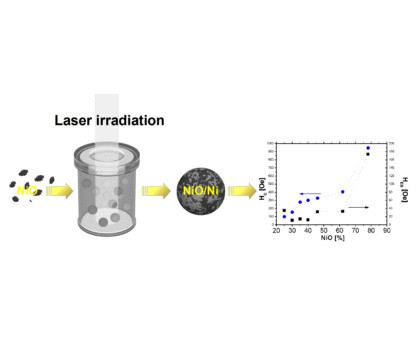
Tailoring of Magnetic Properties of NiO/Ni Composite Particles Fabricated by Pulsed Laser Irradiation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cozzoli, P.D.; Pellegrino, T.; Manna, L. Synthesis, properties and perspectives of hybrid nanocrystal structures. Chem.Soc. Rev. 2006, 35, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Sun, S. Syntheses, properties, and potential applications of multicomponent magnetic nanoparticles. Adv. Func. Mater. 2008, 18, 391–400. [Google Scholar] [CrossRef]
- Férey, G. Some suggested perspectives for multifunctional hybrid porous solids. Dalton Trans. 2009, 23, 4400–4415. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zeng, H.; Sahoo, Y.; Ohulchanskyy, T.Y.; Ding, Y.; Wang, Z.L.; Swihart, M.; Prasad, P.N. A general approach to binary and ternary hybrid nanocrystals. Nano Lett. 2006, 6, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Kagan, C.R.; Murray, C.B.; Bawendi, M.G. Long-range resonance transfer of electronic excitations in close-packed CdSe quantum-dot solids. Phys. Rev. B 1996, 54, 8633. [Google Scholar] [CrossRef]
- Urban, J.J.; Talapin, D.V.; Shevchenko, E.V.; Kagan, C.R.; Murray, C.B. Synergism in binary nanocrystal superlattices leads to enhanced p-type conductivity in self-assembled PbTe/Ag2Te thin films. Nat. Mater. 2007, 6, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Legendre, B.; Sghaier, M. Curie temperature of nickel. J. Therm. Anal. Calorim. 2011, 105, 141–143. [Google Scholar] [CrossRef]
- Lu, L.; Sui, M.L.; Lu, K. Superplastic extensibility of nanocrystalline copper at room temperature. Science 2000, 287, 1463. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M. Preparation and properties of well-defined magnetic particles. Mater. Res. Bull. 1989, 14, 35–40. [Google Scholar] [CrossRef]
- Gleiter, H. Materials with ultrafine microstructures: Retrospectives and perspectives. Nanostruct. Mater. 1992, 1, 1. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Pt nanoparticle anchored molecular self-assemblies of DNA: An extremely stable and efficient HER electrocatalyst with ultralow Pt content. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Shia, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Song, X.F.; Gao, L. Facile synthesis and hierarchical assembly of hollow nickel oxide architectures bearing enhanced photocatalytic properties. J. Phys. Chem. C 2008, 112, 15299. [Google Scholar] [CrossRef]
- Park, J.; Kang, E.; Son, S.U.; Park, H.M.; Lee, M.K.; Kim, J.; Kim, K.W.; Noh, H.J.; Park, J.H.; Bae, C.J.; et al. Monodisperse nanoparticles of Ni and NiO: Synthesis, characterization, self-assembled superlattices, and catalytic applications in the Suzuki coupling reaction. Adv. Mater. 2005, 17, 429. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Zhang, Y.G.; Wang, S.T.; Zhang, Z.D.; Fei, L.F.; Qian, Y.T. High-yield synthesis of NiO nanoplatelets and their excellent electrochemical performance. Cryst. Growth Des. 2006, 6, 2163. [Google Scholar] [CrossRef]
- Zhu, J.X.; Gui, Z.; Ding, Y.Y.; Wang, Z.Z.; Hu, Y.; Zou, M.Q. A facile route to oriented nickel hydroxide nanocolumns and porous nickel oxide. J. Phys. Chem. C 2007, 111, 5622. [Google Scholar] [CrossRef]
- Bader, S. Colloquium: Opportunities in nanomagnetism. ReV. Mod. Phys. 2006, 78, 1. [Google Scholar] [CrossRef]
- Nardi, K.L.; Yang, N.; Dickens, C.F.; Strickler, A.L.; Bent, S.F. Creating highly active atomic layer deposited NiO electrocatalysts for the oxygen evolution reaction. Adv. Energy Mater. 2015, 5, 1500412. [Google Scholar]
- Vernon, M.W. The temperature dependence of the rhombohedral distortion in NiO. Phys. Stat. Sol. 1970, 37, K1–K3. [Google Scholar] [CrossRef]
- Nogués, J.; Langlais, V.; Sort, J.; Doppiu, S.; Suriñach, S.; Baró, M.D. Magnetic properties of Ni-NiO (ferromagnetic-antiferromagnetic) nanocomposites obtained from a partial mechanochemical reduction of NiO. J Nanosci Nanotechnol. 2008, 8, 2923–2928. [Google Scholar] [PubMed]
- Liang, J.; Wang, Y.-Z.; Wang, Ch.-Ch.; Lu, S.-Y. In situ formation of NiO on Ni foam prepared with a novel leaven dough method as an outstanding electrocatalyst for oxygen evolution reactions. J. Mater. Chem. A 2016, 4, 9797–9806. [Google Scholar] [CrossRef]
- Sort, J.; Nogués, J.; Amils, X.; Suriñach, S.; Munõz, J.S.; Baró, M.D. Room temperature magnetic hardening in mechanically milled ferromagnetic–antiferromagnetic composites. J. Magn. Magn. Mater. 2000, 219, 53–57. [Google Scholar] [CrossRef]
- Karimipour, M.; Wikberg, J.M.; Kapaklis, V. Nanoparticles of Ni/NiO embedded in TiO2 synthesized by the complex-polymer sol–gel method. Phys. Scr. 2011, 84, 035702. [Google Scholar] [CrossRef]
- Thakur, M.; Patra, M.; Majumdar, S.; Giri, S. Influence of cooling field on the magnetic properties of Ni/NiO nanostructure. J. Alloys Compd. 2009, 480, 193–197. [Google Scholar] [CrossRef]
- Querejeta-Fernández, A.; Parras, M.; Varela, A.; Monte, F.; García-Hernández, M.; González-Calbet, J.M. Urea-melt assisted synthesis of Ni/NiO nanoparticles exhibiting structural disorder and exchange bias. Chem. Mater. 2010, 22, 6529–6541. [Google Scholar] [CrossRef]
- Kremenovic, A.; Jancar, B.; Ristic, M.; Vucinic-Vasic, M.; Rogan, J.; Pacevski, A.; Antic, B. Exchange-bias and grain-surface relaxations in nanostructured NiO/Ni induced by a particle size reduction. J. Phys. Chem. C 2012, 116, 4356–4364. [Google Scholar] [CrossRef]
- Yao, X.-J.; He, X.-M.; Song, X.-Y.; Ding, Q.; Li, Z.-W.; Zhong, W.; Aub, Ch.-T.; Dua, Y.-W. Enhanced exchange bias and coercivity arising from heterojunctions in Ni–NiO nanocomposites. Phys. Chem. Chem. Phys. 2014, 16, 6925–6930. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Pyatenko, A.; Kawaguchi, K.; Li, X.Y.; Swiatkowska-Warkocka, Z.; Koshizaki, N. Selective pulsed heating for the synthesis of semiconductor and metal submicrometer spheres. Angew. Chem. Int. Ed. 2010, 49, 6361–6364. [Google Scholar] [CrossRef] [PubMed]
- Swiatkowska-Warkocka, Z.; Kawaguchi, K.; Wang, H.Q.; Katou, Y.; Koshizaki, N. Controlling exchange bias in Fe3O4/FeO composite particles prepared by pulsed laser irradiation. Nanoscale Res. Lett. 2011, 6, 226–232. [Google Scholar]
- Li, X.; Shimizu, Y.; Pyatenko, A.; Wang, H.; Koshizaki, N. Carbon-assisted fabrication of submicrometer spheres for low-optical-absorbance materials by selective laser heating in liquid. J. Mater. Chem. 2011, 21, 14406–14409. [Google Scholar] [CrossRef]
- Wang, H.Q.; Koshizaki, N.; Li, L.; Jia, L.; Kawaguchi, K.; Li, X.; Pyatenko, A.; Swiatkowska-Warkocka, Z.; Bando, Y.; Golberg, D. Size-tailored ZnO submicrometer spheres: Bottom-up construction, size-related optical extinction, and selective aniline trapping. Adv. Mater. 2011, 23, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Swiatkowska-Warkocka, Z.; Kawaguchi, K.; Shimizu, Y.; Pyatenko, A.; Wang, H.; Koshizaki, N. Synthesis of Au-based porous magnetic spheres by selective laser heating in liquid. Langmuir 2012, 28, 4903–4907. [Google Scholar] [CrossRef] [PubMed]
- Swiatkowska-Warkocka, Z.; Koga, K.; Kawaguchi, K.; Wang, H.; Pyatenko, A.; Koshizaki, N. Pulsed laser irradiation of colloidal nanoparticles: A new synthesis route for the production of non-equilibrium bimetallic alloy submicrometer spheres. RSC Adv. 2013, 3, 79–83. [Google Scholar] [CrossRef]
- Wang, H.; Jia, L.; Li, L.; Li, X.; Swiatkowska-Warkocka, Z.; Kawaguchi, K.; Pyatenko, A.; Koshizaki, N. Photomediated assembly of single crystalline silver spherical particles with enhanced electro-chemical performance. J. Mater. Chem. A 2013, 1, 692–698. [Google Scholar] [CrossRef]
- Swiatkowska-Warkocka, Z.; Pyatenko, A.; Krok, F.; Jany, B.R.; Marszalek, M. Synthesis of new metastable nanoalloys of immiscible metals with a pulse laser technique. Sci. Rep. 2015, 5, 09849. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Koshizaki, N.; Pyatenko, A.; Saitoh, N.; Yoshizawa, N.; Shimizu, Y. Nano-and submicrometer-sized spherical particle fabrication using a submicroscopic droplet formed using selective laser heating. J. Phys. Chem. C 2016, 120, 2439–2446. [Google Scholar] [CrossRef]
- Pyatenko, A.; Yamaguchi, M.; Suzuki, M. Mechanisms of size reduction of colloidal silver and gold nanoparticles irradiated by Nd:YAG laser. J. Phys. Chem. C 2009, 113, 9078. [Google Scholar] [CrossRef]
- Fullprof Software. Available online: https://www.ill.eu/sites/fullprof/php/tutorials (accessed on 5 October 2018).
- Suh, I.-K.; Ohta, H.; Waseda, Y. High-temperature thermal expansion of six metallic elements measured by dilatation method and X-ray diffraction. J. Mater. Sci. 1988, 23, 757–760. [Google Scholar] [CrossRef]
- Furlan, A.; Lu, J.; Hultman, L.; Janssonand, U.; Magnuson, M. Crystallization characteristics and chemical bonding properties of nickel carbide thin film nanocomposites. J. Phys. Condens. Matter 2014, 26, 415501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portnoi, V.K.; Leonov, A.V.; Mudretsova, S.N.; Fedotov, S.A. Formation of nickel carbide in the course of deformation treatment of Ni-C mixtures. Phys. Metals Metallogr. 2010, 109, 153. [Google Scholar] [CrossRef]
- Swiatkowska-Warkocka, Z.; Pyatenko, A.; Koga, K.; Kawaguchi, K.; Wang, H.; Koshizaki, N. Synthesis and control of various morphologies/phases of Au-based nanocomposite particles by pulsed laser irradiation in liquid media. J. Phys. Chem. C 2017, 121, 8177–8187. [Google Scholar] [CrossRef]
- Pyatenko, A.; Wang, H.; Koshizaki, N.; Tsuji, T. Mechanism of pulse laser interaction with colloidal nanoparticles. Laser Photonics Rev. 2013, 7, 596–604. [Google Scholar] [CrossRef]
- Fulcomer, E.; Charap, S.H. Thermal fluctuation aftereffect model for some systems with ferromagnetic-antiferromagnetic coupling. J. Appl. Phys. 1972, 43, 4190. [Google Scholar] [CrossRef]
- Zhang, S.; Dimitrov, D.V.; Hadjipanayis, G.C.; Cai, J.W. Coercivity induced by random field at ferromagnetic and antiferromagnetic interfaces. J. Magn. Magn. Mater. 1999, 198–199, 468–470. [Google Scholar] [CrossRef]
- Kuch, W.; Chelaru, L.I.; Offi, F.; Wang, J.; Kotsugi, M.; Kirschner, J. Tuning the magnetic coupling across ultrathin antiferromagnetic films by controlling atomic-scale roughness. Nature Mater. 2006, 5, 128. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.L.; Sumiyama, K.; Hihara, T.; Yamamuro, S.; Konno, T.J. Magnetic properties of monodispersed Co/CoO clusters. Phys. Rev. B 2000, 61, 3103. [Google Scholar] [CrossRef]
- Baltz, V.; Sort, J.; Landis, S.; Rodmacq, B.; Dieny, B. Tailoring Size Effects on the exchange bias in ferromagnetic-antiferromagnetic <100 nmnanostructures. Phys. Rev. Lett. 2005, 94, 117201. [Google Scholar] [PubMed]
- Scholl, A.; Nolting, F.; Seo, J.W.; Ohldag, H.; Stohr, J.; Raoux, S.; Locquet, J.P.; Fompeyrine, J. Domain-size-dependent exchange bias in Co/LaFeO3. Appl. Phys. Lett. 2004, 85, 4085. [Google Scholar] [CrossRef]
- Béa, H.; Bibes, M.; Ott, F.; Dupé, B.; Zhu, X.H.; Petit, S.; Fusil, S.; Deranlot, C.; Bouzehouane, K.; Barthélémy, A. Mechanisms of exchange bias with multiferroic BiFeO3 epitaxial thin films. Phys. Rev. Lett. 2008, 100, 017204. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swiatkowska-Warkocka, Z.; Pyatenko, A.; Shimizu, Y.; Perzanowski, M.; Zarzycki, A.; Jany, B.R.; Marszalek, M. Tailoring of Magnetic Properties of NiO/Ni Composite Particles Fabricated by Pulsed Laser Irradiation. Nanomaterials 2018, 8, 790. https://doi.org/10.3390/nano8100790
Swiatkowska-Warkocka Z, Pyatenko A, Shimizu Y, Perzanowski M, Zarzycki A, Jany BR, Marszalek M. Tailoring of Magnetic Properties of NiO/Ni Composite Particles Fabricated by Pulsed Laser Irradiation. Nanomaterials. 2018; 8(10):790. https://doi.org/10.3390/nano8100790
Chicago/Turabian StyleSwiatkowska-Warkocka, Zaneta, Alexander Pyatenko, Yoshiki Shimizu, Marcin Perzanowski, Arkadiusz Zarzycki, Benedykt R. Jany, and Marta Marszalek. 2018. "Tailoring of Magnetic Properties of NiO/Ni Composite Particles Fabricated by Pulsed Laser Irradiation" Nanomaterials 8, no. 10: 790. https://doi.org/10.3390/nano8100790