Use of Cellulose Nanofibers as an Electrode Binder for Lithium Ion Battery Screen Printing on a Paper Separator
Abstract
:1. Introduction
2. Experimental
2.1. Materials
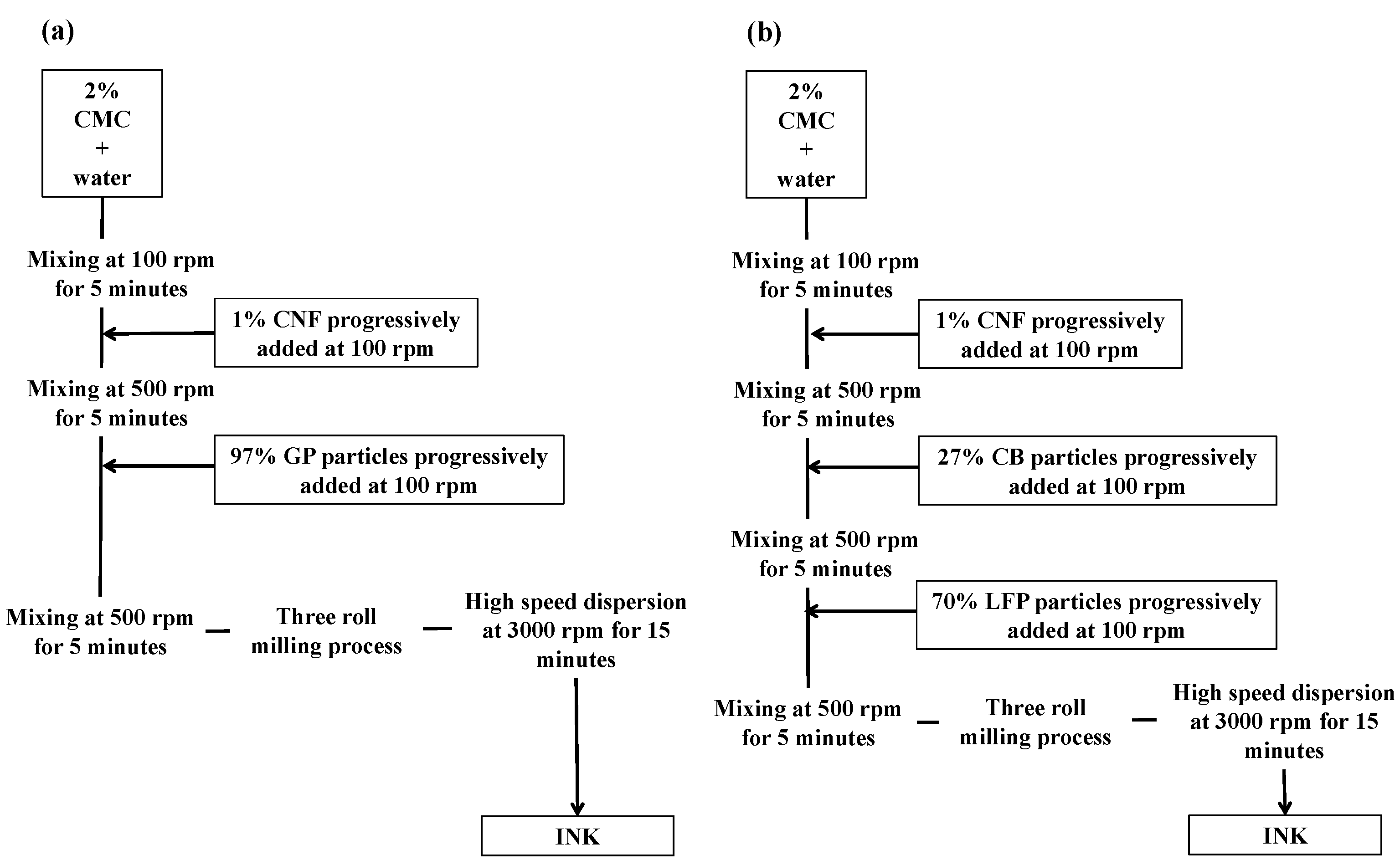
2.2. Ink Formulation
2.3. Printing Process
2.4. Physical and Electrical Tests
2.5. Electrochemical Test
3. Results and Discussions
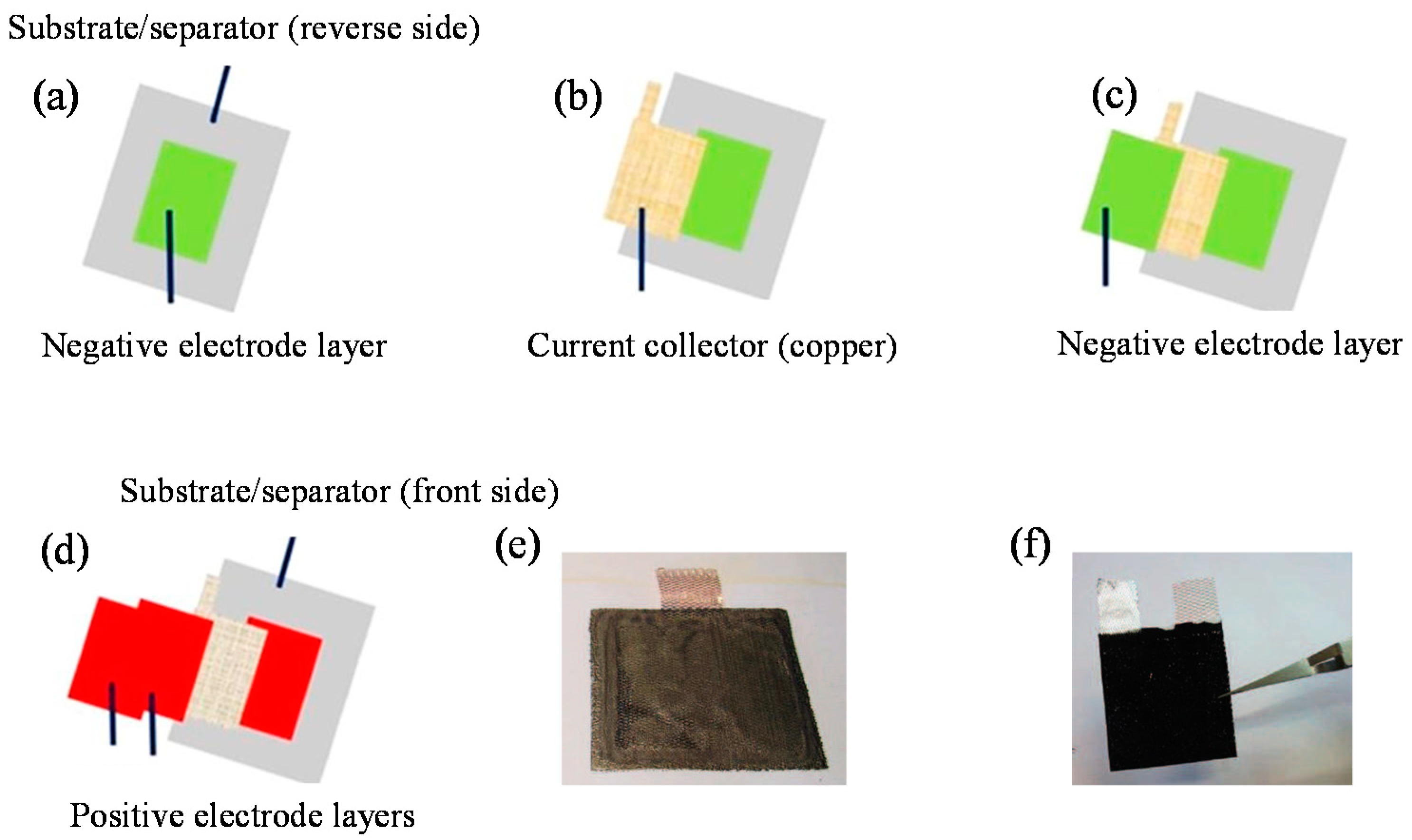
3.1. Assembling Strategy of the Cell
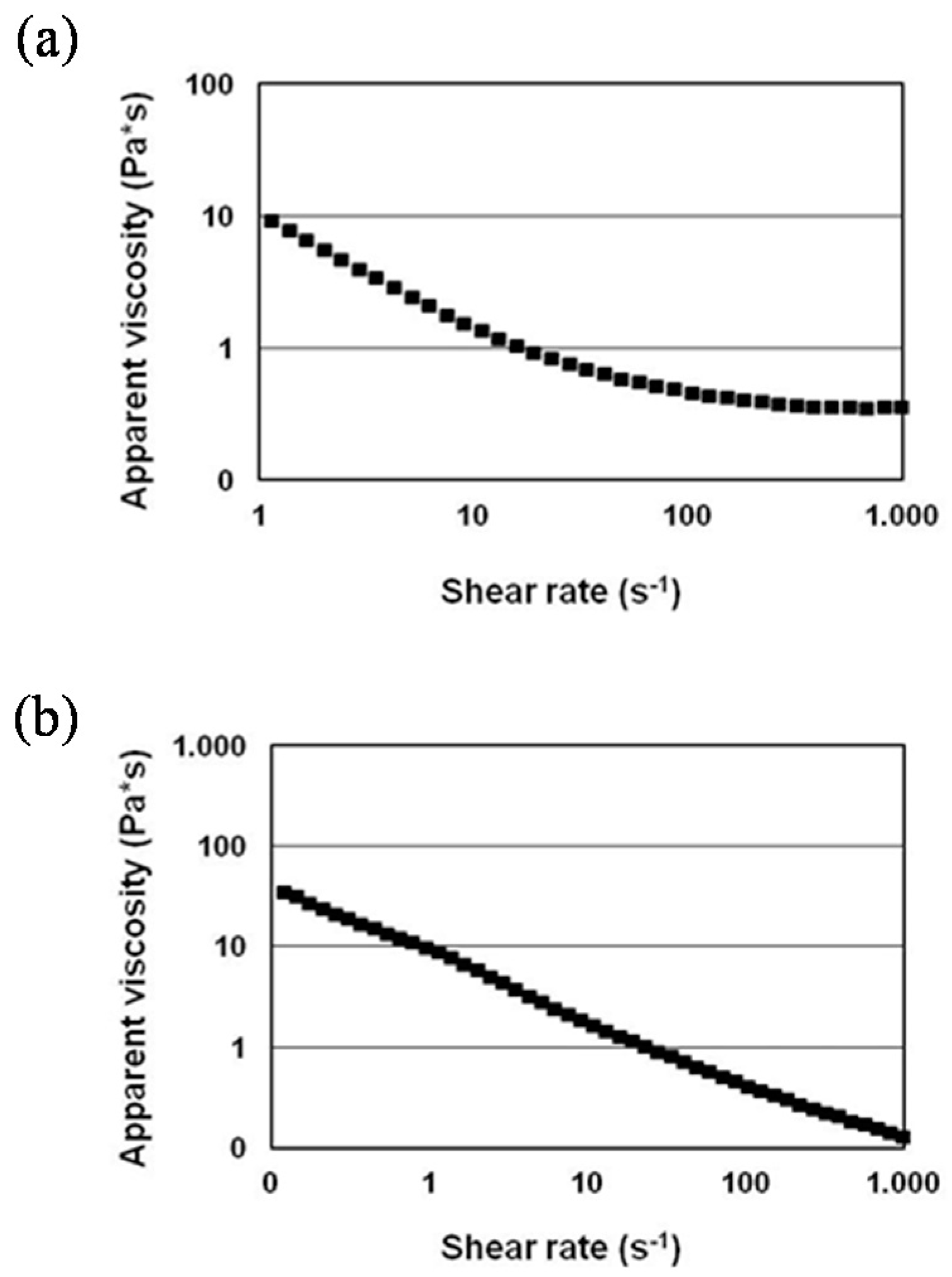
3.2. Formulation and Rheological Characterization of the Inks
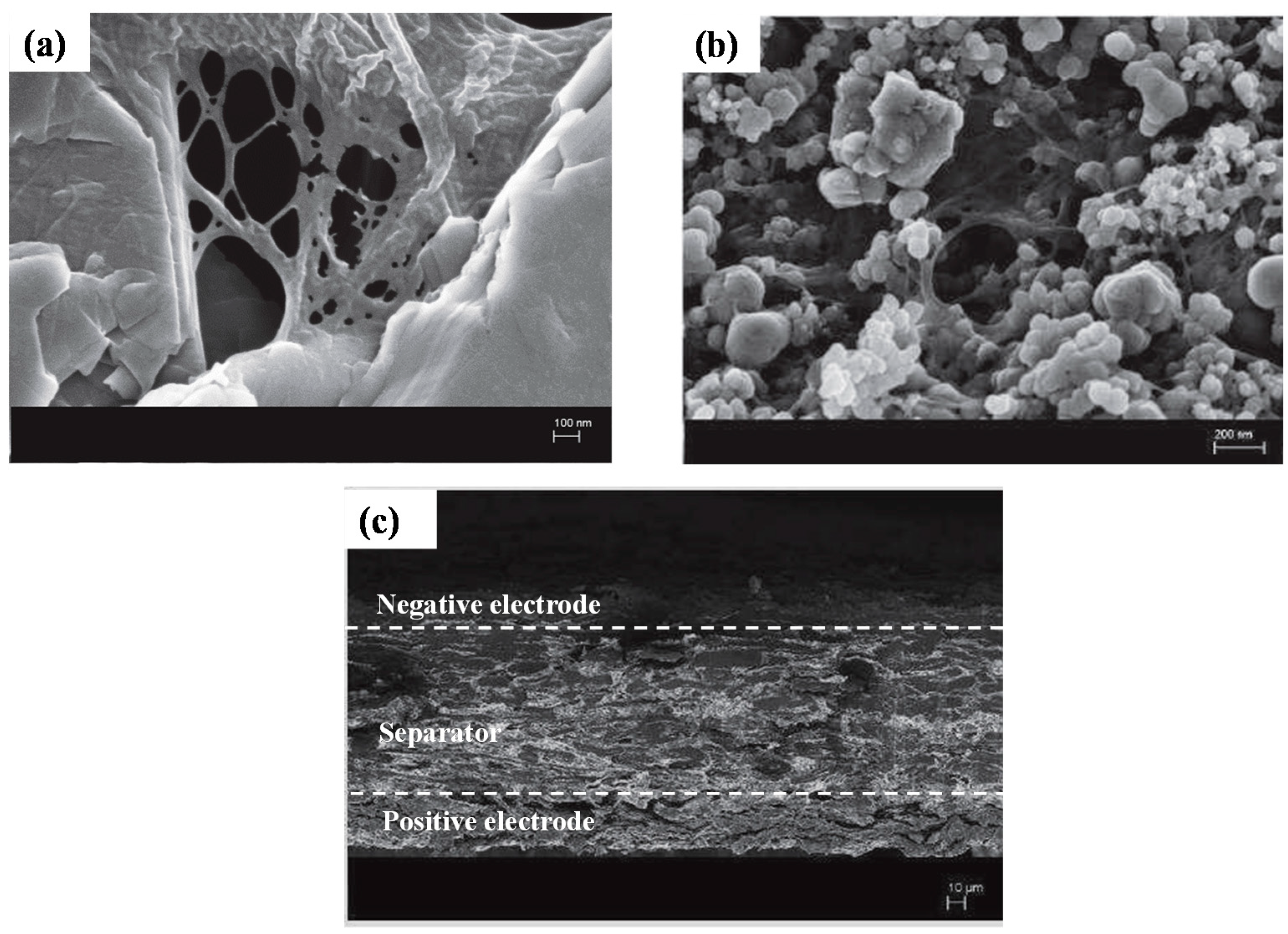
3.3. Physical Characterization of the Electrodes
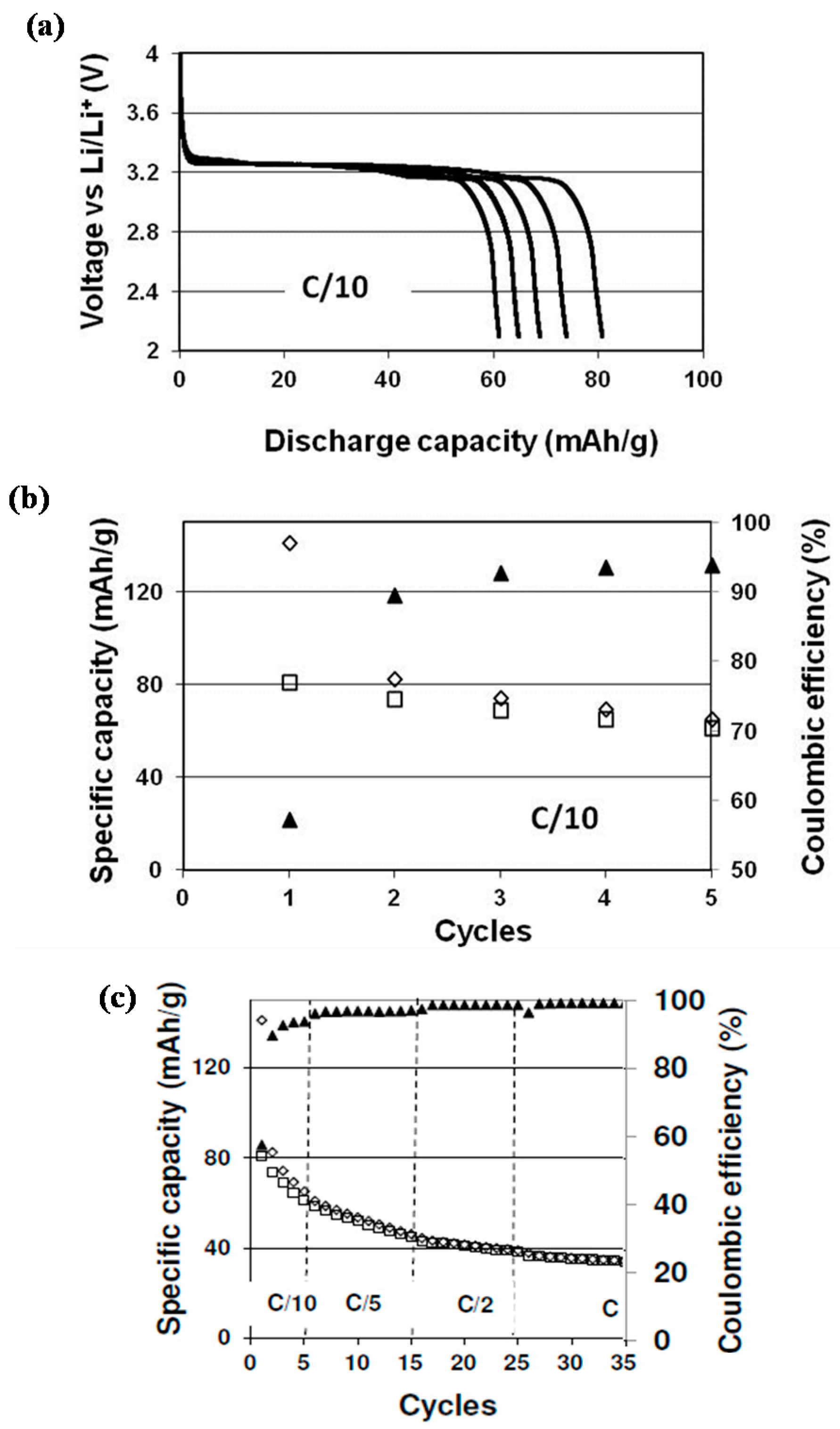
3.4. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalia, S.; Dufresne, A.; Cherian, M.B.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 837875. [Google Scholar] [CrossRef]
- Vilela, C.; Martins, A.P.C.; Sousa, N.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Poly(bis[2-(methacryloyloxy)ethyl] phosphate)/bacterial cellulose nanocomposites: Preparation, characterization and application as polymer electrolyte membranes. Appl. Sci. 2018, 8, 1145. [Google Scholar] [CrossRef]
- Zakuwan, S.Z.; Ahmad, I. Synergistic Effect of Hybridized Cellulose Nanocrystals and Organically Modified Montmorillonite on κ-Carrageenan Bionanocomposites. Nanomaterials 2018, 8, 874. [Google Scholar] [CrossRef] [PubMed]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef] [PubMed]
- Oksmana, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Comp. Part A 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Jabbour, L.; Destro, M.; Gerbaldi, C.; Chaussy, D.; Penazzi, N.; Beneventi, D. Aqueous processing of cellulose based paper-anodes for flexible Li-ion batteries. J. Mater. Chem. 2012, 22, 3227–3233. [Google Scholar] [CrossRef]
- Alloin, F.; D’Aprea, A.; El Kissi, N.; Dufresne, A.; Bossard, F. Nanocomposite polymer electrolyte based on whisker or microfibrils polyoxyethylene nanocomposites. Electrochim. Acta 2010, 55, 5186–5194. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Q.; Liu, L.; Xu, J.; Yan, M.; Jiang, Z. A novel and facile route of ink-jet printing to thin film SnO2 anode for rechargeable lithium ion batteries. Electrochim. Acta 2006, 51, 2639–2645. [Google Scholar] [CrossRef]
- Buqa, H.; Holzapfel, M.; Krumeich, F.; Veit, C.; Novák, P. Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J. Power Sources 2006, 161, 617–622. [Google Scholar] [CrossRef]
- Jabbour, L.; Gerbaldi, C.; Chaussy, D.; Zeno, E.; Bodoardo, S.; Beneventi, D. Microfibrillated cellulose–graphite nanocomposites for highly flexible paper-like Li-ion battery electrodes. J. Mater. Chem. 2010, 20, 7344–7347. [Google Scholar] [CrossRef]
- El Baradai, O.; Beneventi, D.; Alloin, F.; Bongiovanni, R.; Reverdy, N.; Bultel, Y. Microfibrillated cellulose based ink for eco-sustainable screen printed flexible electrodes in lithium ion batteries. J. Mater. Sci. Technol. 2016, 32, 566–572. [Google Scholar] [CrossRef]
- Ohta, S.; Komagata, S.; Seki, J.; Saeki, T.; Morishita, S.; Asaoka, T. All-solid-state lithium ion battery using garnet-type oxide and Li3BO3 solid electrolytes fabricated by screen-printing. J. Power Sources 2013, 238, 53–56. [Google Scholar] [CrossRef]
- Zhou, G.; Li, F.; Cheng, H.-M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Pu, W.; Li, J.; Wan, C. Effect of slurry preparation and dispersion on electrochemical performances of LiFePO4 composite electrode. Ionics 2011, 17, 473–477. [Google Scholar] [CrossRef]
- Lee, G.-W.; Ryu, J.H.; Han, W.; Ahn, K.H.; Oh, S.M. Effect of slurry preparation process on electrochemical performances of LiCoO2 composite electrode. J. Power Sources 2010, 195, 6049–6054. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-S.; Kim, Y.C.; Zang, D.S.; Paik, U. Dispersion properties of aqueous-based LiFePO4 pastes and their electrochemical performance for lithium batteries. Ultramicroscopy 2008, 108, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, Z.; Korochkina, T.; Govindarajan, S.; Thomas, D.J.; O’Mahony, J.; Kettle, J.; Claypole, T.C.; Gethin, D.T. Ultra-thin flexible screen printed rechargeable polymer battery for wearable electronic applications. Organ. Electron. 2015, 26, 386–394. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Chu, H.N.; Qeraj, R.; Zamarayeva, A.M.; Steingart, D.A. Reinforced Electrode Architecture for a Flexible Battery with Paperlike Characteristics. Energy Technol. 2013, 1, 177–185. [Google Scholar] [CrossRef]
- Song, Z.; Ma, T.; Tang, R.; Cheng, Q.; Wang, X.; Krishnaraju, D.; Panat, R.; Chan, C.K.; Yu, H.; Jiang, H. Origami lithium-ion batteries. Nat. Commun. 2014, 5, 3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, V.; Tapin-Lingua, S.; Da Silva Perez, D.; Arndt, T.; Kautto, J. Technical opportunities and economic challenges to produce nanofibrillated cellulose in pilot scale: NFC delivery for applications in demonstrations trials. Presented at the SUNPAP EU Project—Final Conference, Milan, Italy, 19–20 June 2012; Available online: http://sunpap.vtt.fi/finalconference2012.htm (accessed on 12 November 2018).
- Ghiurcan, G.A.; Liu, C.-C.; Webber, A.; Feddrix, F.H. Development and Characterization of a Thick-Film Printed Zinc-Alkaline Battery. J. Electrochem. Soc. 2003, 150, A922–A927. [Google Scholar] [CrossRef]
- Flandrois, S.; Simon, B. Carbon materials for lithium-ion rechargeable batteries. Carbon 1999, 37, 165–180. [Google Scholar] [CrossRef]
- Saarinen, T.; Lille, M.; Seppälä, J. Technical aspects on rheological characterization of microfibrillar cellulose water suspensions. Annu. Trans. Nord. Rheol. Soc. 2009, 17, 121–128. [Google Scholar]
- Yuan, L.-X.; Wang, Z.-H.; Zhang, W.-X.; Hu, X.-L.; Chen, J.-T.; Huang, Y.-H. Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ. Sci. 2011, 4, 269–284. [Google Scholar] [CrossRef]
- Kipphan, H. Handbook of Print Media; Springer: Berlin, Germany, 2001. [Google Scholar]
- Agoda-Tandjawa, G.; Durand, S.; Berot, S.; Blassel, C.; Gaillard, C.; Garnier, C. Rheological characterization of microfibrillated cellulose suspensions after freezing. Carbohydr. Polym. 2010, 80, 677–686. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-S.; Kim, Y.C.; Zang, D.S.; Choi, Y.-M.; Park, W.I. Effect of Carboxymethyl Cellulose on Aqueous Processing of LiFePO4 Cathodes and Their Electrochemical Performance. Electrochem. Solid-State Lett. 2008, 11, A175–A179. [Google Scholar] [CrossRef]
- Chinga, G.; Helle, T. Relationships between the coating surface structural variation and print quality.pdf. J. Pulp Pap. Sci. 2003, 29, 179–184. [Google Scholar]
- Chang, C.-C.; Her, L.-J.; Su, H.-K.; Hsu, S.-H.; Te Yen, Y. Effects of Dispersant on the Conductive Carbon for LiFePO4 Cathode. J. Electrochem. Soc. 2011, 158, A481–A486. [Google Scholar] [CrossRef]
- Rotureau, D.; Viricelle, J.-P.; Pijolat, C.; Caillol, N.; Pijolat, M. Development of a planar SOFC device using screen-printing technology. J. Eur. Ceram. Soc. 2005, 25, 2633–2636. [Google Scholar] [CrossRef]
- Manev, V.; Naidenov, I.; Puresheva, B.; Pistoia, G. Effect of electrode porosity on the performance of natural Brazilian graphite electrodes. J. Power Sources 1995, 57, 133–136. [Google Scholar] [CrossRef]
- Levi, M.D.; Aurbach, D. The mechanism of lithium intercalation in graphite film electrodes in aprotic media. Part 1. High resolution slow scan rate cyclic voltammetric studies and modeling. J. Electroanal. Chem. 1997, 421, 79–88. [Google Scholar] [CrossRef]
- Chen, Z.; Christensen, L.; Dahn, J.R. Large volume-change electrodes for Li-ion batteries of amorphous alloy particles held by elastomeric tethers. Electrochem. Commun. 2003, 5, 919–923. [Google Scholar] [CrossRef]
- Wang, L.; Hong, J.; Chen, G. Comparison study of graphite nanosheets and carbon black as fillers for high density polyethylene. Polym. Eng. Sci. 2010, 50, 2176–2181. [Google Scholar] [CrossRef]
- Tjong, S.G. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Hochun Lee, H.; Choi, S.; Choi, S.; Kim, H.-J.; Choi, Y.; Yoon, S.; Cho, J.-J. SEI layer-forming additives for LiNi0.5Mn1.5O4/graphite5 V Li-ion batteries. Electrochem. Commun. 2007, 9, 801–806. [Google Scholar] [CrossRef]
- Leijonmarck, S.; Cornell, A.; Lindbergh, G.; Wågberg, L. Single-paper flexible Li-ion battery cells through a paper-making process based on nano-fibrillated cellulose. J. Mater. Chem. A 2013, 1, 4671–4677. [Google Scholar] [CrossRef]
| Sample | Comp. | Loading | Thickness | Porosity | Electrical Conductivity |
|---|---|---|---|---|---|
| Weight (%) | g/m2 | µm | % | S/m | |
| Negative electrode (GP_CMC_CNF) | (97_2_1) | 25 ± 2 | 34 ± 3 | 70 ± 3 | 268 ± 69 |
| Separator/substrate | cellulose | 120 ± 1 | 78 ± 2 | 47 ± 1 | - |
| Positive electrode (LFP_CB_CMC_CNF) | (70_27_2_1) | 70 ± 6 | 79 ± 7 | 69 ± 5 | 37 ± 6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Baradai, O.; Beneventi, D.; Alloin, F.; Bultel, Y.; Chaussy, D. Use of Cellulose Nanofibers as an Electrode Binder for Lithium Ion Battery Screen Printing on a Paper Separator. Nanomaterials 2018, 8, 982. https://doi.org/10.3390/nano8120982
El Baradai O, Beneventi D, Alloin F, Bultel Y, Chaussy D. Use of Cellulose Nanofibers as an Electrode Binder for Lithium Ion Battery Screen Printing on a Paper Separator. Nanomaterials. 2018; 8(12):982. https://doi.org/10.3390/nano8120982
Chicago/Turabian StyleEl Baradai, Oussama, Davide Beneventi, Fannie Alloin, Yann Bultel, and Didier Chaussy. 2018. "Use of Cellulose Nanofibers as an Electrode Binder for Lithium Ion Battery Screen Printing on a Paper Separator" Nanomaterials 8, no. 12: 982. https://doi.org/10.3390/nano8120982





