opt. Mater. 2011, 33, 1585–1590. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Nguyen, H.D.; Lazarowska, A.; Mahlik, S.; Grinberg, M.; Liu, R.S. Improvement of the Water Resistance of a Narrow-Band Red-Emitting SrLiAl3N4:Eu2+Phosphor Synthesized under High Isostatic Pressure through Coating with an Organosilica Layer. Angew. Chem. 2016, 55, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tsai, Y.T.; Wu, S.M.; Lin, Y.C.; Lee, J.F.; Sheu, H.S.; Cheng, B.M.; Liu, R.S. Facile Atmospheric Pressure Synthesis of High Thermal Stability and Narrow-Band Red-Emitting SrLiAl3N4:Eu2+ Phosphor for High Color Rendering Index White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2016, 8, 19612–19617. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Xiang, Q.; Song, Z.; Xia, Z.; Liu, Q. The synthesis of narrow-band red-emitting SrLiAl3N4:Eu2+ phosphor and improvement of its luminescence properties. J. Mater. Chem. C 2016, 4, 7332–7338. [Google Scholar] [CrossRef]
- Kim, S.W.; Hasegawa, T.; Hasegawa, S.; Yamanashi, R.; Nakagawa, H.; Toda, K.; Ishigaki, T.; Uematsu, K.; Sato, M. Improved synthesis of SrLiAl3N4:Eu2+ phosphor using complex nitride raw material. RSC Adv. 2016, 6, 61906–61908. [Google Scholar] [CrossRef]
- Chen, L.; Fei, M.; Zhang, Z.; Jiang, Y.; Chen, S.; Dong, Y.; Sun, Z.; Zhao, Z.; Fu, Y.; He, J.; et al. Understanding the Local and Electronic Structures toward Enhanced Thermal Stable Luminescence of CaAlSiN3:Eu2+. Chem. Mater. 2016, 28, 5505–5515. [Google Scholar] [CrossRef]
- Yang, J.J.; Wang, T.; Chen, D.C.; Chen, G.D.; Liu, Q.L. An investigation of Eu2+-doped CaAlSiN3 fabricated by an alloy-nitridation method. Mater. Sci. Eng. B 2012, 177, 1596–1604. [Google Scholar] [CrossRef]
- Li, J.W.; Watanabe, T.; Sakamoto, N.; Wada, H.; Setoyama, T.; Yoshimura, M. Synthesis of a Multinary Nitride, Eu-Doped CaAlSiN3, from Alloy at Low Temperatures. Chem. Mater. 2008, 20, 2095–2105. [Google Scholar] [CrossRef]
- Piao, X.; Machida, K.; Horikawa, T.; Hanzawa, H.; Shimomura, Y.; Kijima, N. Preparation of CaAlSiN3:Eu2+ phosphors by the self-propagating high-temperature synthesis and their luminescent properties. Chem. Mater. 2007, 19, 4592–4599. [Google Scholar] [CrossRef]
- Cai, C.; Qian, J.; Zhang, B.; Hu, W.; Hao, L.; Xu, X.; Wang, Y. Synthesis of Red-Emitting CaAlSiN3:Eu2+ Phosphors through a Cost-Effective Synthetic Route. ECS J. Solid State Sci. Technol. 2014, 3, R169–R172. [Google Scholar] [CrossRef]
- Dierre, B.; Takeda, T.; Sekiguchi, T.; Suehiro, T.; Takahashi, K.; Yamamoto, Y.; Xie, R.J.; Hirosaki, N. Local analysis of Eu2+ emission in CaAlSiN3. Sci. Technol. Adv. Mater. 2013, 14, 064201. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, S.W.; Park, J.H.; Bok, E.; Kim, B.K.; Hong, S.H. Red-Emitting (Sr,Ca)AlSiN3:Eu2+ Phosphors Synthesized by Spark Plasma Sintering. Ecs J. Solid State Sci. Technol. 2013, 2, R3021–R3025. [Google Scholar] [CrossRef]
- Kim, B.H.; Kang, E.H.; Choi, S.W.; Hong, S.H. Luminescence properties of La2Si6O3N8:Eu2+phosphors prepared by spark plasma sintering. Opt. Mater. 2013, 36, 182–185. [Google Scholar] [CrossRef]
- Chung, S.L.; Huang, S.C. Synthesis of CaAlSiN3:Eu2+ Phosphor via the Self-Propagating High Temperature Synthesis Method and its Optical Properties. Adv. Mater. Res. 2014, 904, 33–35. [Google Scholar] [CrossRef]
- Li, S.X.; Peng, X.; Liu, X.J.; Huang, Z.R. Photoluminescence of CaAlSiN3:Eu2+-based fine red-emitting phosphors synthesized by carbothermal reduction and nitridation method. Opt. Mater. 2014, 38, 242–247. [Google Scholar] [CrossRef]
- Li, S.X.; Liu, X.J.; Mao, R.H.; Huang, Z.R.; Xie, R.J. Red-emission enhancement of the CaAlSiN3:Eu2+ phosphor by partial substitution for Ca3N2 by CaCO3and excess calcium source addition. RSC Adv. 2015, 5, 76507–76515. [Google Scholar] [CrossRef]
- Li, S.X.; Liu, X.J.; Liu, J.Q.; Li, H.L.; Mao, R.H.; Huang, Z.R.; Xie, R.J. Synthesis, composition optimization, and tunable red emission of CaAlSiN3:Eu2+ phosphors for white light-emitting diodes. J. Mater. Res. 2015, 30, 2919–2927. [Google Scholar] [CrossRef]
- Kim, H.S.; Horikawa, T.; Hanzawa, H.; Machida, K.-I. Luminescence properties of CaAlSiN3:Eu2+ mixed nitrides prepared by carbothermal process. J. Phys. Conf. Ser. 2012, 379, 012016. [Google Scholar] [CrossRef]
- Aronson, M.C. Fermi surface of the ferromagnetic semimetal, EuB6. Phys. Rev. B 1999, 59, 4720–4724. [Google Scholar] [CrossRef]
- Manna, R.S.; Das, P.; de Souza, M.; Schnelle, F.; Lang, M.; Muller, J.; von Molnar, S.; Fisk, Z. Lattice strain accompanying the colossal magnetoresistance effect in EuB6. Phys. Rev. Lett. 2014, 113, 672021–672025. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Wurentuya, B.; Wei, W.; Li, Y.; Tegus, O. Chemical synthesis and microstructure of nanocrystalline RB6 (R = Ce, Eu). J. Alloys Compd. 2014, 617, 235–239. [Google Scholar]
- Kim, H.S.; Machida, K.; Horikawa, T.; Hanzawa, H. Luminescence properties of CaAlSiN3:Eu2+ phosphor prepared by direct-nitriding method using fine metal hydride powders. J. Alloys Compd. 2015, 633, 97–103. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y.; Xu, J.; Mao, Z.; Chen, J.; Wang, D. Effective suppression of AlN impurity in synthesis of CaAlSiN3:Eu2+ phosphors under condition of atmospheric pressure. Mater. Chem. Phys. 2017, 201, 1–6. [Google Scholar] [CrossRef]
- Qi, J.F.; Matsumoto, T.; Tanaka, M.; Masumoto, Y. Europium silicate thin films on Si substrates fabricated by a radio frequency sputtering method. J. Phys. D Appl. Phys. 2000, 33, 2074–2078. [Google Scholar] [CrossRef]
- Cho, E.J.; Oh, S.J. Surface valence transition in trivalent Eu insulating compounds observed by photoelectron spectroscopy. Phys. Rev. B 1999, 59, R15613–R15616. [Google Scholar] [CrossRef]
- Riviere, J.P.; Pacaud, Y.; Cahoreau, M. Spectroscopic Studies of BN Films Deposited by Dynamic Ion Mixing. Thin Solid Films 1993, 227, 44–53. [Google Scholar] [CrossRef]
- Beshkov, G.; Spassov, D.; Krastev, V.; Stefanov, P.; Georgiev, S.; Nemska, S. XPS study of BNx nanolayers prepared by low pressure rapid thermal annealing in ammonia. J. Phys. Conf. Ser. 2008, 113, 012046. [Google Scholar] [CrossRef]
- Ye, S.; Xiao, F.; Pan, Y.X.; Ma, Y.Y.; Zhang, Q.Y. Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties. Mater. Sci. Eng. R 2010, 71, 1–34. [Google Scholar] [CrossRef]
- Liu, W.Q.; Chao, K.F.; Wu, W.J.; Bao, F.Q.; Zhou, B.Q. CaAlSiN3:Eu2+ red phosphors synthesized by atmospheric nitrogen and their luminescence properties. Acta Phys. Sin. 2016, 65, 207801–207807. [Google Scholar]
- Tian, Y. Development of phosphors with high thermal stability and efficiency for phosphor-converted LEDs. J. Solid State Light. 2014, 1, 1–15. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Takeda, T.; Hirosaki, N.; Tsai, Y.T.; Liu, R.S.; Xie, R.J. Structure, Luminescence, and Application of a Robust Carbidonitride Blue Phosphor (Al1–xSixCxN1–x:Eu2+) for Near UV-LED Driven Solid State Lighting. Chem. Mater. 2015, 27, 8457–8466. [Google Scholar] [CrossRef]
- Huang, W.Y.; Yoshimura, F.; Ueda, K.; Pang, W.K.; Su, B.J.; Jang, L.Y.; Chiang, C.Y.; Zhou, W.; Duy, N.H.; Liu, R.S. Domination of second-sphere shrinkage effect to improve photoluminescence of red nitride phosphors. Inorg. Chem. 2014, 53, 12822–12831. [Google Scholar] [CrossRef] [PubMed]
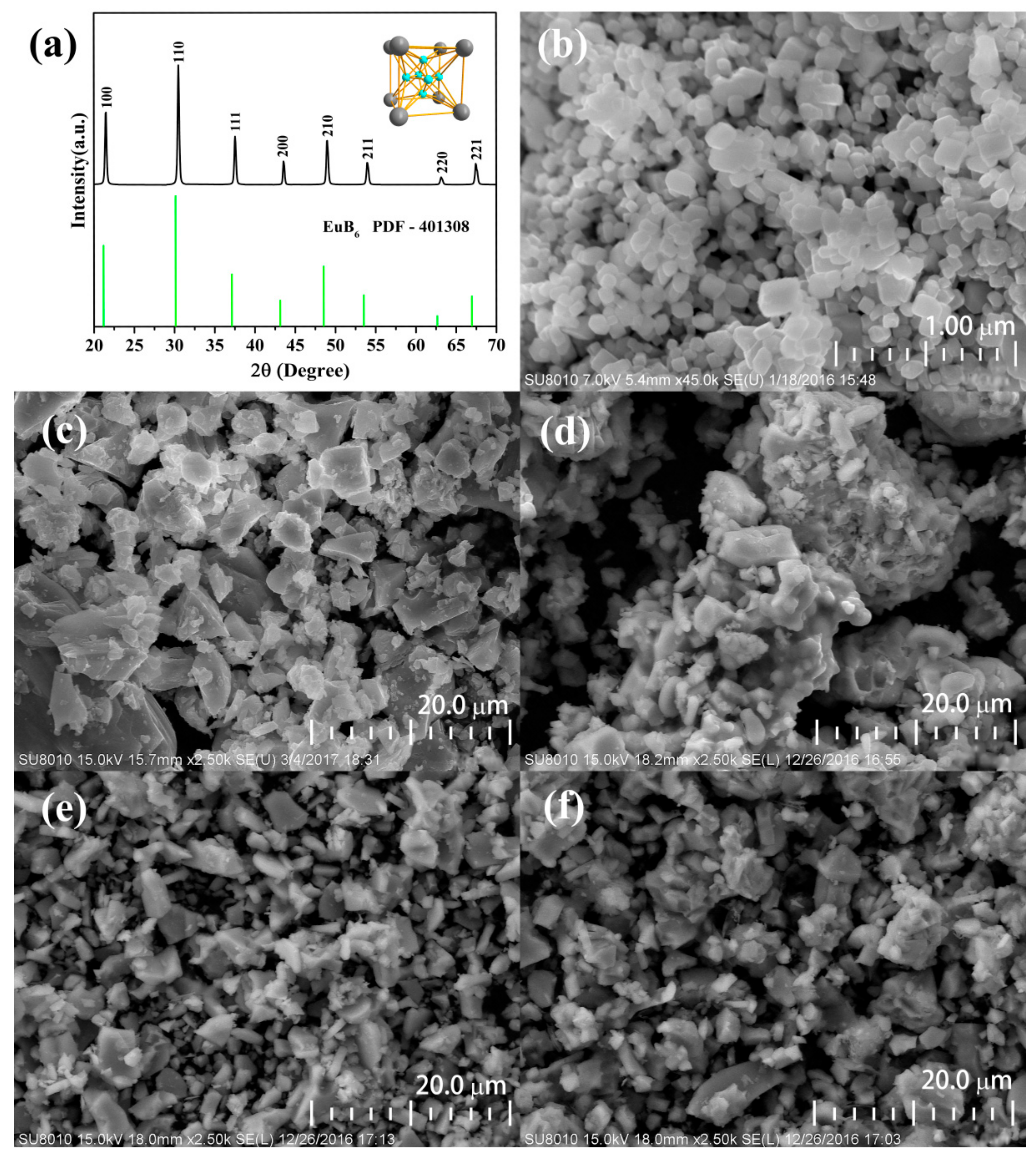

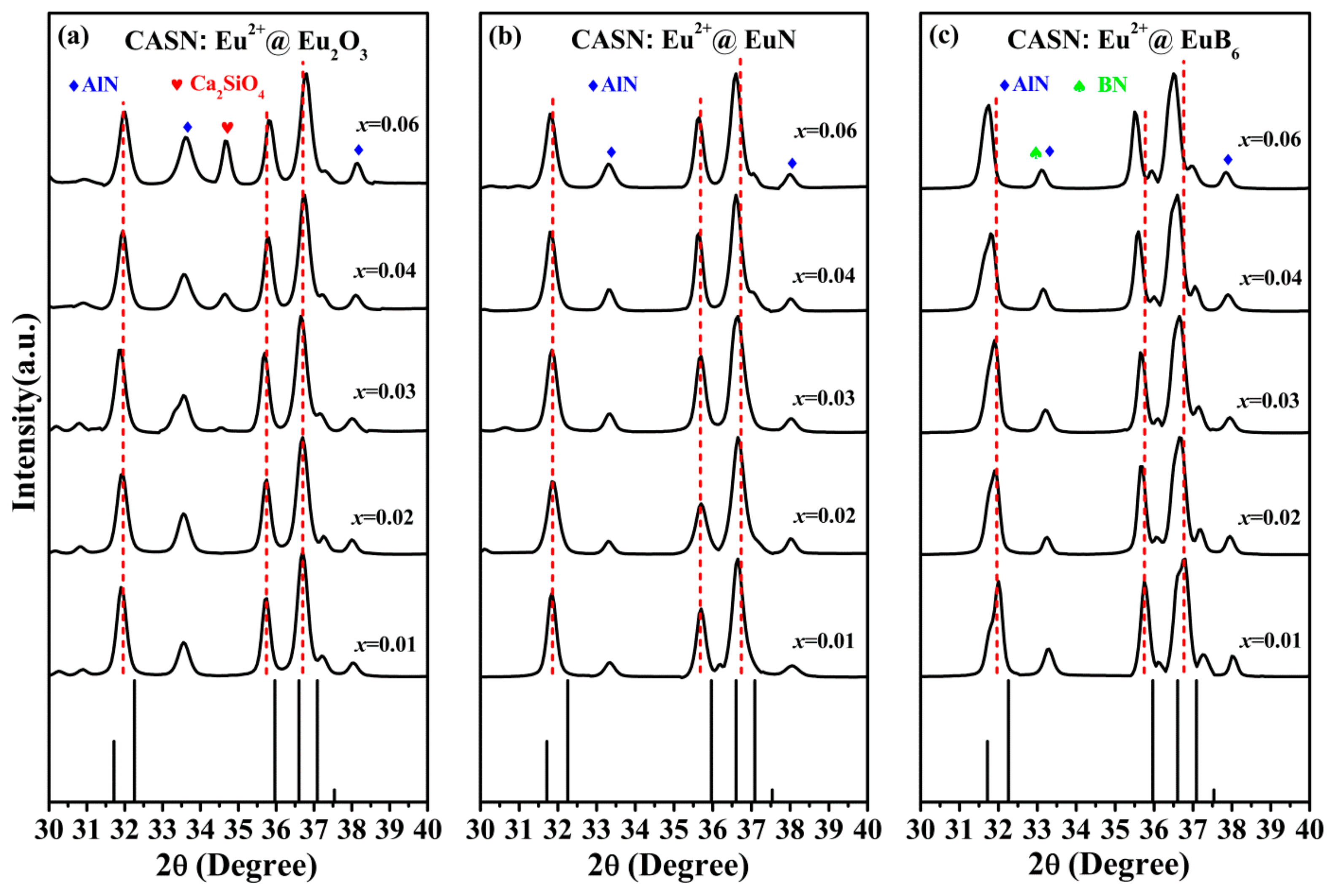
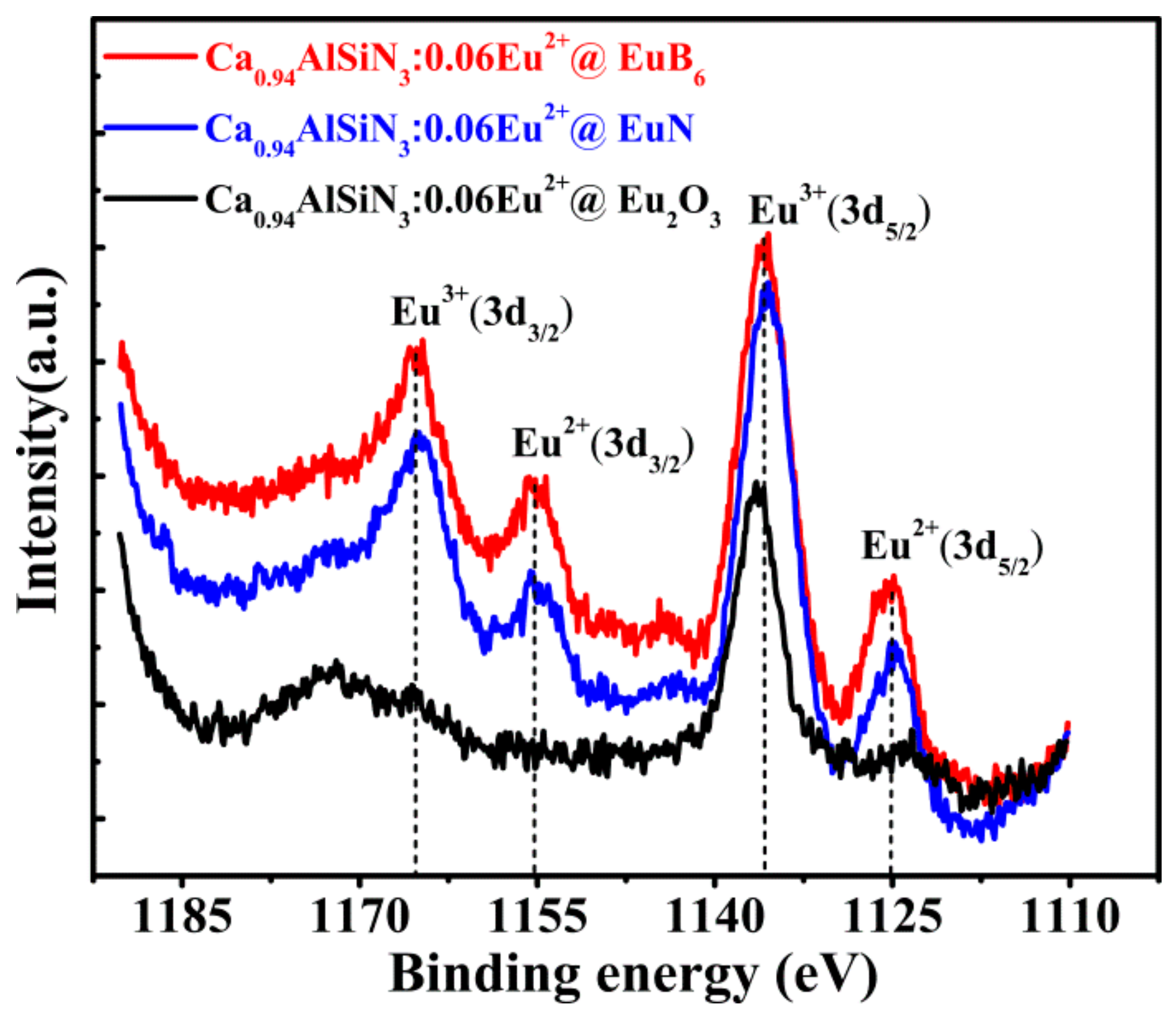
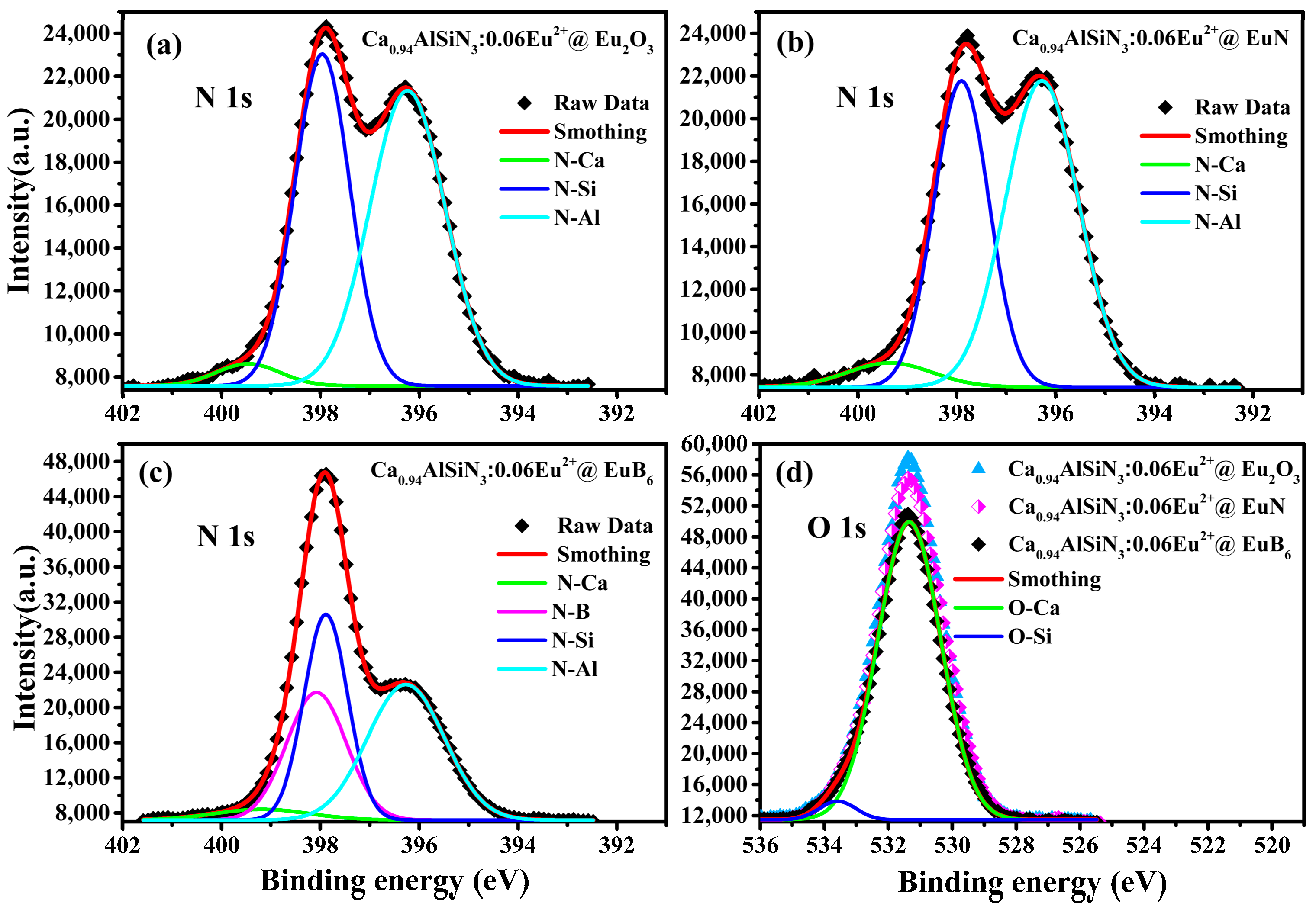


| Element (wt %) | B | N | O | Al | Si | Ca | Eu |
|---|---|---|---|---|---|---|---|
| CaSi alloys | —— | —— | 4.28 | —— | 59.39 | 36.33 | —— |
| Ca0.94AlSiN3:0.06Eu2+@Eu2O3 | —— | 21.52 | 6.19 | 18.05 | 28.53 | 22.53 | 3.26 |
| Ca0.94AlSiN3:0.06Eu2+@EuN | —— | 36.33 | 2.78 | 18.04 | 23.83 | 15.76 | 3.25 |
| Ca0.94AlSiN3:0.06Eu2+@EuB6 | 5.17 | 25.56 | 1.95 | 18.27 | 26.49 | 19.32 | 3.27 |
| XPS Measurements | Intensity of Eu2+ | Intensity of Eu3+ | Eu2+/Eu3+ |
|---|---|---|---|
| Ca0.94AlSiN3:0.06Eu2+@EuN(3d3/2) | 0.8847 | 0.9333 | 9.48/10 |
| Ca0.94AlSiN3:0.06Eu2+@EuN(3d5/2) | 0.8596 | 0.9854 | 8.72/10 |
| Ca0.94AlSiN3:0.06Eu2+@EuB6(3d3/2) | 0.9166 | 0.9633 | 9.52/10 |
| Ca0.94AlSiN3:0.06Eu2+@EuB6(3d5/2) | 0.8817 | 1. 0000 | 8.82/10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-Q.; Wu, D.; Chang, H.; Duan, R.-X.; Wu, W.-J.; Amu, G.; Chao, K.-F.; Bao, F.-Q.; Tegus, O. The Enhanced Red Emission and Improved Thermal Stability of CaAlSiN3:Eu2+ Phosphors by Using Nano-EuB6 as Raw Material. Nanomaterials 2018, 8, 66. https://doi.org/10.3390/nano8020066
Liu W-Q, Wu D, Chang H, Duan R-X, Wu W-J, Amu G, Chao K-F, Bao F-Q, Tegus O. The Enhanced Red Emission and Improved Thermal Stability of CaAlSiN3:Eu2+ Phosphors by Using Nano-EuB6 as Raw Material. Nanomaterials. 2018; 8(2):66. https://doi.org/10.3390/nano8020066
Chicago/Turabian StyleLiu, Wen-Quan, Dan Wu, Hugejile Chang, Ru-Xia Duan, Wen-Jie Wu, Guleng Amu, Ke-Fu Chao, Fu-Quan Bao, and Ojiyed Tegus. 2018. "The Enhanced Red Emission and Improved Thermal Stability of CaAlSiN3:Eu2+ Phosphors by Using Nano-EuB6 as Raw Material" Nanomaterials 8, no. 2: 66. https://doi.org/10.3390/nano8020066





