AuNPs-Based Thermoresponsive Nanoreactor as an Efficient Catalyst for the Reduction of 4-Nitrophenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
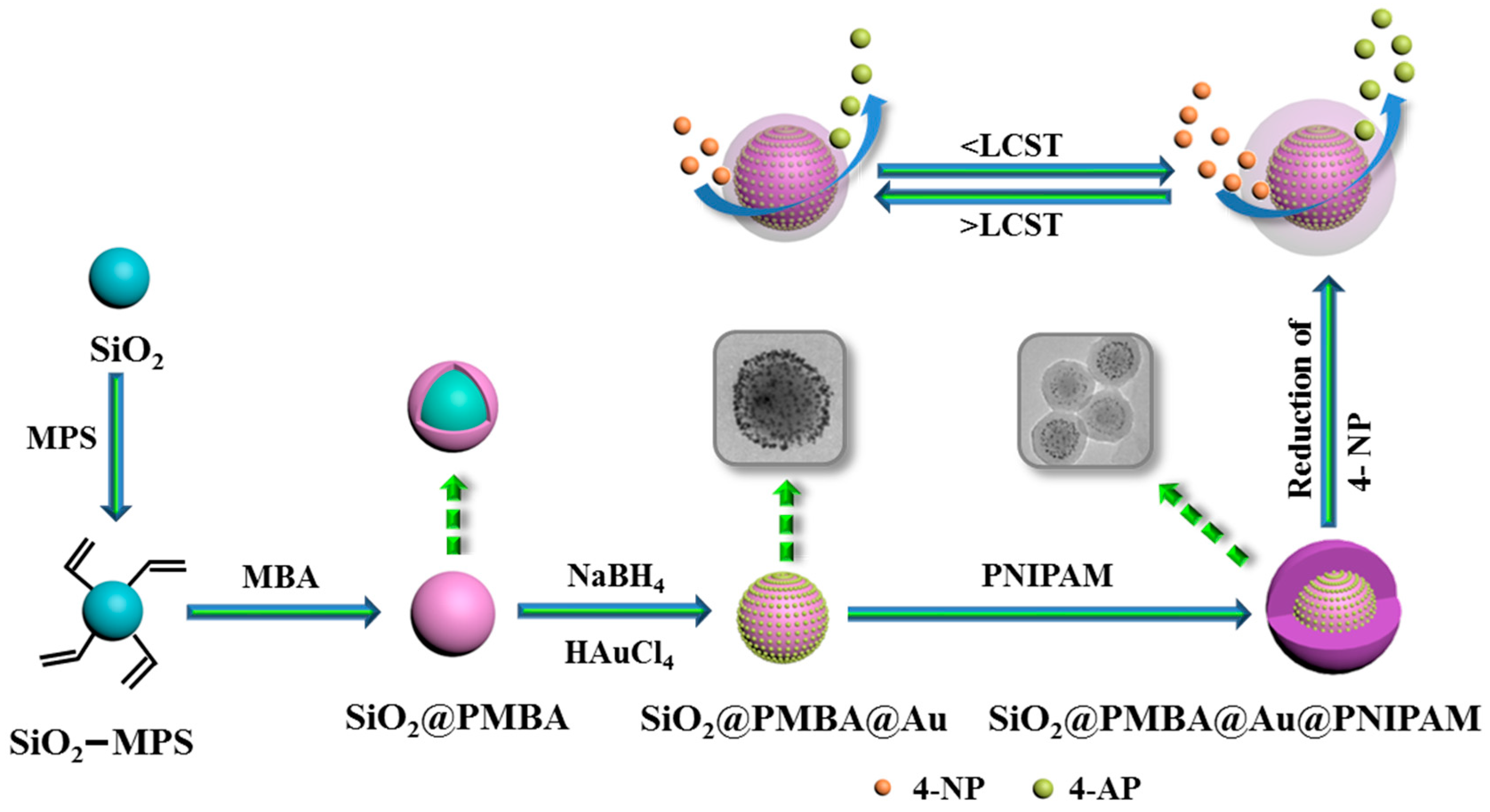
2.2.1. Preparation of MPS-Modified SiO2 Microspheres
2.2.2. Preparation of SiO2@PMBA Core-Shell Microspheres
2.2.3. Preparation of SiO2@PMBA@Au microspheres
2.2.4. Preparation of Thermosensitive SiO2@PMBA@Au@PNIPAM Microspheres
2.3. Characterization
2.4. Catalytic Activity
3. Results and Discussion
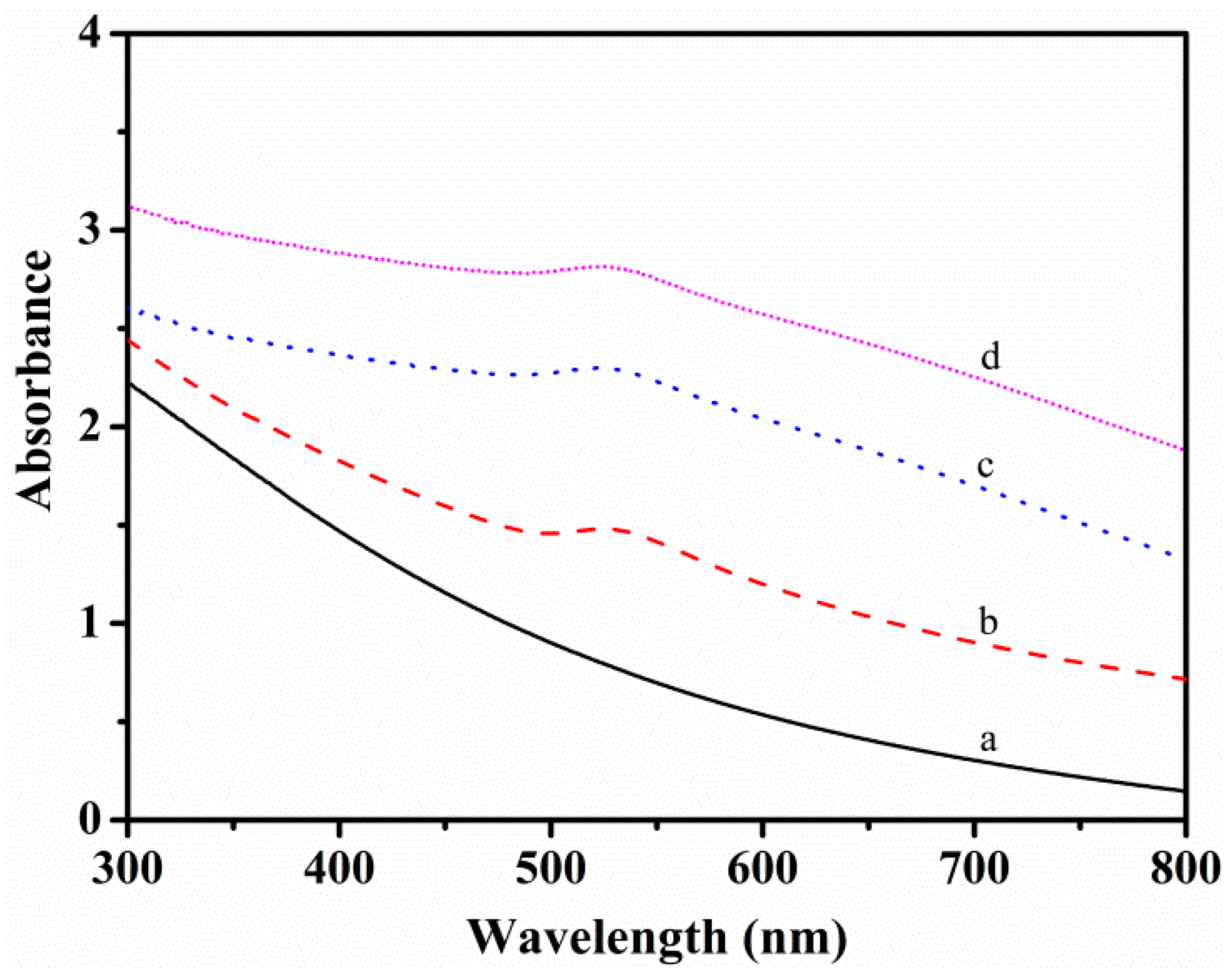
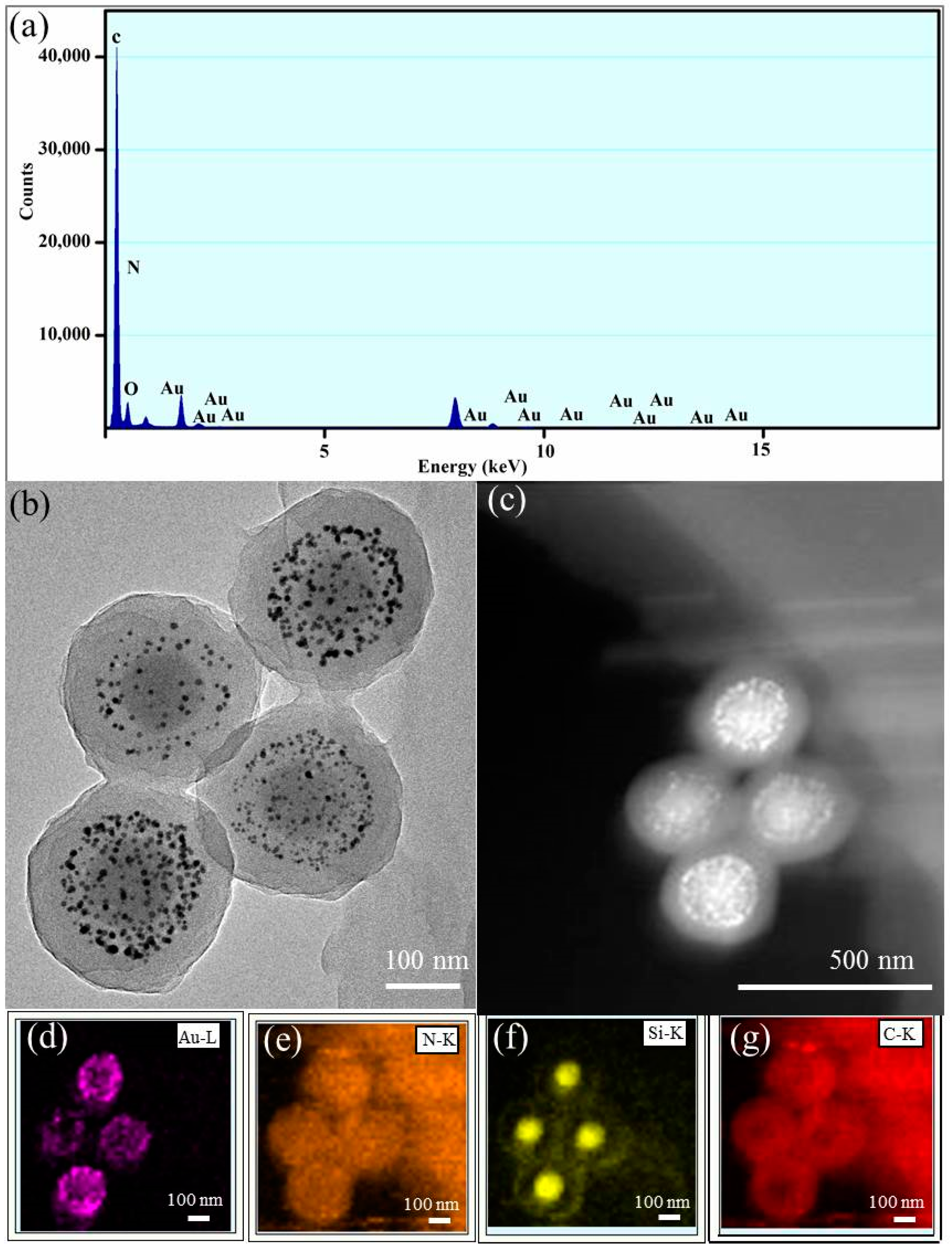
3.1. Preparation and Characterization of the SiO2@PMBA@Au@PNIPAM Microspheres
3.2. The Interaction Nature Between Au NPs and Functional Groups in the New Nanoreactor
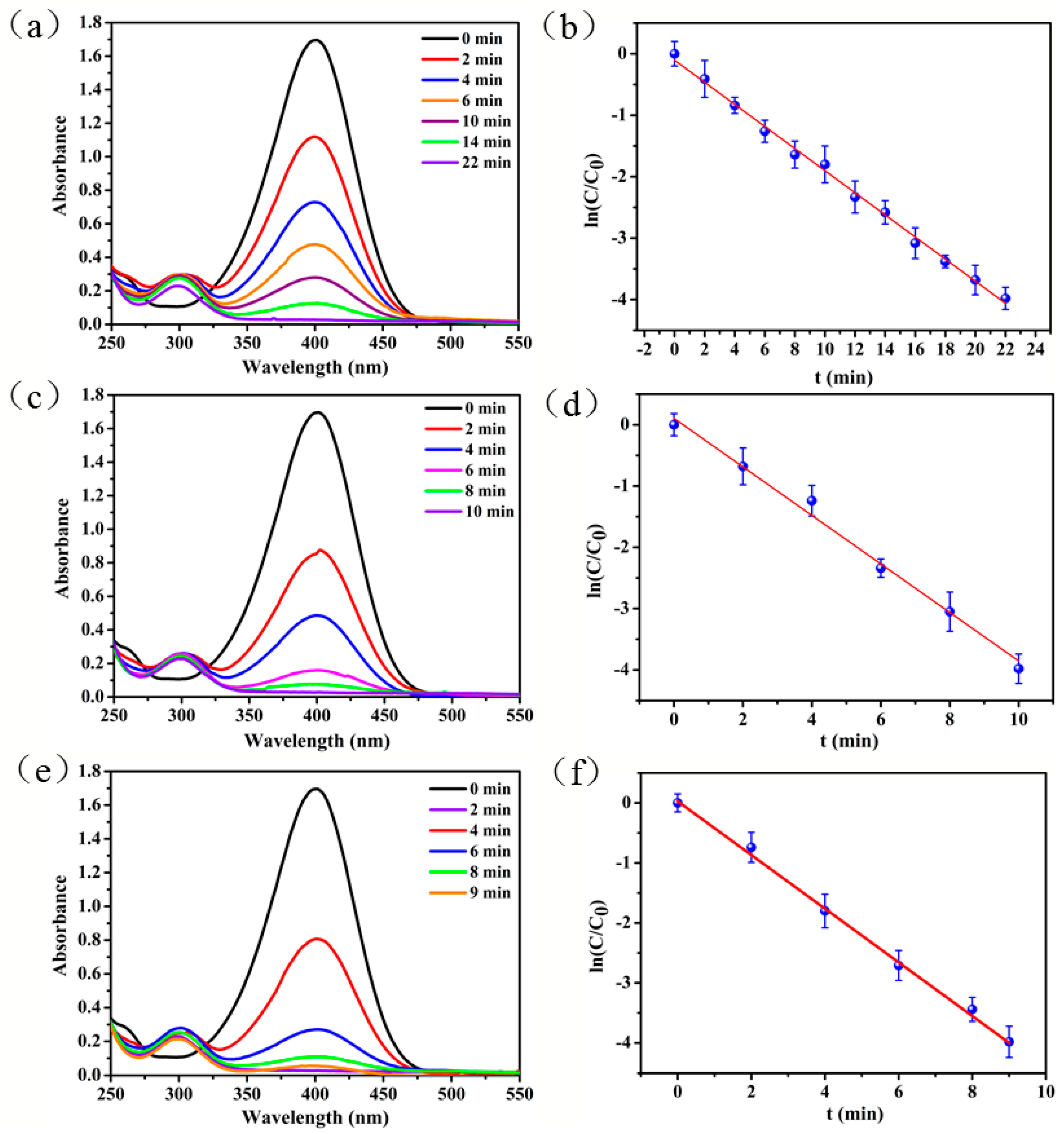
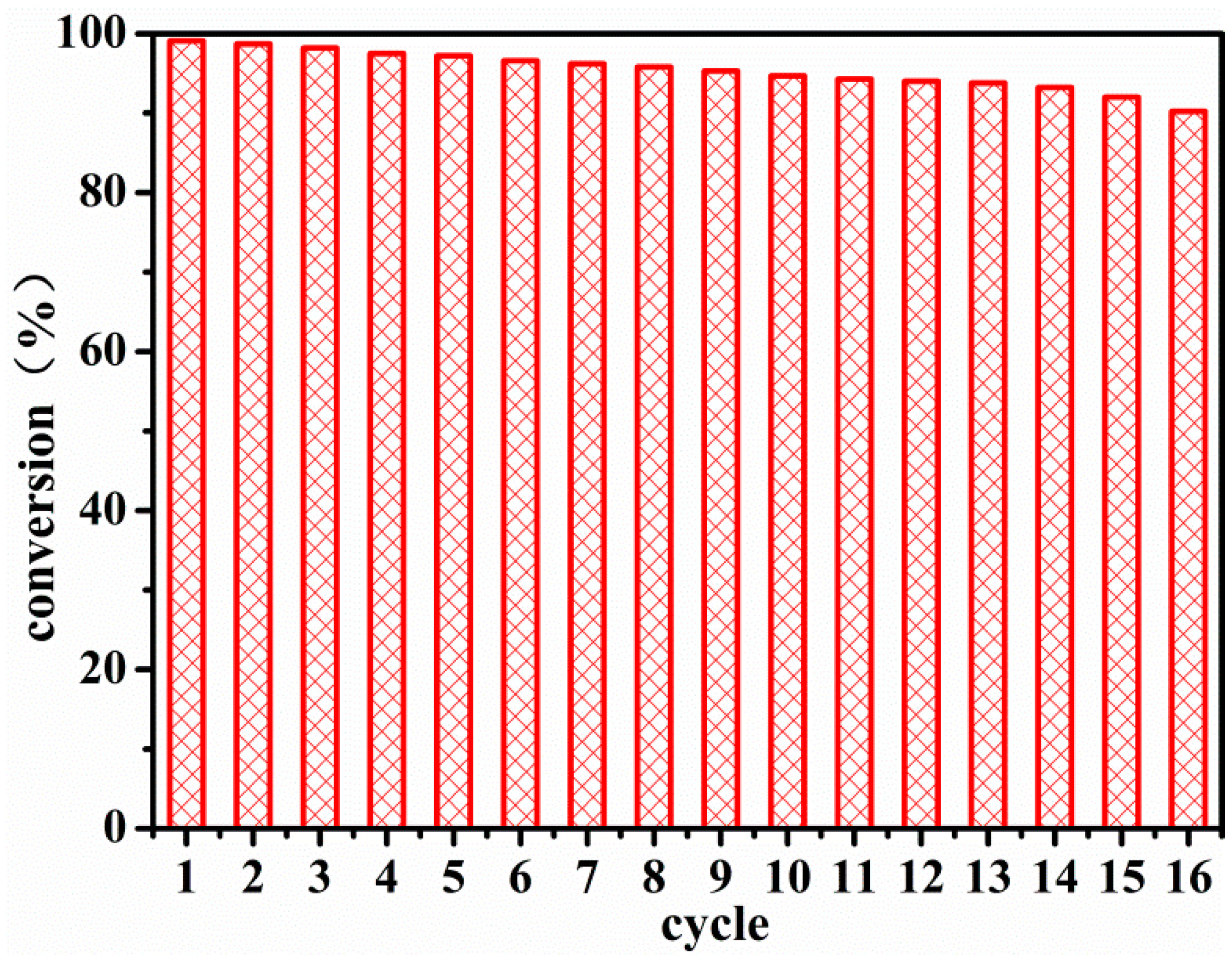
3.3. Catalytic Activity and Thermoresponsive Property of the New Nanoreactor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, Y.Q.; Zhu, M.Y.; Wang, X.T.; Wu, Y.N.; Huang, C.Q.; Fu, W.L.; Meng, X.; Sun, Y.M. Visible-light promoted catalytic activity of dumbbell-like Au nanorods supported on graphene/TiO2 sheets towards hydrogenation reaction. Nanotechnology 2018, 29, 245703. [Google Scholar] [CrossRef] [PubMed]
- Laksee, S.; Puthong, S.; Kongkavitoon, P.; Palaga, T.; Muangsin, N. Facile and green synthesis of pullulan derivative-stabilized Au nanoparticles as drug carriers for enhancing anticancer activity. Carbohydr. Polym. 2018, 198, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak-Silicka, M.; Trukawka, M.; Dudziak, M.; Piotrowska, K.; Mijowska, E. Hexagonal boron nitride functionalized with Au nanoparticles-properties and potential biological applications. Nanomaterials 2018, 8, 605. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.B.; Zou, F.; Hu, X.J.; Zhu, H.; Koh, K.; Chen, H.X. Analyte induced AuNPs aggregation enhanced surface plasmon resonance for sensitive detection of paraquat. Biosens. Bioelectron. 2018, 117, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Adhyapak, P.; Aiyer, R.; Dugasani, S.R.; Kim, H.U.; Song, C.K.; Vinu, A.; Renugopalakrishnan, V.; Park, S.H.; Kim, T.; Lee, H.; Amalnerkar, D. Thickness-dependent humidity sensing by poly(vinyl alcohol) stabilized Au-Ag and Ag-Au core-shell bimetallic nanomorph resistors. Roy. Soc. Open Sci. 2018, 5, 171986. [Google Scholar] [CrossRef] [PubMed]
- Ertem, E.; Diez-Castellnou, M.; Ong, Q.K.; Stellacci, F. Novel sensing strategies based on monolayer protected gold nanoparticles for the detection of metal ions and small molecules. Chem. Rec. 2018, 18, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.C.; Shu, Y.; Sheng, Y.J.; Zhu, H.Y.; Guo, Y.L.; Wang, L.; Guo, Y.; Zhang, J.S.; Lu, G.Z.; Dai, S. Surfactant-assisted stabilization of Au colloids on solids for heterogeneous catalysis. Angew. Chem. Int. Edit. 2017, 56, 4494–4498. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, C.; Ribot, F.; Peiretti, L.F.; Quaino, P.; Tielens, F.; Sanchez, C.; Chaneac, C.; Portehault, D. Quantified binding scale of competing ligands at the surface of gold nanoparticles: The role of entropy and intermolecular forces. Small 2017, 13, 1604028. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, AI.; Nemzer, S.; Pister, I.; Soussan, L.; Harris, T.; Sun, Y.; Rafailovich, M.H. Size-controlled synthesis and characterization of thiol-stabilized gold nanoparticles. J. Chem. Phys. 2005, 123, 184701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.F.; Jiang, Y.Y. A New Method for Immobilization of Polymer-protective Colloidal Platinum metals via Co-ordination capture with anchored ligands. Synthesis of the first example of a mercapto-containing supported metallic catalyst for hydrogenation of alkenes with high activity. J. Chem. Soc. Chem. Commun. 1989, 1878–1879. [Google Scholar]
- Zhang, W.; Liu, B.; Zhang, B.; Bian, G.M.; Qi, Y.L.; Yang, X.L.; Li, C.X. Synthesis of monodisperse magnetic sandwiched gold nanoparticle as an easily recyclable catalyst with a protective polymer shell. Colloid Surf. A-Physicochem. Eng. Asp. 2015, 466, 210–218. [Google Scholar] [CrossRef]
- Maji, S.; Cesur, B.; Zhang, Z.Y.; De Geest, B.G.; Hoogenboom, R. Poly(N-isopropylacrylamide) coated gold nanoparticles as colourimetric temperature and salt sensors. Polym. Chem. 2016, 7, 1705–1710. [Google Scholar] [CrossRef]
- Kusolkamabot, K.; Sae-ung, P.; Niamnont, N.; Wongravee, K.; Sukwattanasinitt, M.; Hoven, V.P. Poly(N-isopropylacrylamide)-Stabilized Gold Nanoparticles in Combination with Tricationic Branched Phenylene-Ethynylene Fluorophore for Protein Identification. Langmuir 2013, 29, 12317–12327. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Bishnoi, S.W.; Perez-Luna, V.H. Gold Nanoparticles with Poly(N-isopropylacrylamide) formed via surface initiated atom transfer free radical polymerization exhibit unusually slow aggregation kinetics. J. Phys. Chem. C 2010, 114, 5947–5955. [Google Scholar] [CrossRef]
- Satapathy, S.S.; Bhol, P.; Chakkarambath, A.; Mohanta, J.; Samantaray, K.; Bhat, S.K.; Panda, S.K.; Mohanty, P.S.; Si, S. Thermo-responsive PNIPAM-metal hybrids: An efficient nanocatalyst for the reduction of 4-Nitrophenol. Appl. Surf. Sci. 2017, 420, 753–763. [Google Scholar] [CrossRef]
- Chen, Z.; Cui, Z.M.; Cao, C.Y.; He, W.D.; Jiang, L.; Song, W.G. Temperature-responsive smart nanoreactors: poly(N-isopropylacrylamide)-coated Au@mesoporous-SiO2 hollow nanospheres. Langmuir 2012, 28, 13452–13458. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, Y.Y.; Wu, Q.; Yu, H.B.; Zhao, Y.H.; Qu, J.; Huo, M.X.; Yuan, X. Synthesis of Cu2O nanocrystals/TiO2 photonic crystal composite for efficient p-nitrophenol removal. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 539, 291–300. [Google Scholar] [CrossRef]
- Zhao, H.H.; Kong, C.H. Enhanced removal of p-nitrophenol in a microbial fuel cell after long-term operation and the catabolic versatility of its microbial community. Chem. Eng. J. 2018, 339, 424–431. [Google Scholar] [CrossRef]
- Naseer, F.; Ajmal, M.; Bibi, F.; Farooqi, Z.H.; Siddiq, M. Copper and cobalt nanoparticles containing poly(acrylic acid-co-acrylamide) hydrogel composites for rapid reduction of 4-nitrophenol and fast removal of malachite green from aqueous medium. Polym. Composite. 2018, 39, 3187–3198. [Google Scholar] [CrossRef]
- Hasan, Z.; Cho, D.W.; Chon, C.M.; Yoon, K.; Song, H. Reduction of p-nitrophenol by magnetic Co-carbon composites derived from metal organic frameworks. Chem. Eng. J. 2016, 298, 183–190. [Google Scholar] [CrossRef]
- Lv, J.J.; Wang, A.J.; Ma, X.H.; Xiang, R.Y.; Chen, J.R.; Feng, J.J. One-pot synthesis of porous Pt-Au nanodendrites supported on reduced graphene oxide nanosheets toward catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2015, 3, 290–296. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.Y.; Lü, J.H.; Lü, C.L. A facile construction of Au nanoparticles stabilized by thermo-responsive polymer-tethered carbon dots for enhanced catalytic performance. Appl. Surf. Sci. 2018, 454, 181–191. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E.J. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Liu, W.; Li, P.S.; Zhang, H.M. Preparation of thermosensitive microcapsules and application on the controllable drug delivery of ibuprofen. Int. J. Polym. Mater. Polym. 2017, 67, 978–986. [Google Scholar] [CrossRef]
- Matsoukas, T.; Gulari, E. Dynamics of Growth of Silica Particles from Ammonia-Catalyzed Hydrolysis of Tetra-ethyl-orthosilicate. J. Colloid Interface Sci. 1988, 124, 252–261. [Google Scholar] [CrossRef]
- Wu, S.; Kaiser, J.; Drechsler, M.; Ballauff, M.; Lu, Y. Thermosensitive Au-PNIPA yolk-shell particles as "nanoreactors" with tunable optical properties. Colloid Polym. Sci. 2013, 291, 231–237. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, C.; Hu, X.Z.; Xu, W.J. General avenue to multifunctional aqueous nanocrystals stabilized by hyperbranched polyglycerol. Chem. Mater. 2011, 23, 1461–1470. [Google Scholar] [CrossRef]
- Song, T.; Zhou, M.J.; Liu, W.; Bian, G.M.; Qi, Y.L.; Bai, F.; Yang, X.L. Preparation of polymer microspheres with reactive epoxy group and amino groups as stabilizers for gold nanocolloids with recoverable catalysis. Colloid Polym. Sci. 2015, 293, 187–197. [Google Scholar] [CrossRef]
- Liu, W.; Yang, X.L.; Huang, W.Q. Catalytic properties of carboxylic acid functionalized-polymer microsphere-stabilized gold metallic colloids. J. Colloid Interface Sci. 2006, 304, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Li, D.X.; He, Q.; Cui, Y.; Wang, K.W.; Zhang, X.M.; Li, J.B. Thermosensitive copolymer networks modify gold nanoparticles for nanocomposite entrapment. Chem-Eur J. 2007, 13, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Jiao, T.F.; Xing, R.R.; Chen, Y.; Guo, W.C.; Zhou, J.X.; Zhang, L.X.; Peng, Q.M. Hierarchical AuNPs-Loaded Fe3O4/Polymers nanocomposites constructed by electrospinning with enhanced and magnetically recyclable catalytic capacities. Nanomaterials 2017, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Ishida, T.; Haruta, M. Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. J. Mol. Catal. A-Chem. 2009, 298, 7–11. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Liu, S.; Lu, W.B.; Wang, L.; Tian, J.Q.; Sun, X.P. In situ green synthesis of Au nanostructures on graphene oxide and their application for catalytic reduction of 4-nitrophenol. Catal. Sci. Technol. 2011, 1, 1142–1144. [Google Scholar] [CrossRef]
- Marcelo, G.; Lopez-Gonzalez, M.; Mendicuti, F.; Tarazona, M.P.; Valiente, M. Poly(N-isopropylacrylamide)/Gold hybrid hydrogels prepared by catechol redox chemistry. Characterization and smart tunable catalytic activity. Macromolecules 2014, 47, 6028–6036. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhu, X.; Xu, C.; Dai, Z.; Meng, Z. AuNPs-Based Thermoresponsive Nanoreactor as an Efficient Catalyst for the Reduction of 4-Nitrophenol. Nanomaterials 2018, 8, 963. https://doi.org/10.3390/nano8120963
Liu W, Zhu X, Xu C, Dai Z, Meng Z. AuNPs-Based Thermoresponsive Nanoreactor as an Efficient Catalyst for the Reduction of 4-Nitrophenol. Nanomaterials. 2018; 8(12):963. https://doi.org/10.3390/nano8120963
Chicago/Turabian StyleLiu, Wei, Xiaolian Zhu, Chengcheng Xu, Zhao Dai, and Zhaohui Meng. 2018. "AuNPs-Based Thermoresponsive Nanoreactor as an Efficient Catalyst for the Reduction of 4-Nitrophenol" Nanomaterials 8, no. 12: 963. https://doi.org/10.3390/nano8120963




