Rapid Tartrazine Determination in Large Yellow Croaker with Ag Nanowires Using Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation and Characterization of Ag Nanowires
2.3. Preparation of Standard Solution
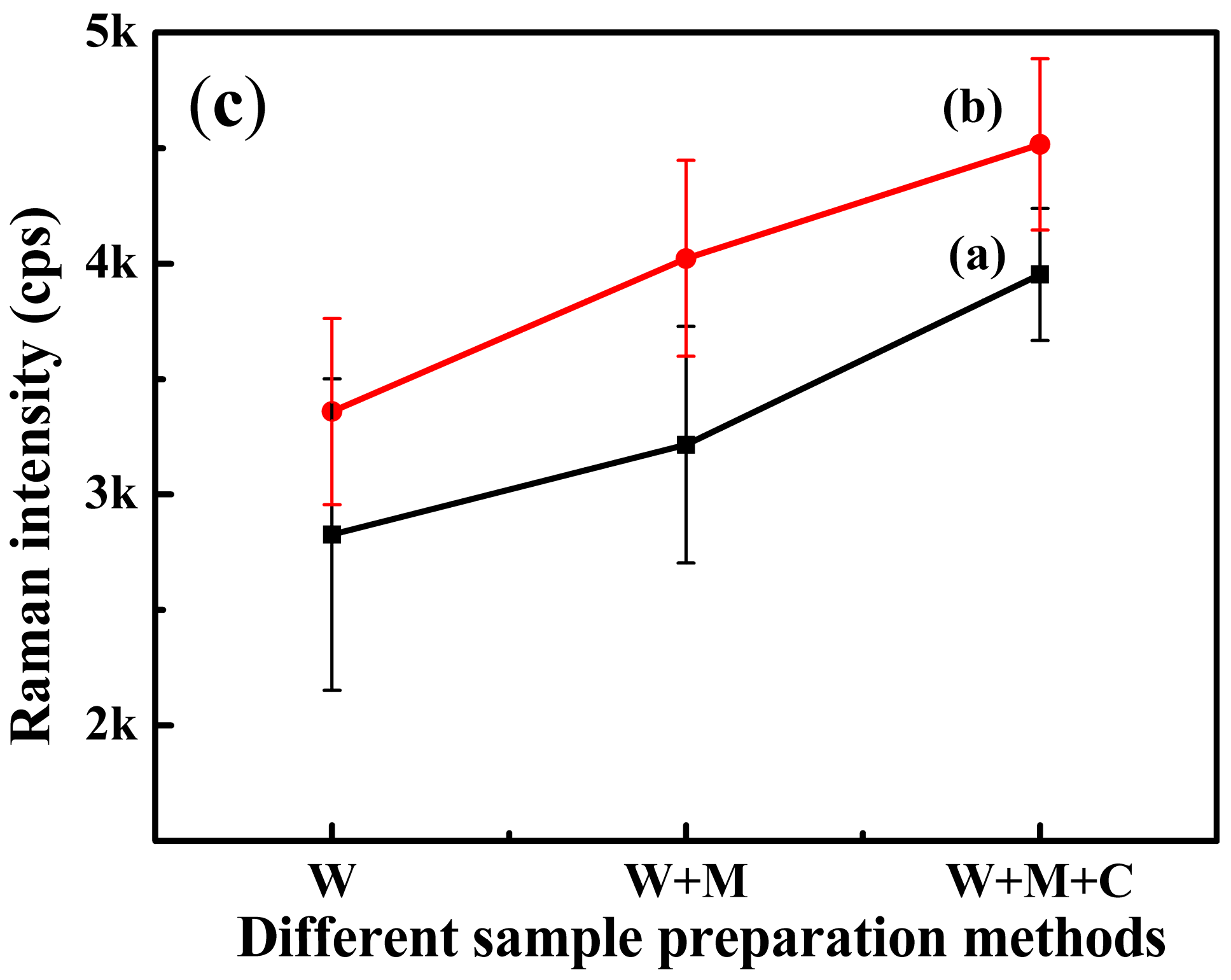
2.4. Pretreatment of Large Yellow Croaker Sample
2.5. Raman and SERS Measurements
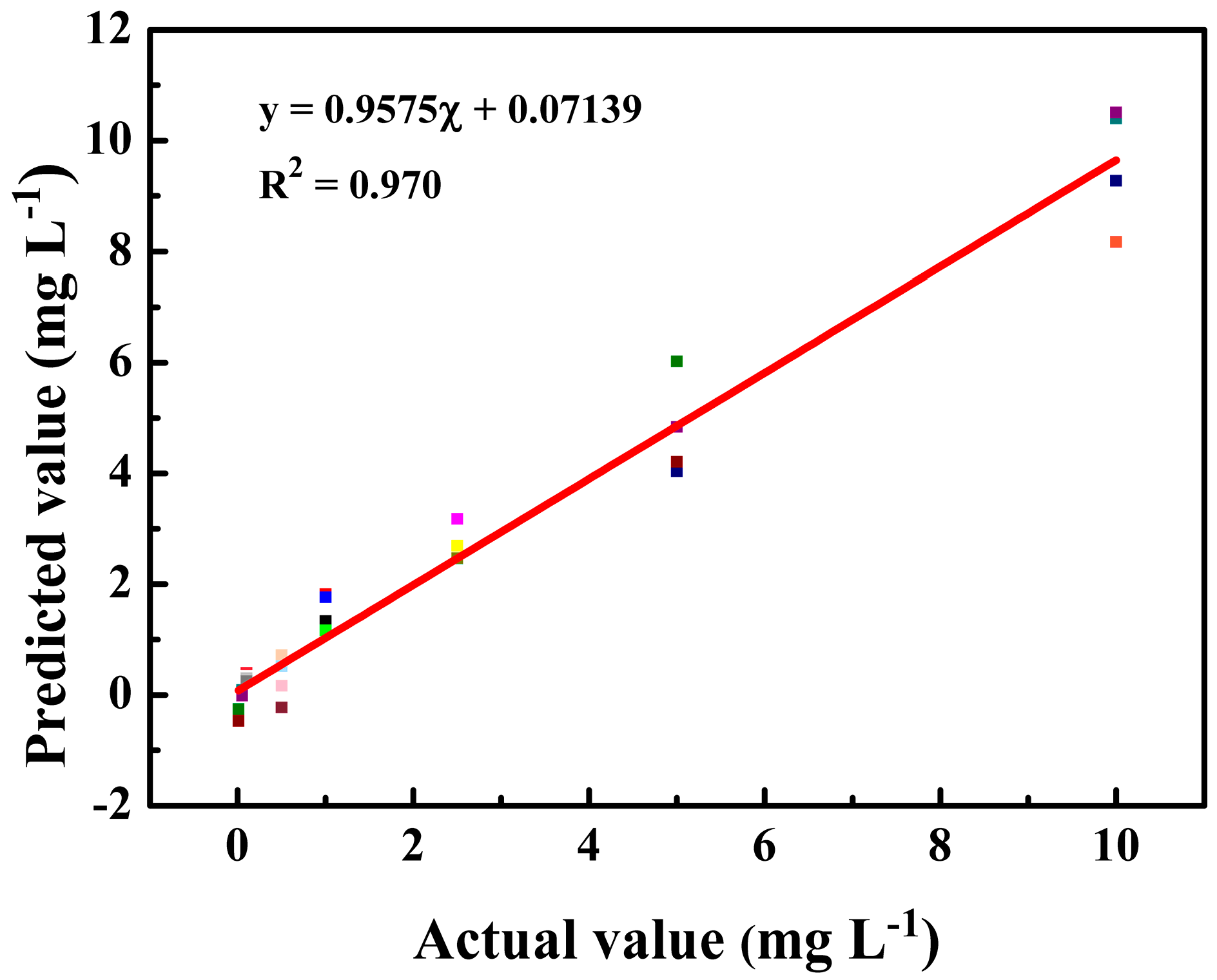
2.6. Data Analysis
3. Results
3.1. Spectral Features of Tartrazine Dye
3.2. Ag Nanowire Characterization
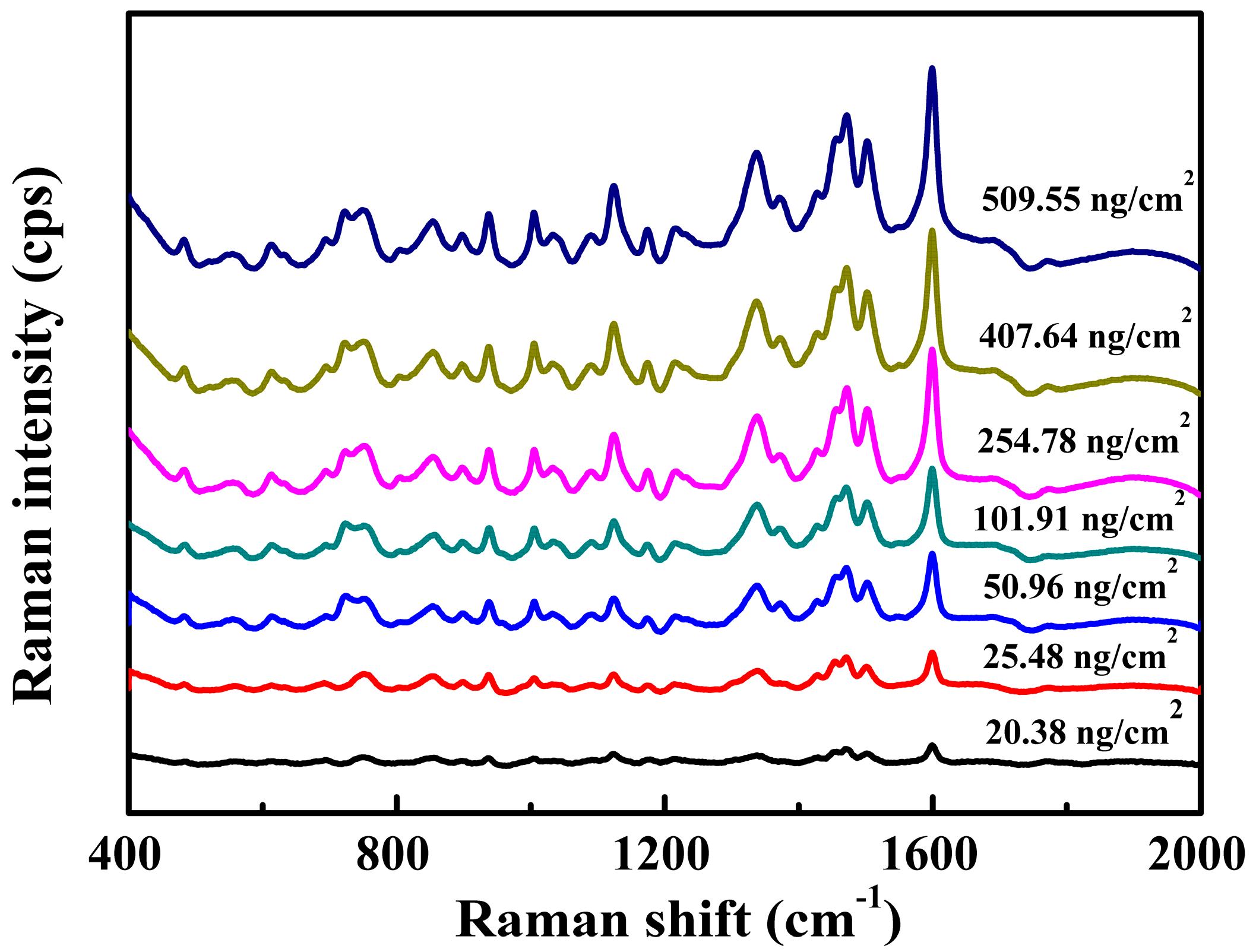
3.3. SERS Analysis of Tartrazine
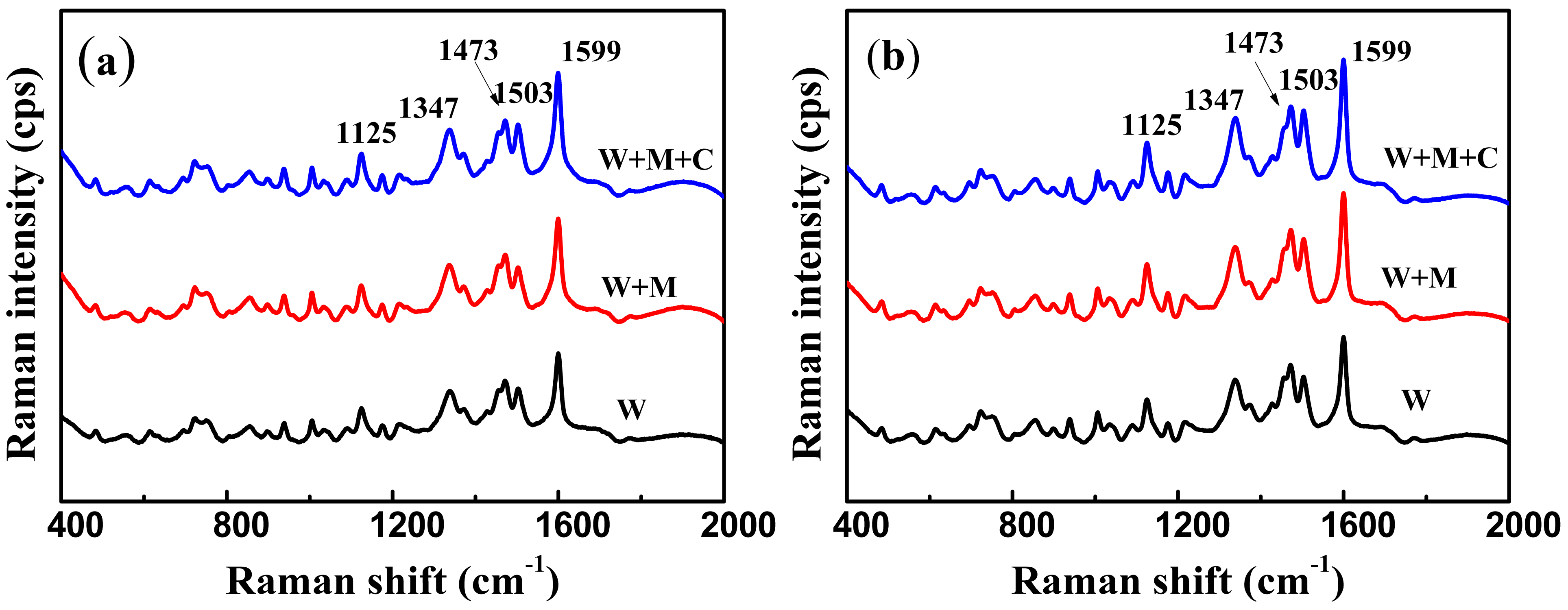
3.4. SERS Analysis of Tartrazine in Large Yellow Croaker
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Llamas, N.E.; Garrido, M.; Di Nezio, M.S.; Fernandez Band, B.S. Second order advantage in the determination of amaranth, sunset yellow FCF and tartrazine by UV-vis and multivariate curve resolution-alternating least squares. Anal. Chim. Acta 2009, 655, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Jain, R.; Nayak, A.; Agarwal, S.; Shrivastava, M. Removal of the hazardous dye—Tartrazine by photodegradation on titanium dioxide surface. Mater. Sci. Eng. C 2011, 31, 1062–1067. [Google Scholar] [CrossRef]
- Rowe, K.S.; Rowe, K.J. Synthetic food coloring and behavior: A dose response effect in a double-blind, placebo-controlled, repeated-measures study. J. Pediatr. 1994, 125, 691–698. [Google Scholar] [CrossRef]
- Guyot, S.; Serrand, S.; Le Quéré, J.M.; Sanoner, P.; Renard, C.M.G.C. Enzymatic synthesis and physicochemical characterisation of phloridzin oxidation products (POP), a new water-soluble yellow dye deriving from apple. Innov. Food Sci. Emerg. 2007, 8, 443–450. [Google Scholar] [CrossRef]
- National Food Safety Standard: Standards for the Uses of Food Additives (GB2760-2014). Available online: http://www.nhfpc.gov.cn/zwgkzt/cybz/201412/d9a9f04bc35f42ecac0600e0360f8c89.shtml (accessed on 7 August 2018).
- A List of Non-Edible Substances that May Be Added Illegally and Food Additives that May Be Abused (Summary of Batches 1–5). Available online: http://www.nhfpc.gov.cn/sps/s7892/201406/38e5c8a53615486888d93ed05ac9731a.shtml (accessed on 7 August 2018).
- Elena, D.; Cornelia, P.E. Study of analytical parameters of the HPLC method for tartrazine and sunset yellow analysis in soft drinks. Rev. Chim. Buchar. 2010, 61, 1177–1182. [Google Scholar]
- Chen, X.; Zhao, Y.; Shen, H.; Zhou, L.; Pan, S.; Jin, M. Fast determination of seven synthetic pigments from wine and soft drinks using magnetic dispersive solid-phase extraction followed by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1346, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.M.S.; Hashem, E.Y.; Al-Salahi, N.O.A. Oxidation and complexation-based spectrophotometric methods for sensitive determination of tartrazine E102 in some commercial food samples. Comput. Chem. 2016, 4, 51–64. [Google Scholar] [CrossRef]
- Gomez, M.; Arancibia, V.; Rojas, C.; Nagles, E. Adsorptive stripping voltammetric determination of tartrazine and sunset yellow in gelatins and soft drink powder in the presence of cetylpyridinium bromide. Int. J. Electrochem. Sci. 2012, 7, 7493–7502. [Google Scholar]
- De Andrade, F.I.; Guedes, M.I.F.; Vieira, Í.G.P.; Mendes, F.N.P.; Rodrigues, P.A.S.; Maia, C.S.C.; Ávila, M.M.M.; Ribeiro, L.d.M. Determination of synthetic food dyes in commercial soft drinks by TLC and ion-pair HPLC. Food Chem. 2014, 157, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Pei, L.; Lai, K.; Huang, Y.; Rasco, B.A.; Wang, X.; Fan, Y. Rapid analysis of multiple Sudan dyes in chili flakes using surface-enhanced Raman spectroscopy coupled with Au–Ag core-shell nanospheres. Food Anal. Methods 2017, 10, 565–574. [Google Scholar] [CrossRef]
- Xu, M.; Gao, Y.; Han, X.X.; Zhao, B. Detection of pesticide residues in food using surface-enhanced Raman spectroscopy: A review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Analyses of phosmet residues in apples with surface-enhanced Raman spectroscopy. Food Control 2014, 37, 153–157. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, P.; Liu, X.; Sun, X.; Li, H.; Lin, M. Detection of pesticides in fruits by surface-enhanced Raman spectroscopy coupled with gold nanostructures. Food Bioprocess Technol. 2013, 6, 710–718. [Google Scholar] [CrossRef]
- Luo, H.; Huang, Y.; Lai, K.; Rasco, B.A.; Fan, Y. Surface-enhanced Raman spectroscopy coupled with gold nanoparticles for rapid detection of phosmet and thiabendazole residues in apples. Food Control 2016, 68, 229–235. [Google Scholar] [CrossRef]
- Futamata, M.; Yu, Y.; Yajima, T. Elucidation of electrostatic interaction between cationic dyes and Ag nanoparticles generating enormous SERS enhancement in aqueous solution. J. Phys. Chem. C 2011, 115, 5271–5279. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhai, F.; Du, R.; Liu, Y.; Lai, K. Analyses of enrofloxacin, furazolidone and malachite green in fish products with surface-enhanced Raman spectroscopy. Food Chem. 2012, 135, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Liang, P.; Wu, Y.; Dong, Q.; Li, J.; Bai, Y.; Xu, B.; Yu, Z.; Ni, D. Rapid qualitative and quantitative determination of food colorants by both Raman spectra and surface-enhanced Raman scattering (SERS). Food Chem. 2018, 241, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Saviello, D.; Di Gioia, A.; Turenne, P.-I.; Trabace, M.; Giorgi, R.; Mirabile, A.; Baglioni, P.; Iacopino, D. Handheld su31rface-enhanced Raman scattering identification of dye chemical composition in felt-tip pen drawings. J. Raman Spectrosc. 2018. [Google Scholar] [CrossRef]
- Saviello, D.; Trabace, M.; Alyami, A.; Mirabile, A.; Giorgi, R.; Baglioni, P.; Iacopino, D. A combined surface enhanced Raman spectroscopy (SERS)/UV-vis approach for the investigation of dye content in commercial felt tip pens inks. Talanta 2018, 181, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Qin, S.; Zhang, L.; Yang, L. Designing of a novel gold nanodumbbells SERS substrate for detection of prohibited colorants in drinks. Appl. Surf. Sci. 2016, 366, 181–186. [Google Scholar] [CrossRef]
- Song, J.; Huang, Y.; Fan, Y.; Zhao, Z.; Yu, W.; Rasco, B.; Lai, K. Detection of prohibited fish drugs using silver nanowires as substrate for surface-enhanced Raman scattering. Nanomaterials 2016, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cavinato, A.G.; Mayes, D.M.; Bledsoe, G.E.; Rasco, B.A. Nondestructive prediction of moisture and sodium chloride in cold smoked Atlantic salmon (Salmo salar). J. Food Sci. 2002, 67, 2543–2547. [Google Scholar] [CrossRef]
- Peica, N.; Pavel, I.; Pînzaru, S.C.; Rastogi, V.K.; Kiefer, W. Vibrational characterization of E102 food additive by Raman and surface-enhanced Raman spectroscopy and theoretical studies. J. Raman Spectrosc. 2005, 36, 657–666. [Google Scholar] [CrossRef]
- Gao, Y.; Song, L.; Jiang, P.; Liu, L.F.; Yan, X.Q.; Zhou, Z.P.; Liu, D.F.; Wang, J.X.; Yuan, H.J.; Zhang, Z.X.; et al. Silver nanowires with five-fold symmetric cross-section. J. Cryst. Growth 2005, 276, 606–612. [Google Scholar] [CrossRef]
- Sha, O.; Zhu, X.; Feng, Y.; Ma, W. Determination of sunset yellow and tartrazine in food samples by combining ionic liquid-based aqueous two-phase system with high performance liquid chromatography. J. Anal. Methods Chem. 2014, 2014, 964273. [Google Scholar] [CrossRef] [PubMed]
- Seney, C.S.; Gutzman, B.M.; Goddard, R.H. Correlation of size and surface-enhanced Raman scattering activity of optical and spectroscopic properties for silver nanoparticles. J. Phys. Chem. C 2009, 113, 74–80. [Google Scholar] [CrossRef]
- Nardou, E.; Vouagner, D.; Jurdyc, A.M.; Berthelot, A.; Pillonnet, A.; Sablonière, V.; Bessueille, F.; Champagnon, B. Surface enhanced Raman scattering in an amorphous matrix for Raman amplification. J. Non-Cryst. Solids 2011, 357, 1895–1899. [Google Scholar] [CrossRef]
- Ru, E.C.L.; Blackie, E.; Meyer, M.; Etchegoint, P.G. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar]
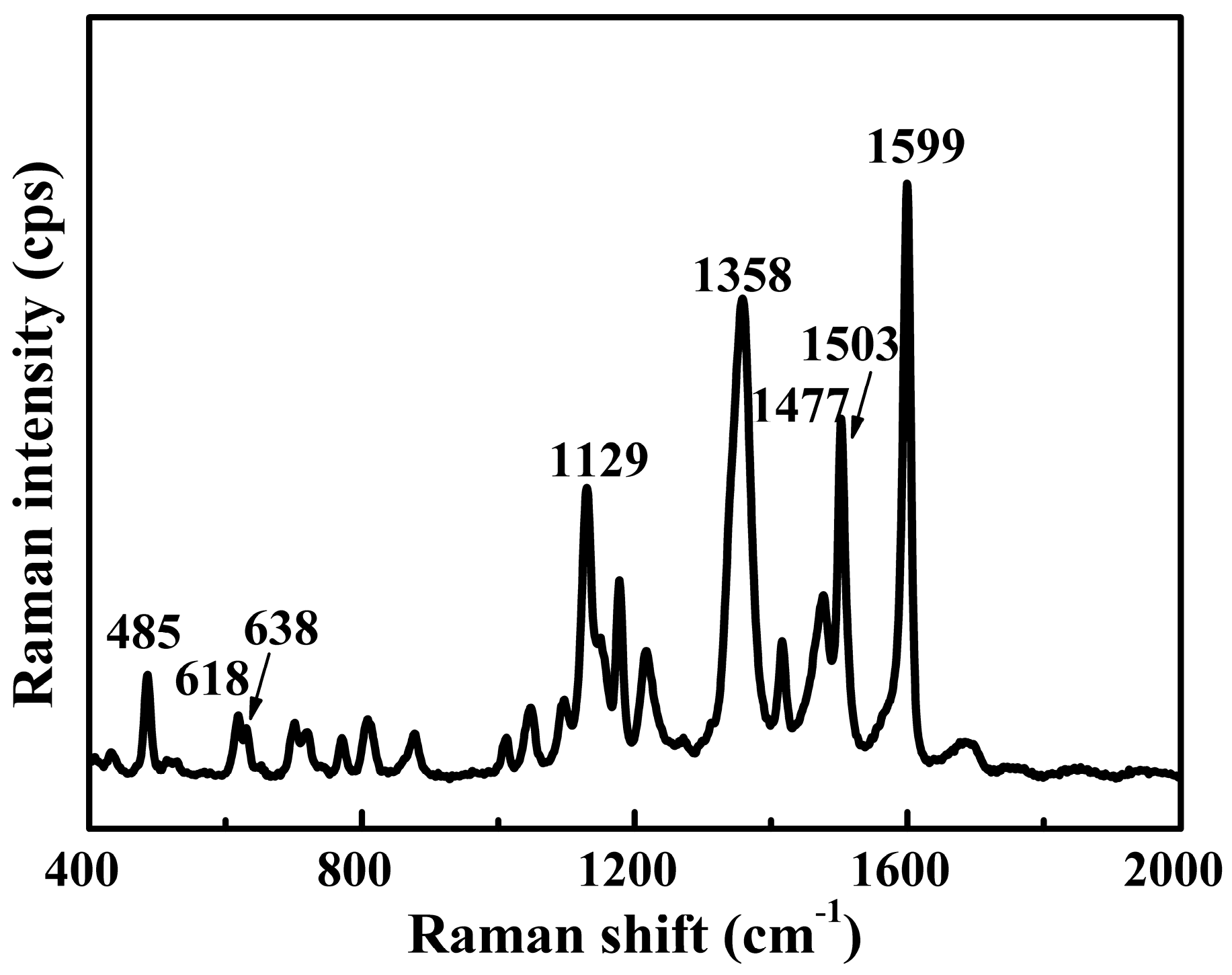

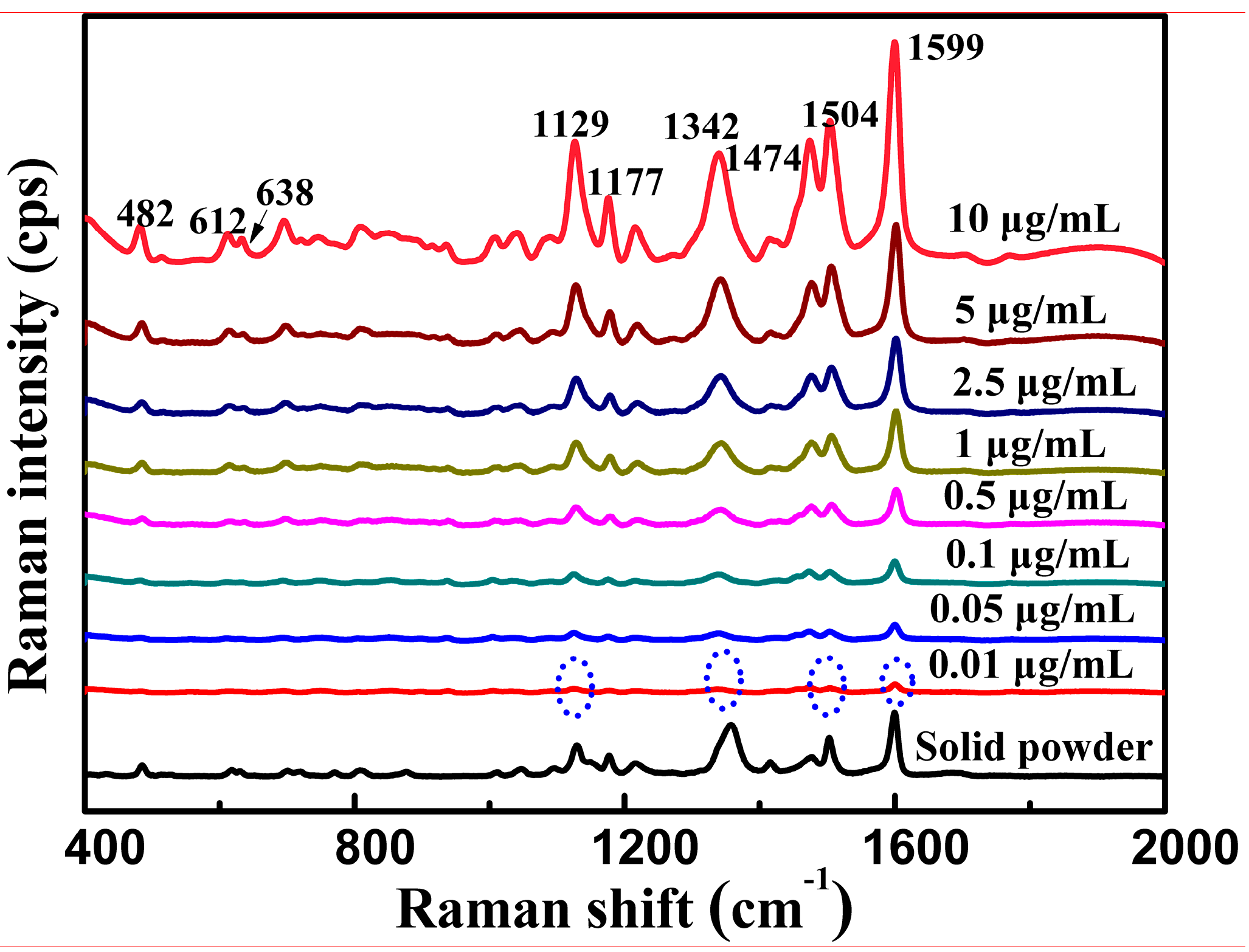
| Raman Shift (cm−1) | Vibrational Assignment |
|---|---|
| 485 | ρ (SO3−), Defop (C–H)ph1, pyr |
| 618 | Defop (C–H)ph1, ph2, δ (pyr) |
| 638 | Defop (ph1, ph2) |
| 1129 | Defop (C–H)ph1, ph2, δ (ph1) |
| 1358 | υ (–C–N=N–C–), υs (COO−), ρ (ph2) |
| 1477 | δ (C–H)ph1, ph2, υas (N=N–C) |
| 1503 | δ (ph2), δ (C=C) |
| 1599 | υ (ph1, ph2), Defop (C–H)ph1, ph2, δ (OH), υas (COO−) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Zhang, Y.; Huang, Y.; Fan, Y.; Lai, K. Rapid Tartrazine Determination in Large Yellow Croaker with Ag Nanowires Using Surface-Enhanced Raman Spectroscopy. Nanomaterials 2018, 8, 967. https://doi.org/10.3390/nano8120967
Song J, Zhang Y, Huang Y, Fan Y, Lai K. Rapid Tartrazine Determination in Large Yellow Croaker with Ag Nanowires Using Surface-Enhanced Raman Spectroscopy. Nanomaterials. 2018; 8(12):967. https://doi.org/10.3390/nano8120967
Chicago/Turabian StyleSong, Jia, Yuanyi Zhang, Yiqun Huang, Yuxia Fan, and Keqiang Lai. 2018. "Rapid Tartrazine Determination in Large Yellow Croaker with Ag Nanowires Using Surface-Enhanced Raman Spectroscopy" Nanomaterials 8, no. 12: 967. https://doi.org/10.3390/nano8120967




