PEG 400-Based Phase Change Materials Nano-Enhanced with Functionalized Graphene Nanoplatelets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Graphene and Poly(Ethylene Glycol) Characterization
2.3. NePCM Preparation, Stability and Chemical Interactivity Analysis
2.4. Thermophysical Characterization
3. Results
3.1. Phase Change Characterization
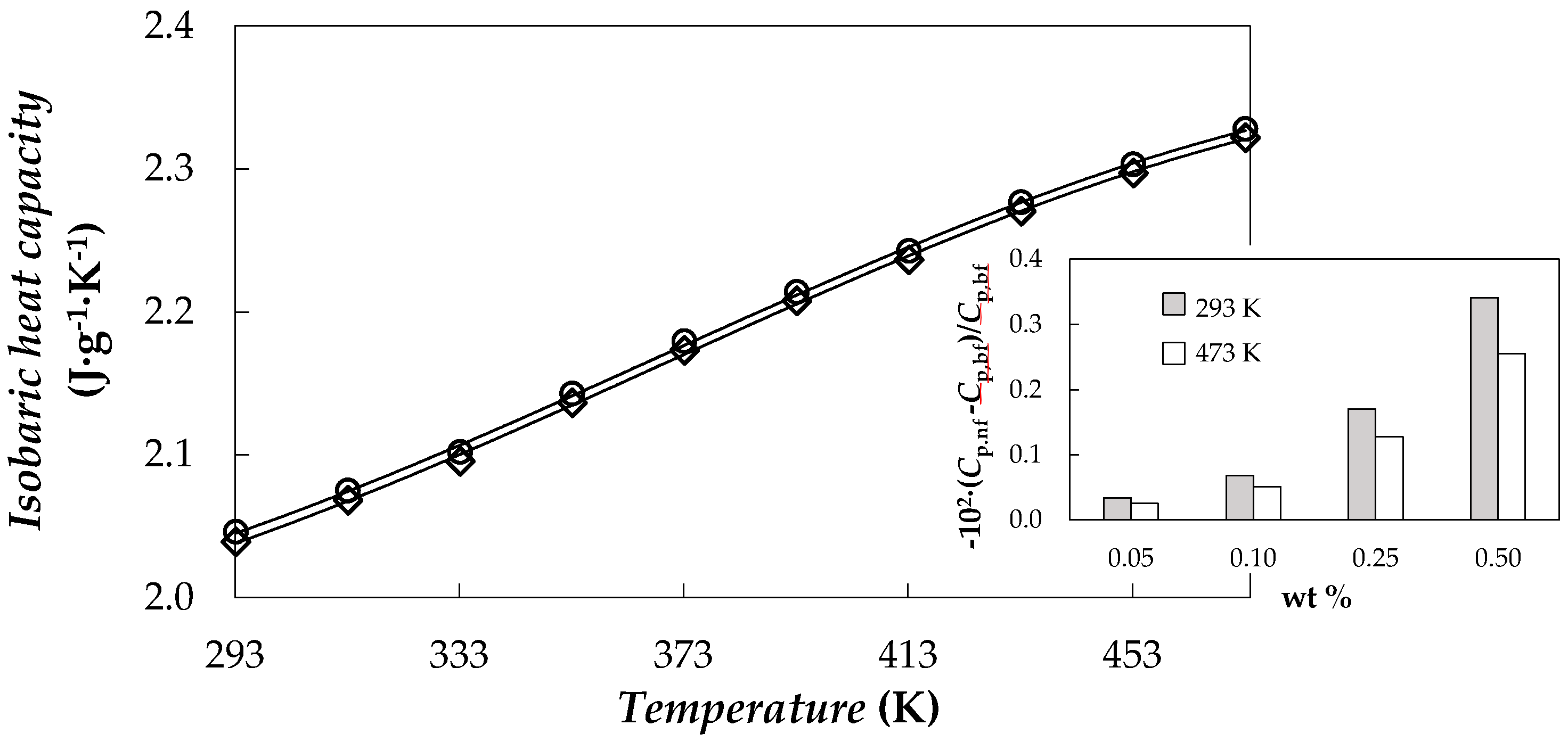
3.2. Isobaric Heat Capacity
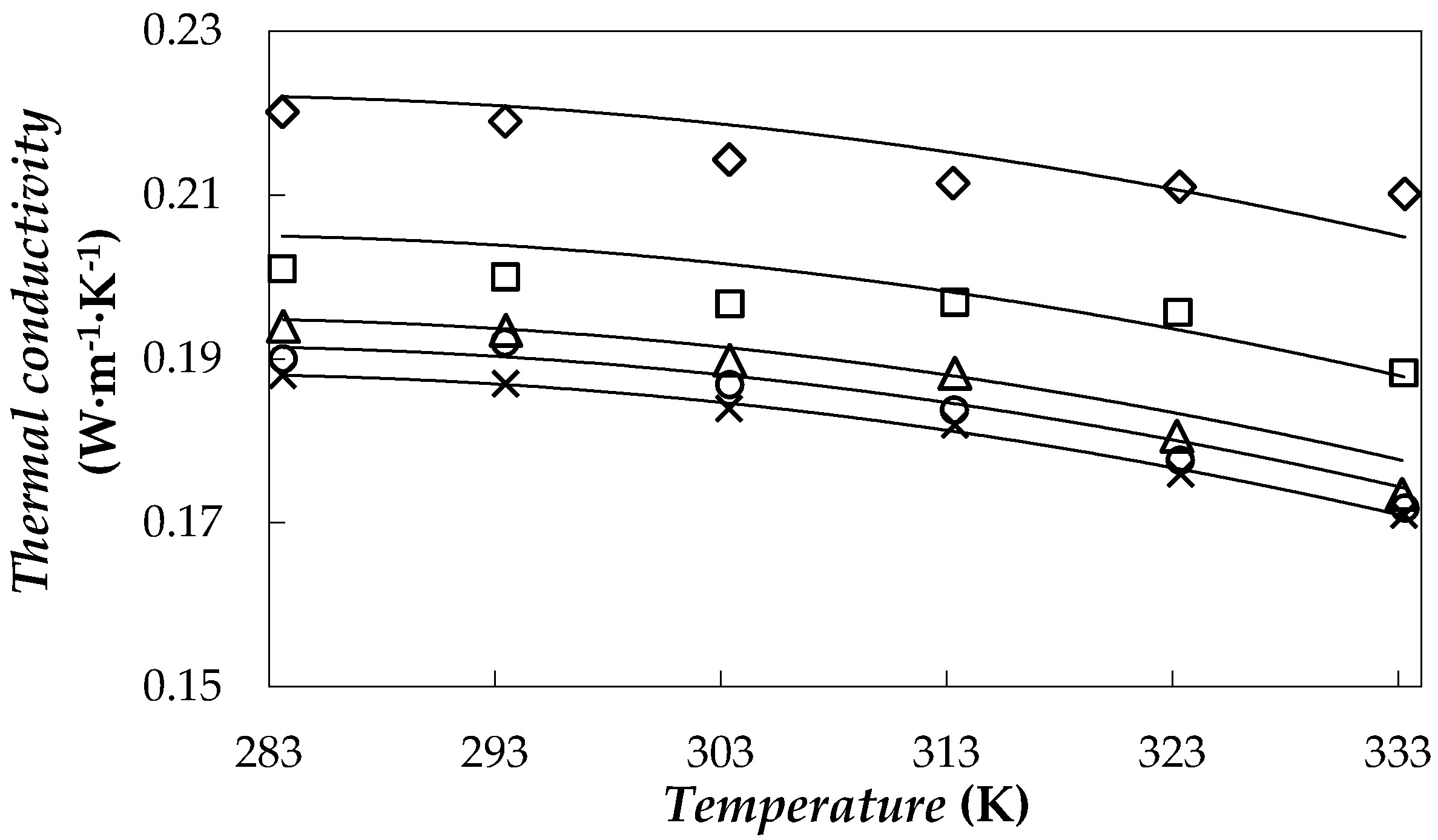
3.3. Thermal Conductivity
3.4. Volumetric Behaviour
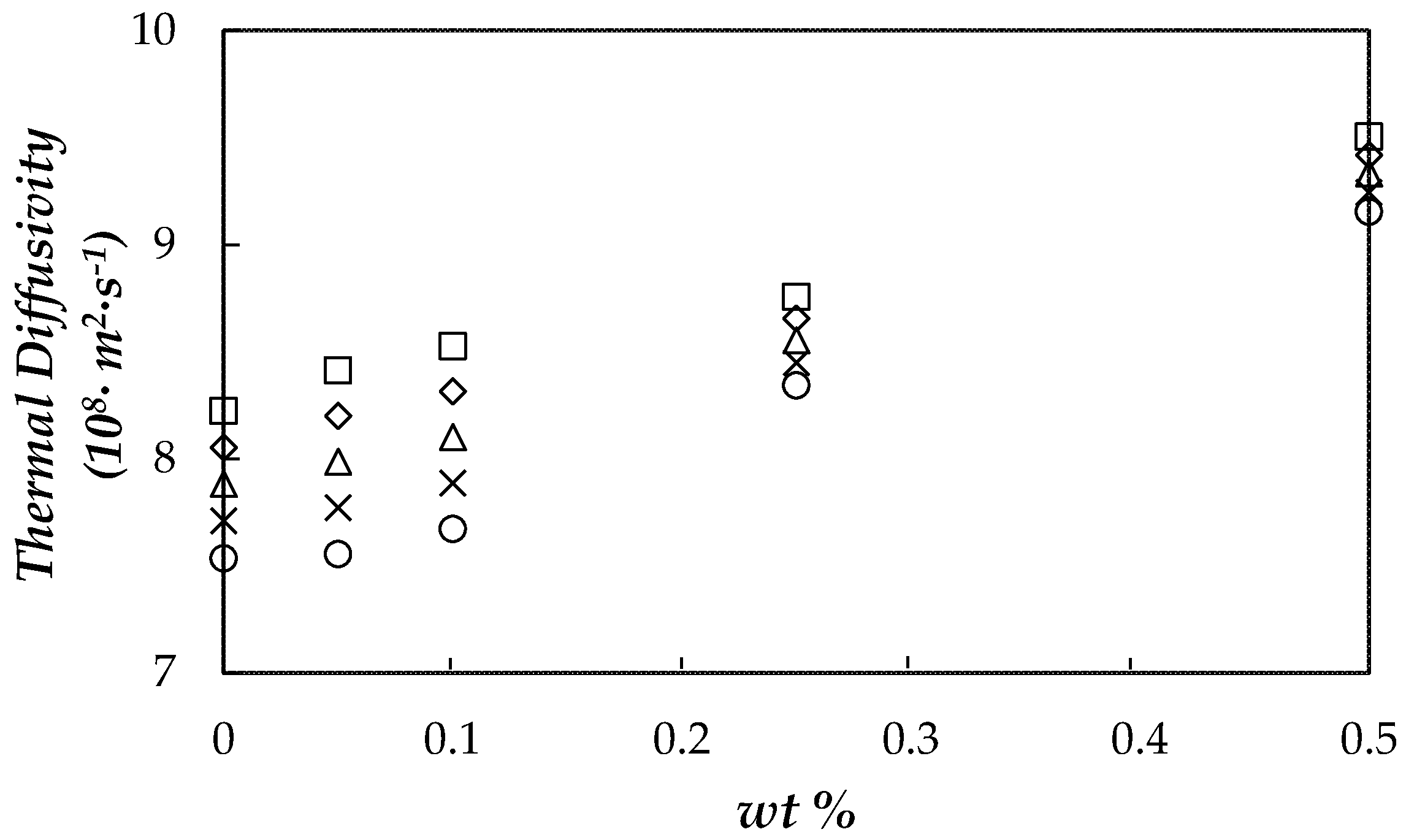
3.5. Thermal Diffusivity
3.6. Viscosity
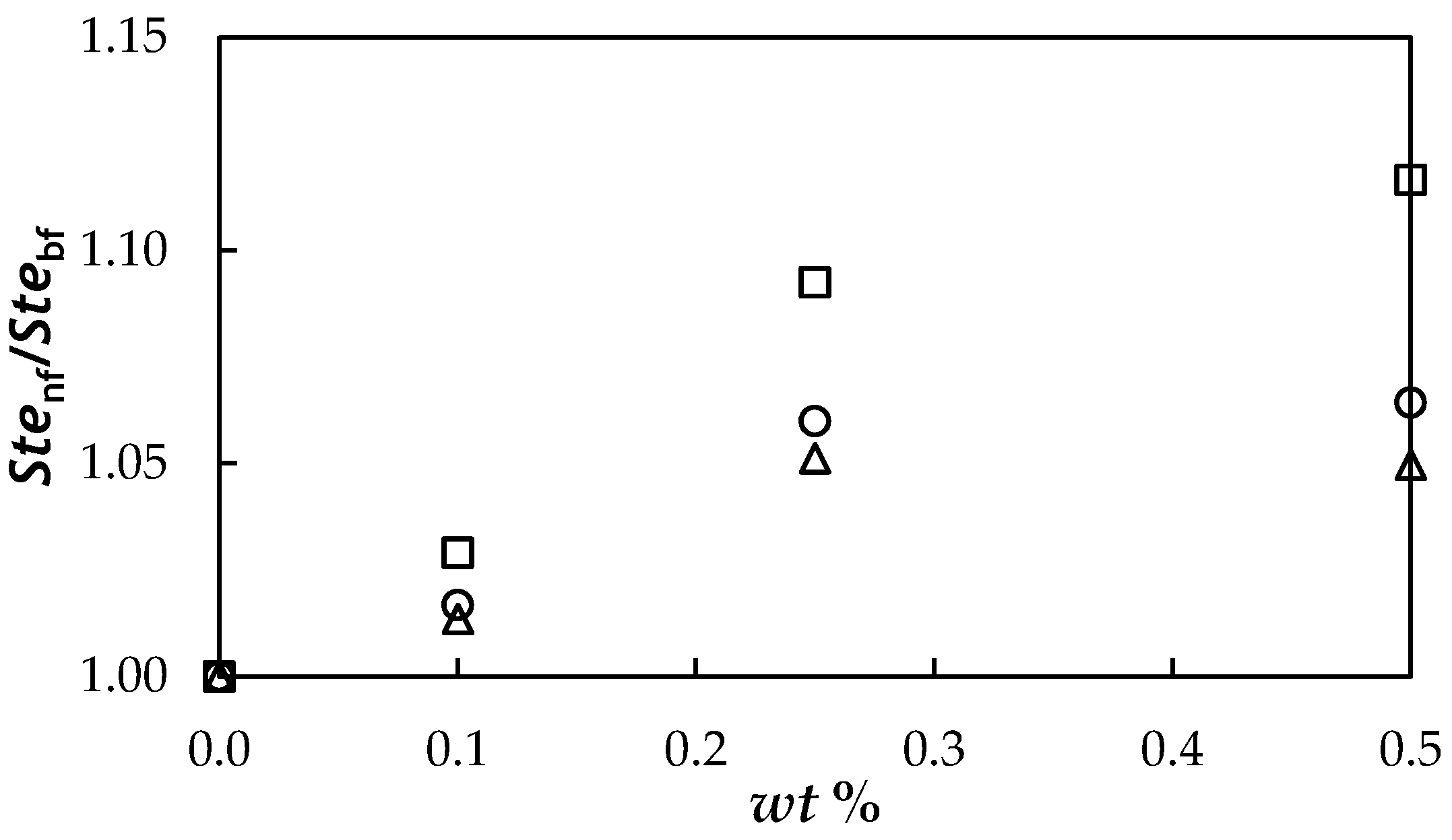
3.7. Evaluation Based on the Studied Properties
4. Conclusions and Future Works
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karaman, S.; Karaipekli, A.; Sarı, A.; Biçer, A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1647–1653. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Martorell, I.; Miró, L.; Fernández, A.I.; Barreneche, C. Introduction to Thermal Energy Storage (TES) Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 1–28. [Google Scholar]
- Sun, Q.; Yuan, Y.; Zhang, H.; Cao, X.; Sun, L. Thermal properties of polyethylene glycol/carbon microsphere composite as a novel phase change material. J. Therm. Anal. Calorim. 2017, 130, 1741–1749. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Ma, H.; Yang, J. The preparation of a green shape-stabilized composite phase change material of polyethylene glycol/SiO2 with enhanced thermal performance based on oil shale ash via temperature-assisted sol–gel method. Sol. Energy Mater. Sol. Cells 2015, 132, 29–39. [Google Scholar] [CrossRef]
- Tang, B.; Wu, C.; Qiu, M.; Zhang, X.; Zhang, S. PEG/SiO2–Al2O3 hybrid form-stable phase change materials with enhanced thermal conductivity. Mater. Chem. Phys. 2014, 144, 162–167. [Google Scholar] [CrossRef]
- Ma, Z.; Lin, W.; Sohel, M.I. Nano-enhanced phase change materials for improved building performance. Renew. Sustain. Energy Rev. 2016, 58, 1256–1268. [Google Scholar] [CrossRef]
- Gerard, F.; Camila, B.; Aran, S.; José Enrique, J.; Luisa, F.C. Recent patents on nano-enhanced materials for use in thermal energy storage (TES). Recent Pat. Nanotechnol. 2017, 11, 101–108. [Google Scholar]
- Fandiño, O.; Lugo, L.; Comuñas, M.J.P.; López, E.R.; Fernández, J. Temperature and pressure dependences of volumetric properties of two poly(propylene glycol) dimethyl ether lubricants. J. Chem. Thermodyn. 2010, 42, 84–89. [Google Scholar] [CrossRef]
- Paredes, X.; Fandiño, O.; Pensado, A.S.; Comuñas, M.J.P.; Fernández, J. Pressure-viscosity coefficients for polyalkylene glycol oils and other ester or ionic lubricants. Tribol. Lett. 2012, 45, 89–100. [Google Scholar] [CrossRef]
- Zin, V.; Barison, S.; Agresti, F.; Colla, L.; Pagura, C.; Fabrizio, M. Improved tribological and thermal properties of lubricants by graphene based nano-additives. RSC Adv. 2016, 6, 59477–59486. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Dash, S.; Tyagi, A.K. Energy efficient reduced graphene oxide additives: Mechanism of effective lubrication and antiwear properties. Sci. Rep. 2016, 6, 18372. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Fang, M.; Huang, Z.; Liu, Y.; Huang, Y.; Wen, R.; Qian, T.; Wu, X. Enhanced thermal properties of novel shape-stabilized PEG composite phase change materials with radial mesoporous silica sphere for thermal energy storage. Sci. Rep. 2015, 5, 12964. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Cui, J.; Wang, Y.; Jia, C.; Zhang, S. Facile synthesis and performances of PEG/SiO2 composite form-stable phase change materials. Sol. Energy 2013, 97, 484–492. [Google Scholar] [CrossRef]
- Wang, P.; Li, N.; Zhao, C.; Wu, L.; Han, G. A phase change storage material that may be used in the fire resistance of building structure. Procedia Eng. 2014, 71, 261–264. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J. Preparation and performance of form-stable polyethylene glycol/silicon dioxide composites as solid–liquid phase change materials. Appl. Energy 2009, 86, 170–174. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J.; Yan, J. Enhanced thermal conductivity and thermal performance of form-stable composite phase change materials by using β-aluminium nitride. Appl. Energy 2009, 86, 1196–1200. [Google Scholar] [CrossRef]
- Tang, B.; Qiu, M.; Zhang, S. Thermal conductivity enhancement of PEG/SiO2 composite PCM by in situ Cu doping. Sol. Energy Mater. Sol. Cells 2012, 105, 242–248. [Google Scholar] [CrossRef]
- Tang, B.; Wei, H.; Zhao, D.; Zhang, S. Light-heat conversion and thermal conductivity enhancement of PEG/SiO2 composite PCM by in situ Ti4O7 doping. Sol. Energy Mater. Sol. Cells 2017, 161, 183–189. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Majdan-Cegincara, R. Investigation on stability and rheological properties of nanofluid of ZnO nanoparticles dispersed in poly(ethylene glycol). Fluid Phase Equilib. 2013, 354, 102–108. [Google Scholar] [CrossRef]
- Zendehasbagh, S.; Majdan-Cegincara, R. Magnetorheological and volumetric properties of starch and polyethylene glycol solutions in the presence of nio nanoparticles. Phys. Chem. Res. 2018, 6, 45–66. [Google Scholar]
- Amiri, A.; Sadri, R.; Shanbedi, M.; Ahmadi, G.; Chew, B.T.; Kazi, S.N.; Dahari, M. Performance dependence of thermosyphon on the functionalization approaches: An experimental study on thermo-physical properties of graphene nanoplatelet-based water nanofluids. Energy Convers. Manag. 2015, 92, 322–330. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Kazi, S.N.; Mehrali, M.; Zubir, M.N.B.M.; Metselaar, H.S.C. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.Q.; Liang, C.L.; Bao, R.Y.; Liu, Z.Y.; Yang, W.; Xie, B.H.; Yang, M.B. Polyethylene glycol based shape-stabilized phase change material for thermal energy storage with ultra-low content of graphene oxide. Sol. Energy Mater. Sol. Cells 2014, 123, 171–177. [Google Scholar] [CrossRef]
- Żyła, G.; Fal, J.; Estellé, P. The influence of ash content on thermophysical properties of ethylene glycol based graphite/diamonds mixture nanofluids. Diam. Relat. Mater. 2017, 74, 81–89. [Google Scholar] [CrossRef]
- Khodadadi, J.M.; Fan, L.; Babaei, H. Thermal conductivity enhancement of nanostructure-based colloidal suspensions utilized as phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2013, 24, 418–444. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, X. Study on paraffin/expanded graphite composite phase change thermal energy storage material. Energy Convers. Manag. 2006, 47, 303–310. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Thermal conductivity and latent heat thermal energy storage characteristics of paraffin/expanded graphite composite as phase change material. Appl. Therm. Eng. 2007, 27, 1271–1277. [Google Scholar] [CrossRef]
- Kim, S.D.L.T. High latent heat storage and high thermal conductive phase change materials using exfoliated graphite nanoplatelets. Sol. Energy Mater. Sol. Cells 2009, 93, 136–142. [Google Scholar] [CrossRef]
- Bahiraei, F.; Fartaj, A.; Nazri, G.-A. Experimental and numerical investigation on the performance of carbon-based nanoenhanced phase change materials for thermal management applications. Energy Convers. Manag. 2017, 153, 115–128. [Google Scholar] [CrossRef]
- Sari, A.; Karapekl, A.; Kaygusuz, K. Fatty acid/expanded graphite composites as phase change material for latent heat thermal energy storage. Energy Sources Part A 2008, 30, 464–474. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of palmitic acid/expanded graphite composite as form-stable PCM for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 571–576. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A.; Kaygusuz, K. Thermal conductivity improvement of stearic acid using expanded graphite and carbon fiber for energy storage applications. Renew. Energy 2007, 32, 2201–2210. [Google Scholar] [CrossRef]
- Li, C.; Xie, B.; Chen, J. Graphene-decorated silica stabilized stearic acid as a thermal energy storage material. RSC Adv. 2017, 7, 30142–30151. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Xin, G.; Li, G.; Zheng, J.; Tian, W.; Li, X. Phase change behaviors of PEG on modified graphene oxide mediated by surface functional groups. Eur. Polym. J. 2016, 74, 43–50. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, P.; Mou, Y.; Kang, M.; Liu, M.; Song, L.; Lu, A.; Rong, J. Synthesis of the polyethylene glycol solid-solid phase change materials with a functionalized graphene oxide for thermal energy storage. Polym. Test. 2017, 63, 494–504. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Q.; Lin, J.; Chen, J.; Xu, Z. Thermal conductivity of polyethylene glycol nanofluids containing carbon coated metal nanoparticles. J. Appl. Phys. 2010, 108, 124304. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Z.; Ma, B.; Wen, R.; Min, X.; Huang, Y.; Yin, Z.; Liu, Y.; Fang, M.; Wu, X. Preparation and performance of novel form-stable composite phase change materials based on polyethylene glycol/white carbon black assisted by super-ultrasound-assisted. Thermochim. Acta 2016, 638, 35–43. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Jia, S.; Zhu, Y.; Chen, L.; Fu, L. Influences of dynamic impregnating on morphologies and thermal properties of polyethylene glycol-based composite as shape-stabilized PCMs. J. Therm. Anal. Calorim. 2017, 128, 1039–1048. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Tang, B.; Xu, S.; Shufen, Z. Novel light–driven CF/PEG/SiO2 composite phase change materials with high thermal conductivity. Sol. Energy Mater. Sol. Cells 2018, 174, 538–544. [Google Scholar] [CrossRef]
- Tang, B.; Wang, Y.; Qiu, M.; Zhang, S. A full-band sunlight-driven carbon nanotube/PEG/SiO2 composites for solar energy storage. Sol. Energy Mater. Sol. Cells 2014, 123, 7–12. [Google Scholar] [CrossRef]
- Pop, E.; Varshney, V.; Roy, A.K. Thermal properties of graphene: Fundamentals and applications. MRS Bull. 2012, 37, 1273–1281. [Google Scholar] [CrossRef]
- Mehrali, M.; Tahan Latibari, S.; Mahlia, T.M.I.; Metselaar, H.S.C.; Naghavi, M.S.; Sadeghinezhad, E.; Akhiani, A.R. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material. Appl. Therm. Eng. 2013, 61, 633–640. [Google Scholar] [CrossRef]
- Agromayor, R.; Cabaleiro, D.; Pardinas, A.A.; Vallejo, J.P.; Fernandez-Seara, J.; Lugo, L. Heat transfer performance of functionalized graphene nanoplatelet aqueous nanofluids. Materials 2016, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Li, J.; Feng, W.; Nian, H.E. Single-walled carbon nanotube for shape stabilization and enhanced phase change heat transfer of polyethylene glycol phase change material. Energy Convers. Manag. 2017, 143, 96–108. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Yang, H.; Xin, G.; Li, W.; Zheng, J.; Tian, W.; Li, X. Graphene oxide stabilized polyethylene glycol for heat storage. Phys. Chem. Chem. Phys. 2012, 14, 13233–13238. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Li, J.; Ma, H.; Yang, J. Adjustable thermal property of polyethylene glycol/diatomite shape-stabilized composite phase change material. Polym. Compos. 2016, 37, 854–860. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Gracia-Fernández, C.; Lugo, L. (Solid + liquid) phase equilibria and heat capacity of (diphenyl ether + biphenyl) mixtures used as thermal energy storage materials. J. Chem. Thermodyn. 2014, 74, 43–50. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Colla, L.; Agresti, F.; Lugo, L.; Fedele, L. Transport properties and heat transfer coefficients of ZnO/(ethylene glycol + water) nanofluids. Int. J. Heat Mass Transf. 2015, 89, 433–443. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Nimo, J.; Pastoriza-Gallego, M.J.; Piñeiro, M.M.; Legido, J.L.; Lugo, L. Thermal conductivity of dry anatase and rutile nano-powders and ethylene and propylene glycol-based TiO2 nanofluids. J. Chem. Thermodyn. 2015, 83, 67–76. [Google Scholar] [CrossRef]
- Gaciño, F.M.; Regueira, T.; Lugo, L.; Comuñas, M.J.P.; Fernández, J. Influence of molecular structure on densities and viscosities of several ionic liquids. J. Chem. Eng. Data 2011, 56, 4984–4999. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K. Differential scanning calorimetry studies on poly(ethylene glycol) with different molecular weights for thermal energy storage materials. Polym. Adv. Technol. 2002, 13, 690–696. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics: Crystal Nucleation, Growth, Annealing; Academic Press: New York, NY, USA, 1976; pp. 11–12. [Google Scholar]
- Francesconi, R.; Bigi, A.; Rubini, K.; Comelli, F. Molar heat capacities, densities, viscosities and refractive indices of poly(ethylene glycols) + 2-methyltetrahydrofuran at (293.15, 303.15 and 313.15) K. J. Chem. Eng. Data 2007, 52, 2020–2025. [Google Scholar] [CrossRef]
- O’Hanley, H.; Buongiorno, J.; McKrell, T.; Hu, L.W. Measurement and model validation of nanofluid specific heat capacity with differential scanning calorimetry. Adv. Mech. Eng. 2015, 4, 181079. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Ni, R. Measurement of the specific heat capacity of water-based Al2O3 nanofluid. Appl. Phys. Lett. 2008, 92, 093123. [Google Scholar] [CrossRef]
- Nan, C.W.; Birringer, R.; Clarke, D.R.; Gleiter, H. Effective thermal conductivity of particulate composites with interfacial thermal resistance. J. Appl. Phys. 1997, 81, 6692–6699. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T.K. Investigation of thermal conductivity, viscosity and electrical conductivity of graphene based nanofluids. J. Appl. Phys. 2013, 113, 084307. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Colla, L.; Barison, S.; Lugo, L.; Fedele, L.; Bobbo, S. Heat transfer capability of (ethylene glycol + water)-based nanofluids containing graphene nanoplatelets: Design and thermophysical profile. Nanoscale Res. Lett. 2017, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Afzal, W.; Mohammadi, A.H.; Richon, D. Volumetric properties of mono-, di-, tri- and polyethylene glycol aqueous solutions from (273.15 to 363.15) K: Experimental measurements and correlations. J. Chem. Eng. Data 2009, 54, 1254–1261. [Google Scholar] [CrossRef]
- Niu, Y.; Gao, F.; Zhu, R.; Sun, S.; Wei, X. Solubility of dilute SO2 in mixtures of n,n-dimethylformamide + polyethylene glycol 400 and the density and viscosity of the mixtures. J. Chem. Eng. Data 2013, 58, 639–647. [Google Scholar] [CrossRef]
- Živković, N.V.; Šerbanović, S.S.; Kijevčanin, M.L.; Živković, E.M. Volumetric and viscometric behavior of binary systems 2-butanol + PEG 200, + PEG 400, + tetraethylene glycol dimethyl ether and + n-methyl-2-pyrrolidone. J. Chem. Eng. Data 2013, 58, 3332–3341. [Google Scholar] [CrossRef]
- Trivedi, S.; Pandey, S. Densities of 1-butyl-3-methylimidazolium hexafluorophosphate + poly(ethylene glycol) in the temperature range (283.15 to 363.15) K. J. Chem. Eng. Data 2011, 56, 2168–2174. [Google Scholar] [CrossRef]
- Yoon, D.; Son, Y.-W.; Cheong, H. Negative thermal expansion coefficient of graphene measured by raman spectroscopy. Nano Lett. 2011, 11, 3227–3231. [Google Scholar] [CrossRef] [PubMed]
- Babapoor, A.; Karimi, G. Thermal properties measurement and heat storage analysis of paraffinnanoparticles composites phase change material: Comparison and optimization. Appl. Therm. Eng. 2015, 90, 945–951. [Google Scholar] [CrossRef]
- Cheng, X.; Li, G.; Yu, G.; Li, Y.; Han, J. Effect of expanded graphite and carbon nanotubes on the thermal performance of stearic acid phase change materials. J. Mater. Sci. 2017, 52, 12370–12379. [Google Scholar] [CrossRef]
- Bajić, D.M.; Ivaniš, G.R.; Visak, Z.P.; Živković, E.M.; Šerbanović, S.P.; Kijevčanin, M.L. Densities, viscosities and refractive indices of the binary systems (PEG 200 + 1,2-propanediol, + 1,3-propanediol) and (PEG 400 + 1,2-propanediol, + 1,3-propanediol) at (288.15 to 333.15) K and atmospheric pressure: Measurements and modeling. J. Chem. Thermodyn. 2013, 57, 510–529. [Google Scholar] [CrossRef]
- Maron, S.H.; Pierce, P.E. Application of ree-eyring generalized flow theory to suspensions of spherical particles. J. Colloid Sci. 1956, 11, 80–95. [Google Scholar] [CrossRef]
- Halelfadl, S.; Estellé, P.; Aladag, B.; Doner, N.; Maré, T. Viscosity of carbon nanotubes water-based nanofluids: Influence of concentration and temperature. Int. J. Therm. Sci. 2013, 71, 111–117. [Google Scholar] [CrossRef] [Green Version]

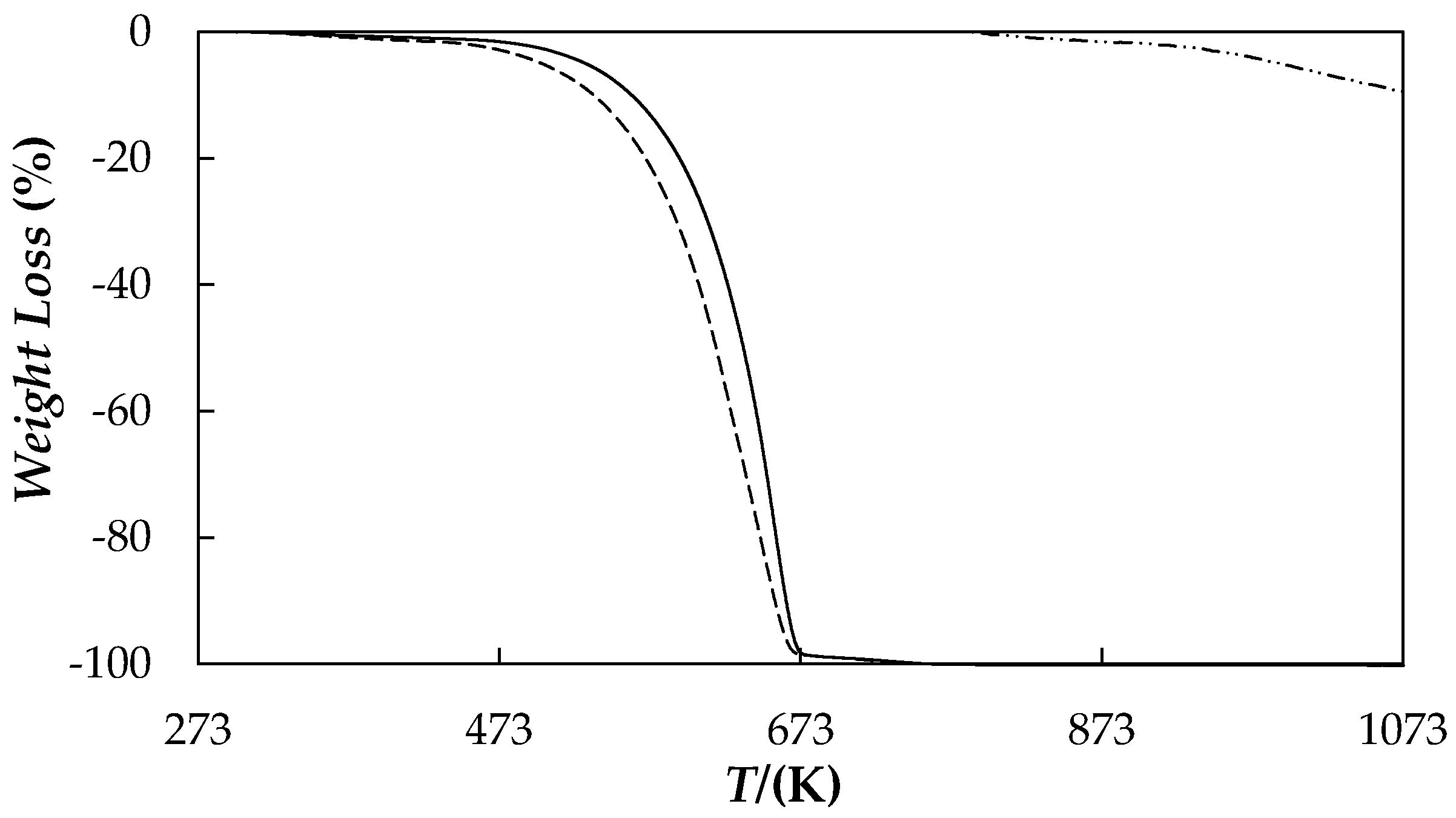
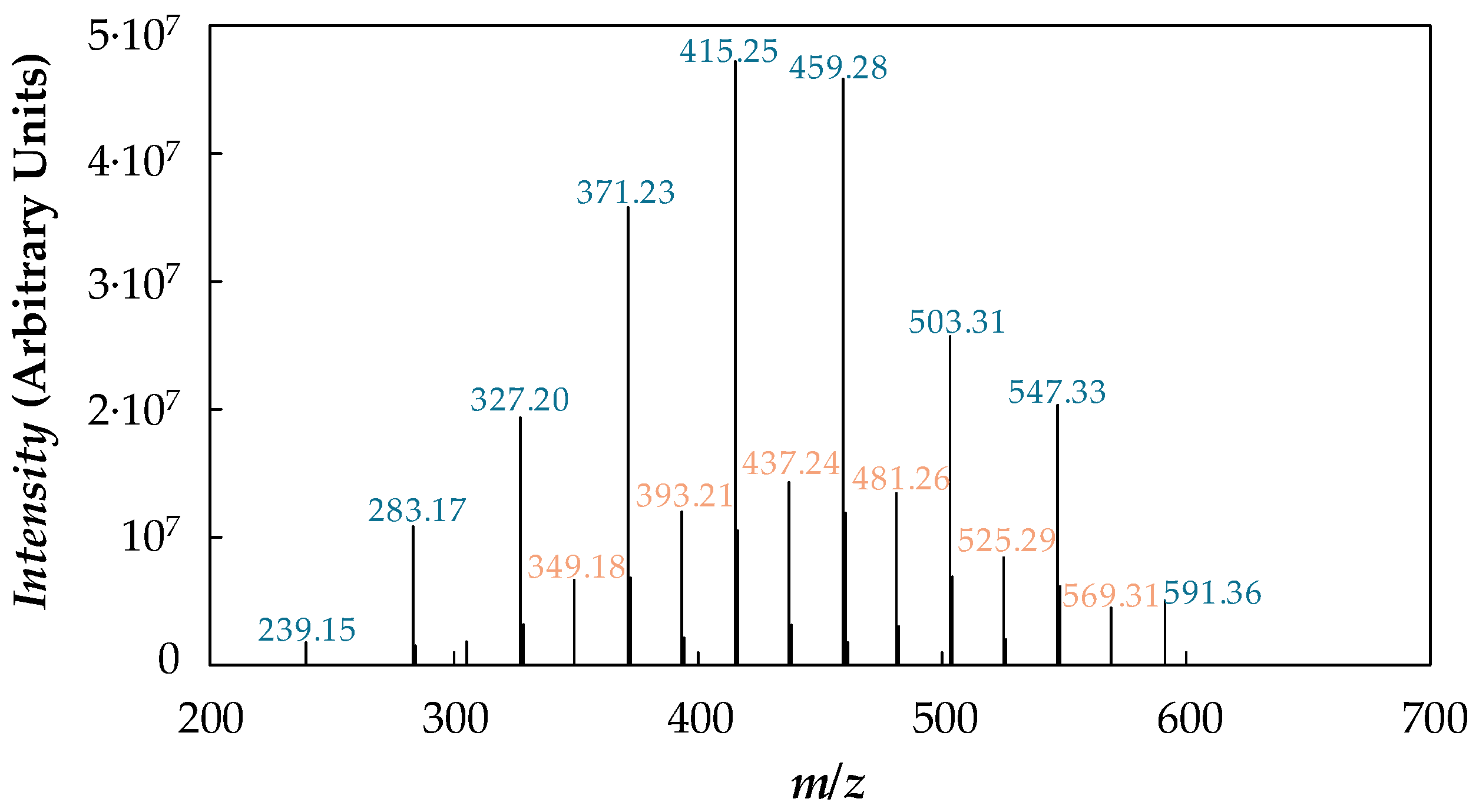
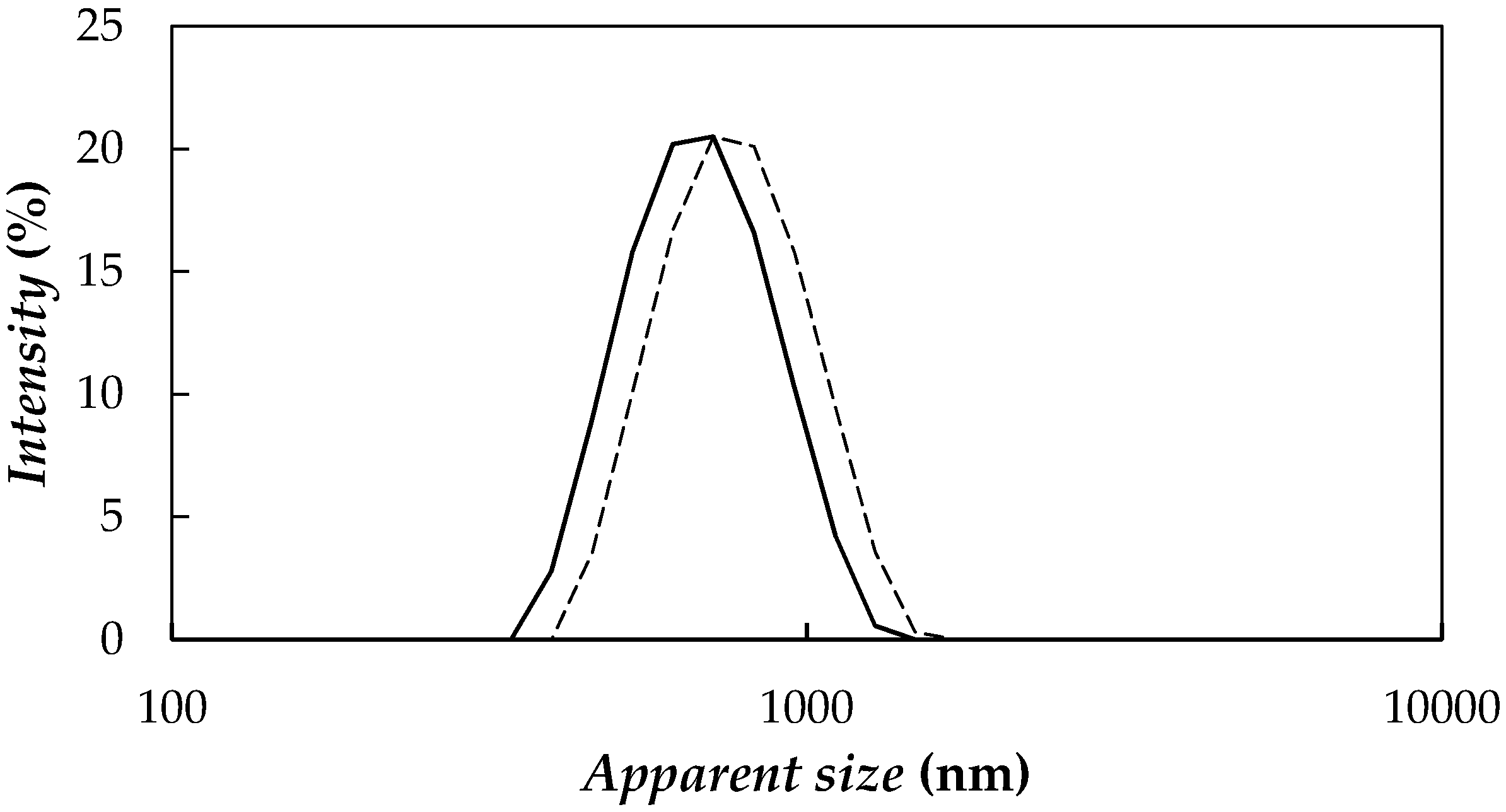
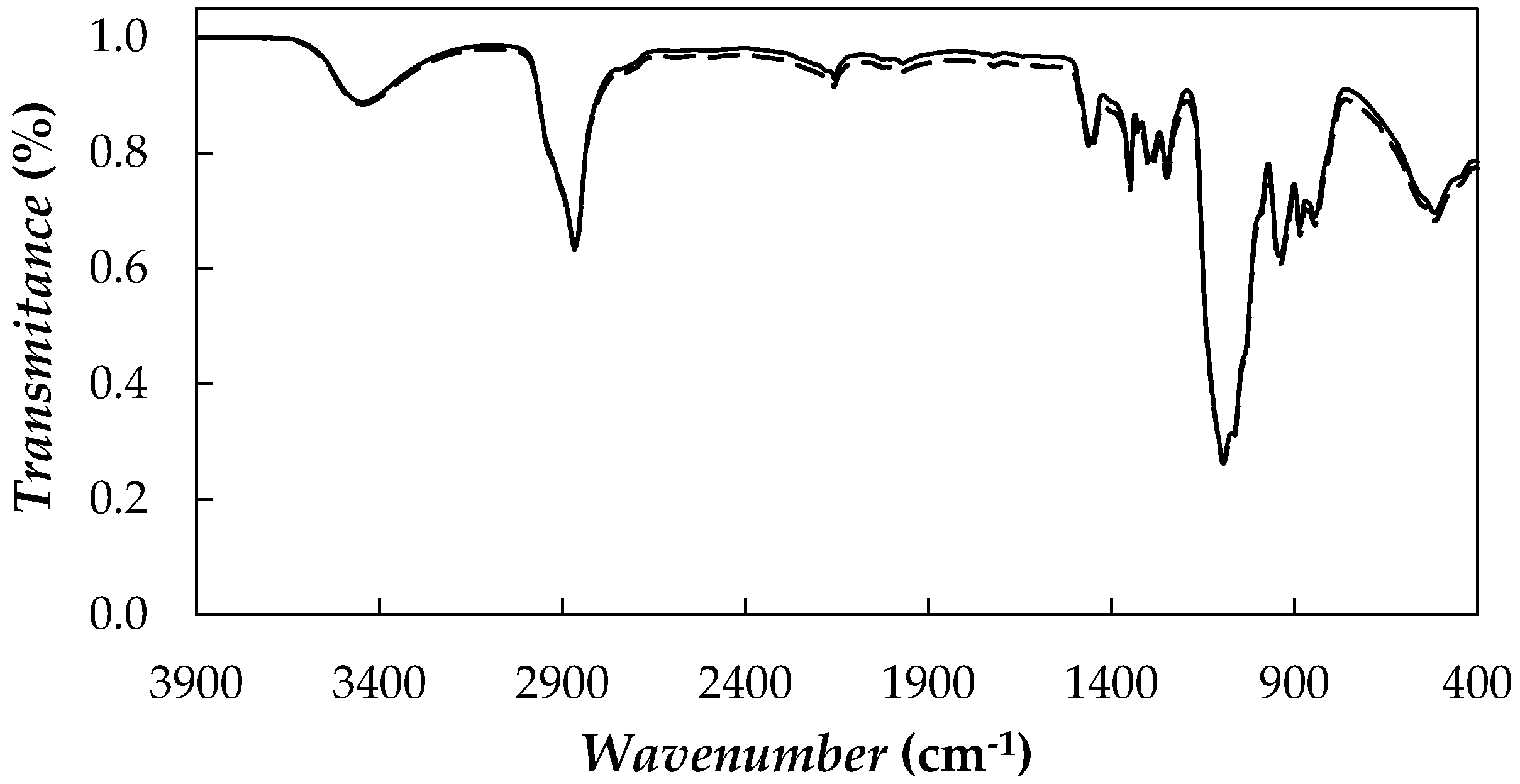



| Nanoparticle Concentration, wt % | Tcrys (K) | Tm (K) | ΔHm (J·g−1) |
|---|---|---|---|
| PEG 400, base fluid 0% | 271.6 | 278.8 | 105.3 |
| fGnP/PEG400 NePCM, 0.10 wt % | 269.9 | 278.5 | 104.4 |
| fGnP/PEG400 NePCM, 0.25 wt % | 268.3 | 277.7 | 100.7 |
| fGnP/PEG400 NePCM, 0.50 wt % | 267.6 | 276.3 | 97.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos, M.A.; Cabaleiro, D.; Guimarey, M.J.G.; Comuñas, M.J.P.; Fedele, L.; Fernández, J.; Lugo, L. PEG 400-Based Phase Change Materials Nano-Enhanced with Functionalized Graphene Nanoplatelets. Nanomaterials 2018, 8, 16. https://doi.org/10.3390/nano8010016
Marcos MA, Cabaleiro D, Guimarey MJG, Comuñas MJP, Fedele L, Fernández J, Lugo L. PEG 400-Based Phase Change Materials Nano-Enhanced with Functionalized Graphene Nanoplatelets. Nanomaterials. 2018; 8(1):16. https://doi.org/10.3390/nano8010016
Chicago/Turabian StyleMarcos, Marco A., David Cabaleiro, María J. G. Guimarey, María J. P. Comuñas, Laura Fedele, Josefa Fernández, and Luis Lugo. 2018. "PEG 400-Based Phase Change Materials Nano-Enhanced with Functionalized Graphene Nanoplatelets" Nanomaterials 8, no. 1: 16. https://doi.org/10.3390/nano8010016






