The Electrospun Ceramic Hollow Nanofibers
Abstract
:1. Introduction
2. Electrospinning-Based Fabrication Methods of Ceramic Hollow Nanofibers
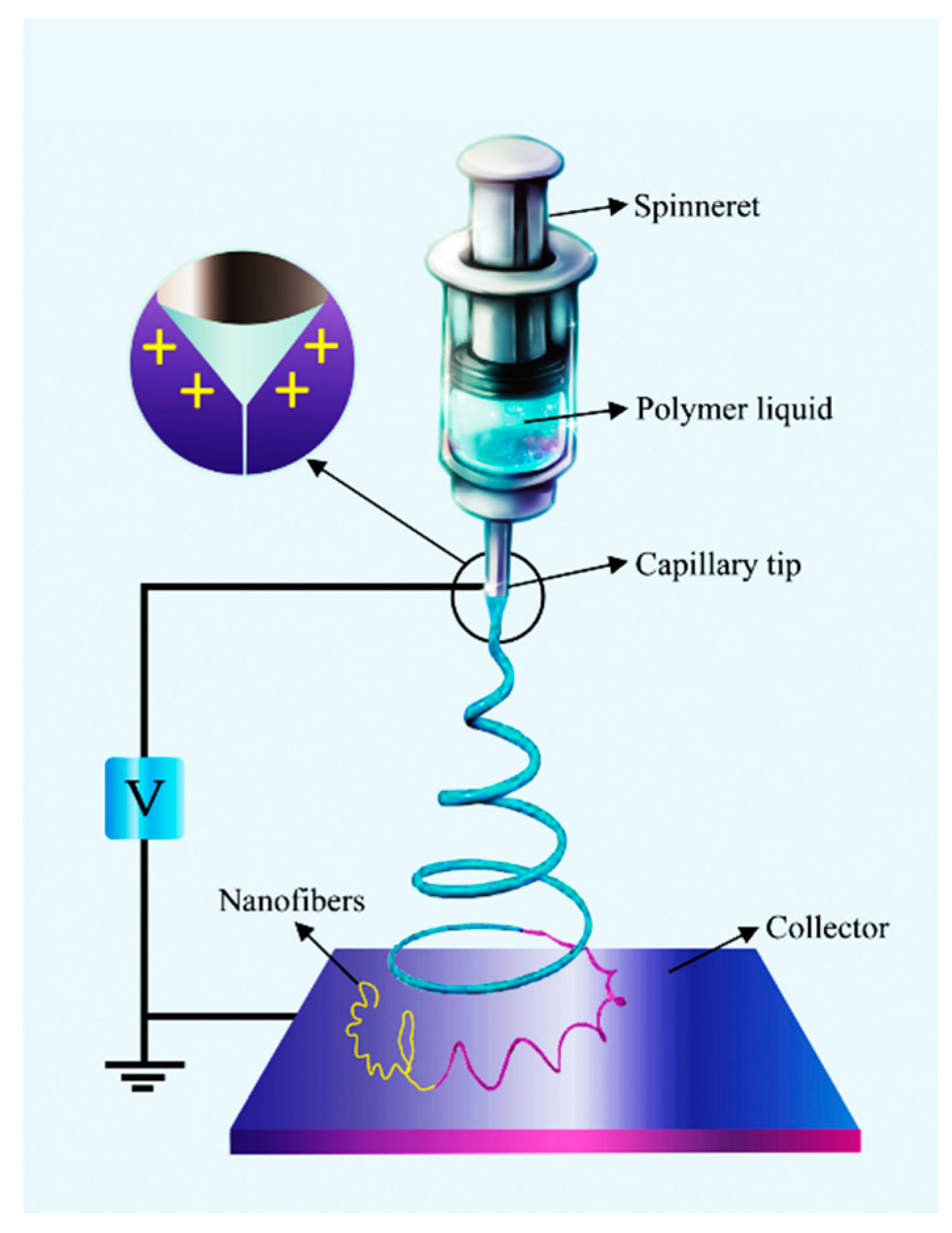
2.1. Electrospinning with a Single Spinneret
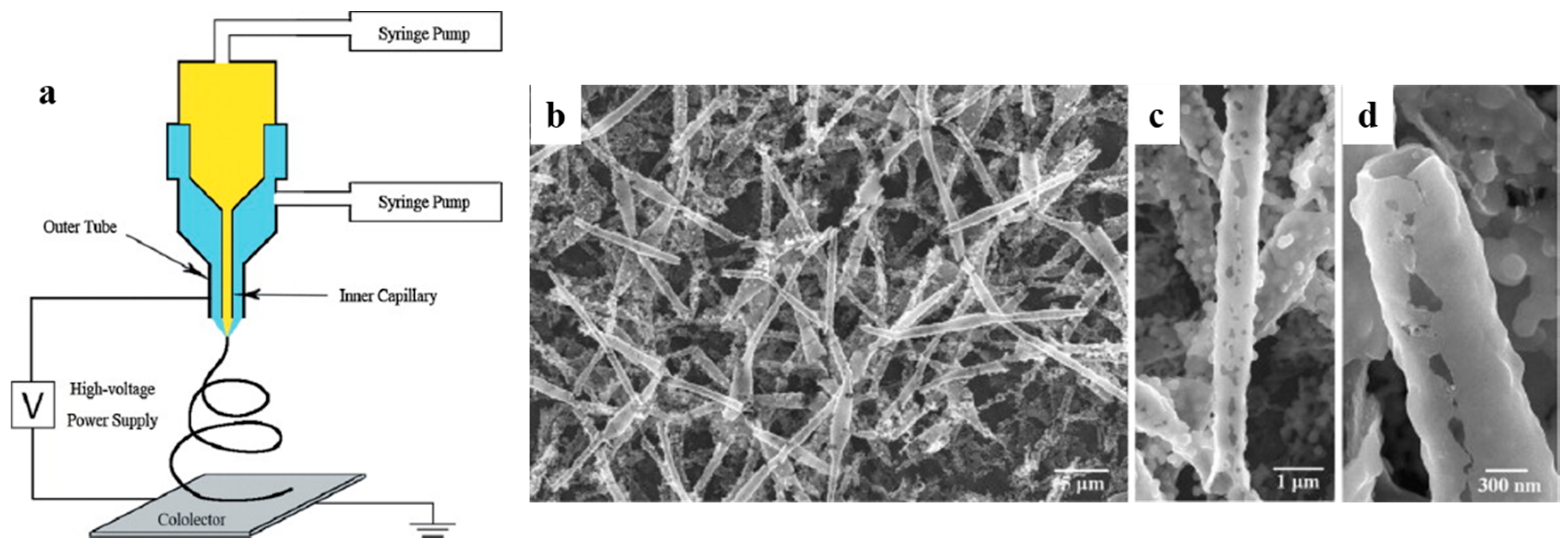
2.2. Coaxial Electrospinning with a Two-Capillary Spinneret
- The sheath layer must be strong enough to retain the hollow structure, otherwise the produced hollow nanofibers will collapse.
- Despite the easiness of this method, continuous and perfect hollow nanofibers are hardly made because of the post-treatment processes applied to remove the core.
- Complete elimination of the core is challenging.
- The hollow nanofibers prepared by this method can consist of only one layer wall.
2.3. Microfluidic Electrospinning
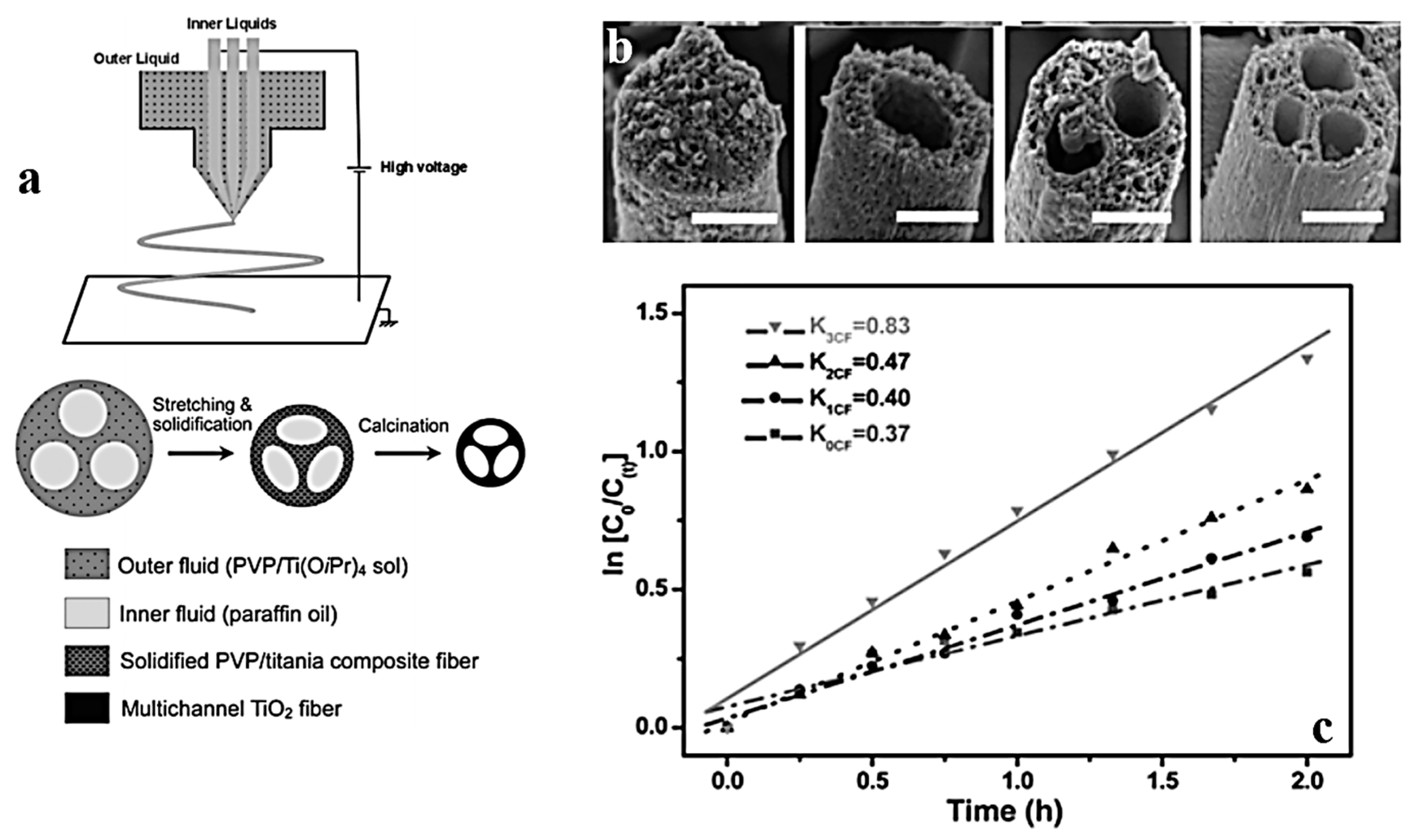
2.4. Triaxial Electrospinning
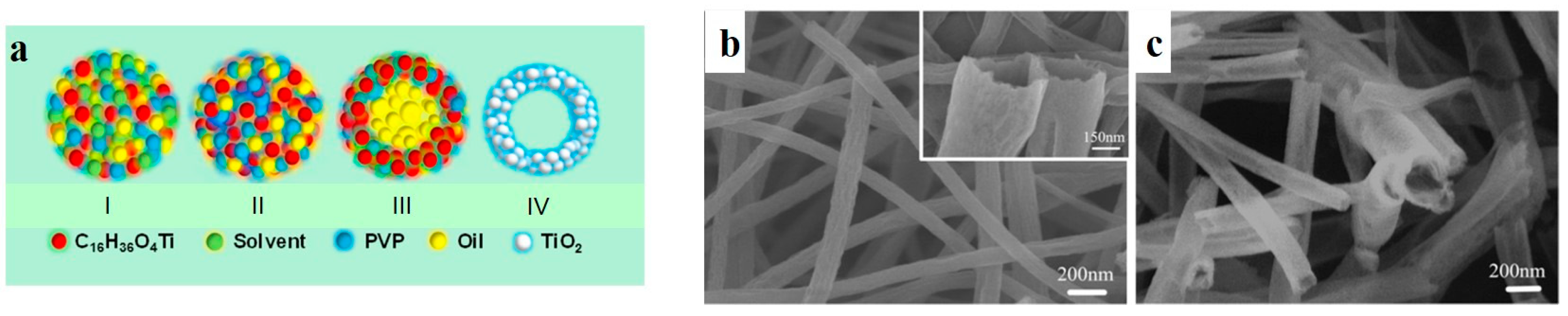
2.5. Emulsion Electrospinning
3. Most Studied Ceramic Hollow Nanofibers and Their Applications
3.1. Titanium Dioxide (TiO2) Hollow Nanofibers for Photodecomposition of Organic Pollutants
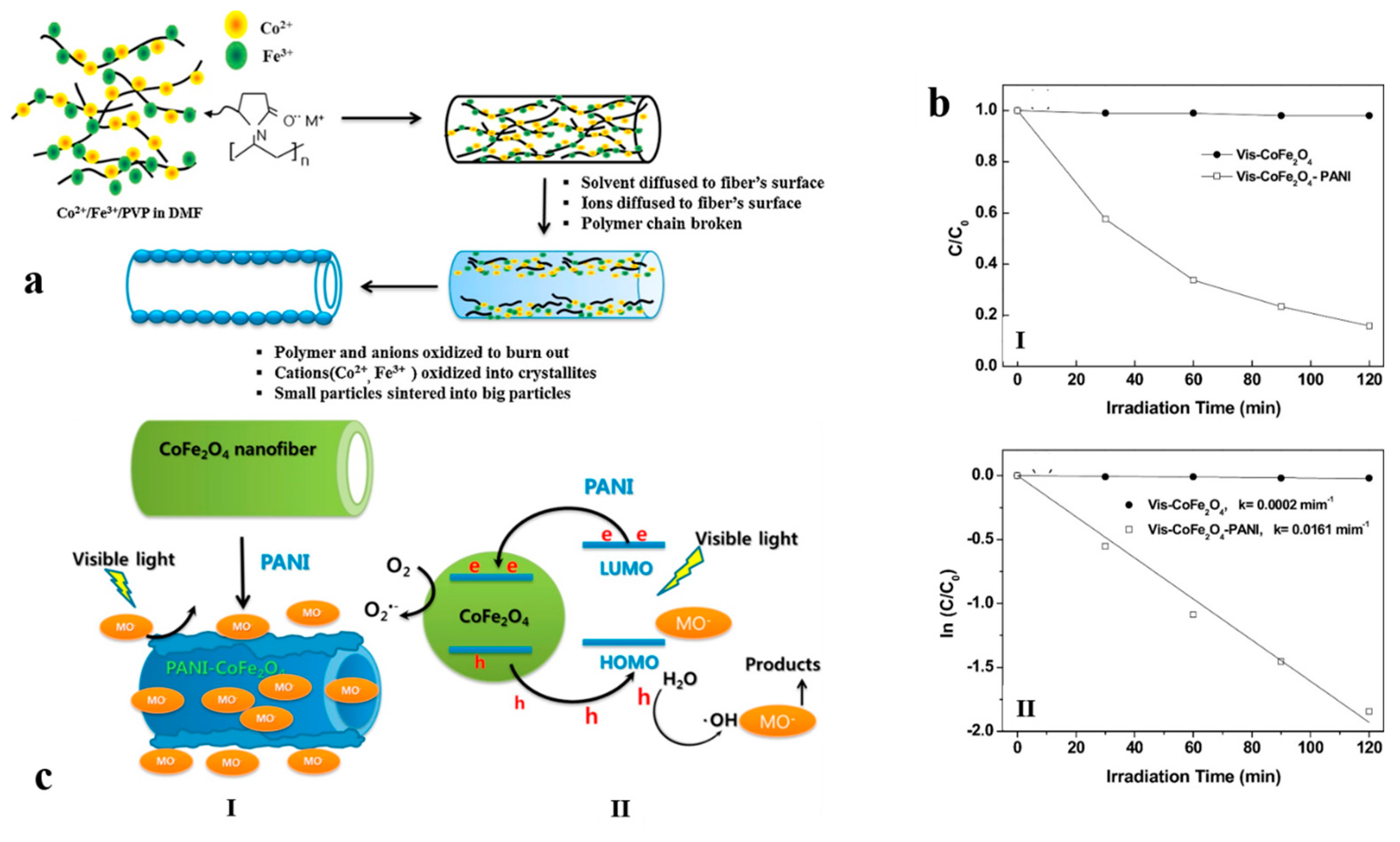
3.2. Ferrite Hollow Nanofibers for Electromagnetic and Photocatalytic Devices
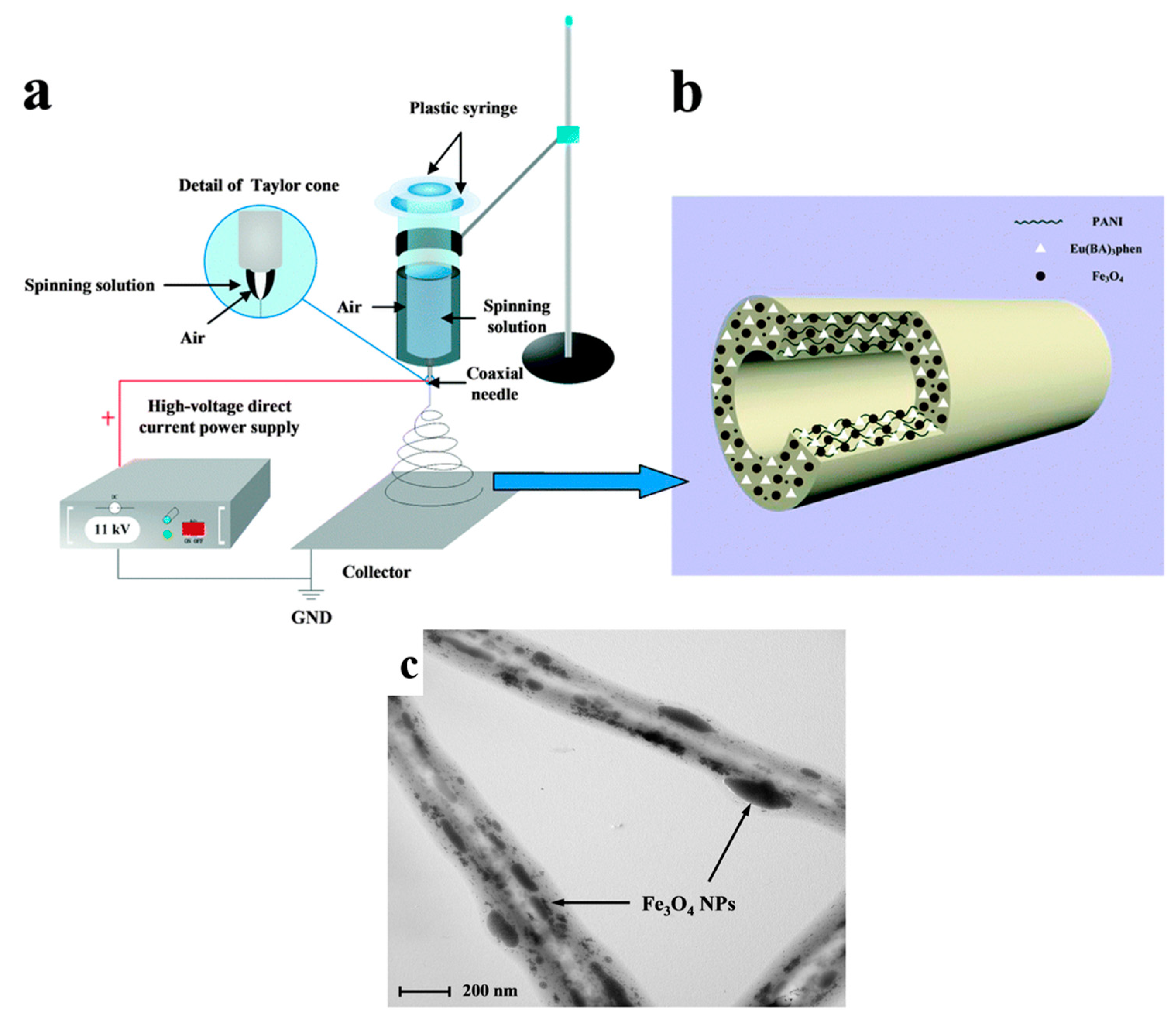
3.3. Iron Compound-Based Hollow Nanofibers for Ferromagnetic Devices
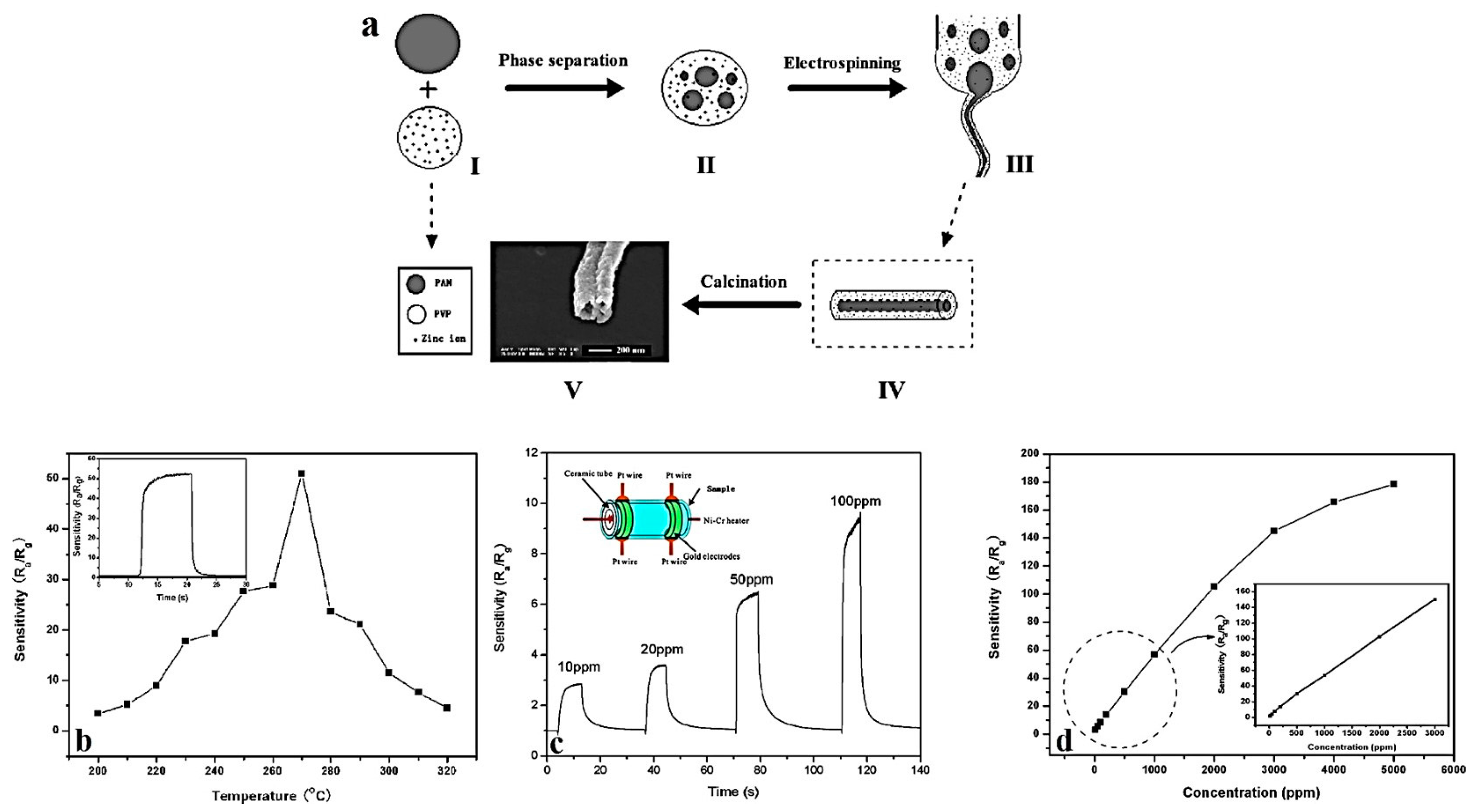
3.4. Zinc Oxide Hollow Nanofibers for Gas Sensing
3.5. Tin Oxide Hollow Nanofibers for Magneto-Optoelectronic Devices
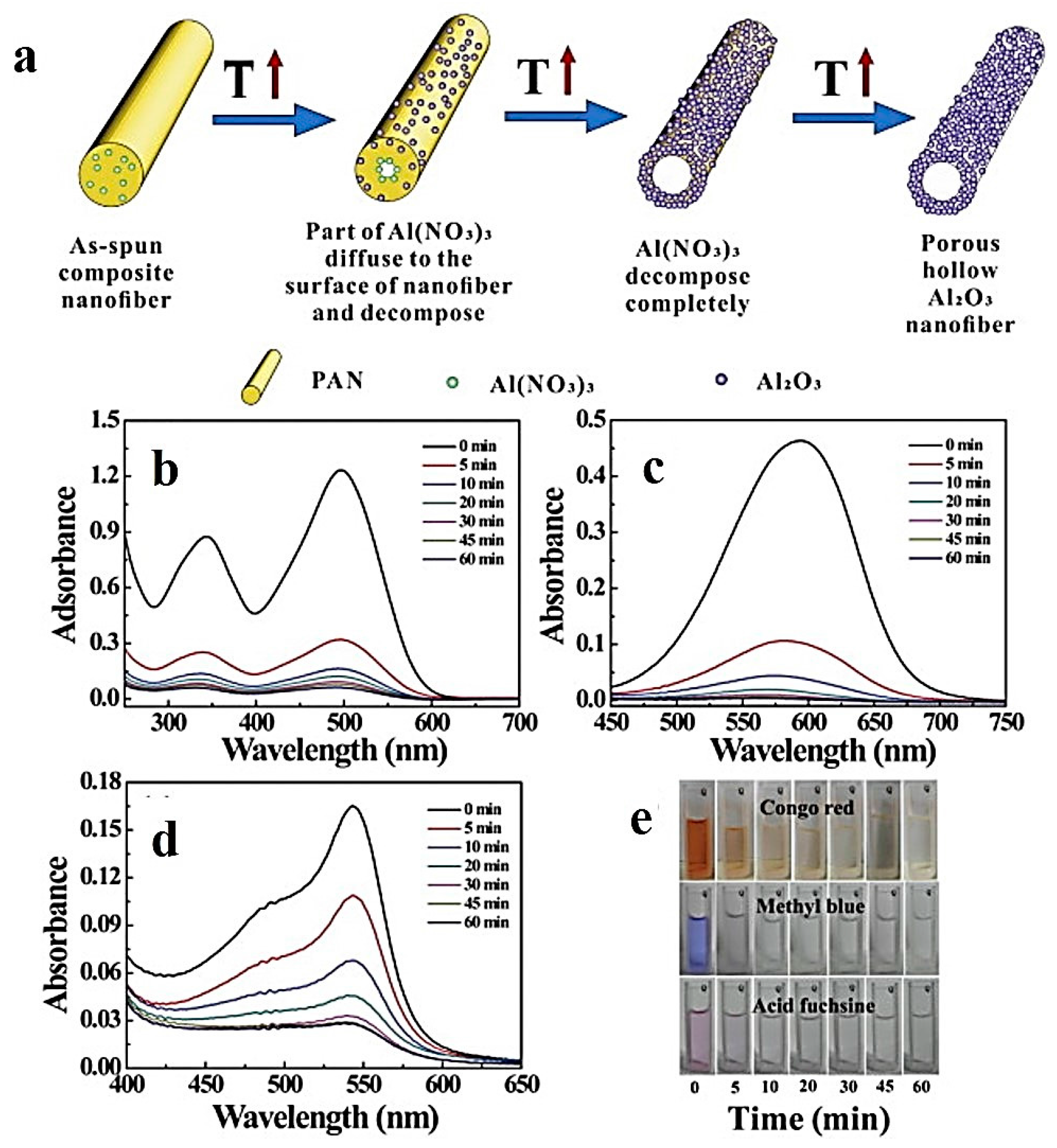
3.6. Aluminium Oxide Hollow Nanofibers for Dye Adsorption
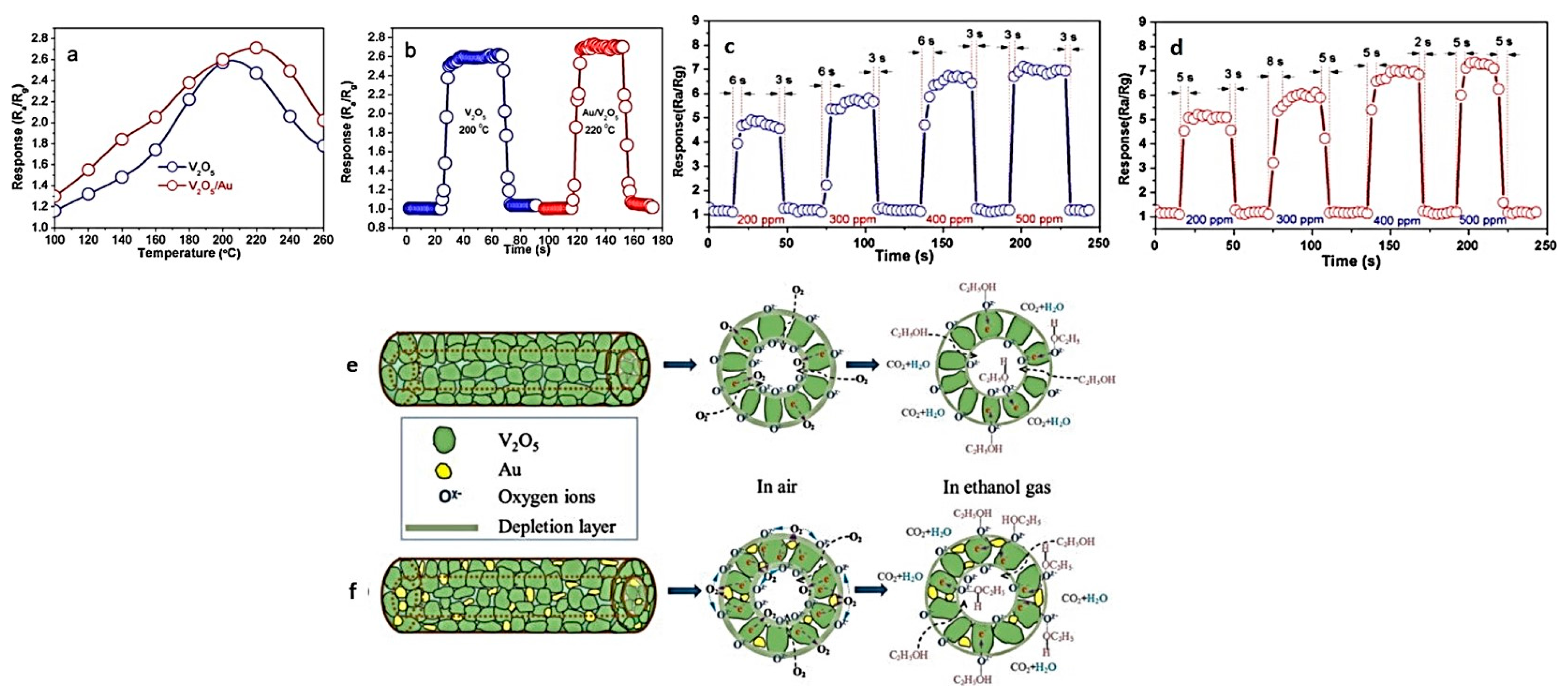
3.7. Vanadium Oxide Hollow Nanofibers for Gas Sensing
4. Summary and Remarks on Future Challenges
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Kim, J.S.; Reneker, D.H. Mechanical properties of composites using ultrafine electrospun fibers. Polym. Compos. 1999, 20, 124–131. [Google Scholar] [CrossRef]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Greiner, A.; Wendorff, J.H. Nanostructured fibers via electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Dai, T.; Elbahri, M. Biofunctionalized nanofibrous membranes as super separators of protein and enzyme from water. J. Colloid Interface Sci. 2013, 406, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Disci-Zayed, D.; Dai, T.; Elbahri, M. Biofunctionalized nanofibrous membranes mimicking carnivorous plants. Bioinspired Biomim. Nanobiomater. 2013, 2, 186–193. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Nanocomposite electrospun nanofiber membranes for environmental remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Zillohu, A.U.; Abdelaziz, R.; Hedayati, M.K.; Elbahri, M. A novel nanohybrid nanofibrous adsorbent for water purification from dye pollutants. Materials 2016, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.S.; Buhr, K.; Ebert, K. Polyethersulfone electrospun nanofibrous composite membrane for liquid filtration. J. Membr. Sci. 2010, 365, 68–77. [Google Scholar] [CrossRef]
- Homaeigohar, S.S.; Elbahri, M. Novel compaction resistant and ductile nanocomposite nanofibrous microfiltration membranes. J. Colloid Interface Sci. 2012, 372, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Reneker, D. DNA fibers by electrospinning. J. Macromol. Sci. B 1997, 36, 169–173. [Google Scholar] [CrossRef]
- Fong, H.; Reneker, D.H. Elastomeric nanofibers of styrene-butadiene-styrene triblock copolymer. J. Polym. Sci. Polym. Phys. 1999, 37, 3488–3493. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [PubMed]
- He, J.H.; Wan, Y.Q.; Yu, J.Y. Application of vibration technology to polymer electrospinning. Int. J. Nonlinear Sci. Numer. Simul. 2004, 5, 253–262. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Dzenis, Y. Spinning continuous fibers for nanotechnology. Science 2004, 304, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Electrospinning of manmade and biopolymer nanofibers-progress in techniques, materials, and applications. Adv. Funct. Mater. 2009, 19, 2863–2879. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Wang, C.; Wei, L.; Liu, Y.; Shao, C. Zno hollow nanofibers: Fabrication from facile single capillary electrospinning and applications in gas sensors. J. Phys. Chem. C 2009, 113, 19397–19403. [Google Scholar] [CrossRef]
- Zhang, T.; Ge, L.; Wang, X.; Gu, Z. Hollow TiO2 containing multilayer nanofibers with enhanced photocatalytic activity. Polymer 2008, 49, 2898–2902. [Google Scholar] [CrossRef]
- Choi, K.-I.; Kim, H.-R.; Lee, J.-H. Enhanced co sensing characteristics of hierarchical and hollow In2O3 microspheres. Sens. Actuators B-Chem. 2009, 138, 497–503. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, F.; Liu, L.; Xu, H.; Sun, C. Synthesis of indium tin oxide nanotubes using 2-methoxyethanol as solvent via simple template method. J. Alloys Compd. 2010, 504, 171–176. [Google Scholar] [CrossRef]
- Ab Kadir, R.; Li, Z.; Sadek, A.Z.; Abdul Rani, R.; Zoolfakar, A.S.; Field, M.R.; Ou, J.Z.; Chrimes, A.F.; Kalantar-Zadeh, K. Electrospun granular hollow SnO2 nanofibers hydrogen gas sensors operating at low temperatures. J. Phys. Chem. C 2014, 118, 3129–3139. [Google Scholar] [CrossRef]
- Chu, Z.; Cheng, H.; Xie, W.; Sun, L. Effects of diameter and hollow structure on the microwave absorption properties of short carbon fibers. Ceram. Int. 2012, 38, 4867–4873. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, L.; Wang, P.; Guo, L.; Yu, J. Low material density and high microwave-absorption performance of hollow strontium ferrite nanofibers prepared via coaxial electrospinning. J. Alloys Compd. 2016, 687, 541–547. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, J.; Dong, X.; Yu, W.; Liu, G.; Xu, J. Electrospinning preparation and properties of magnetic-photoluminescent bifunctional coaxial nanofibers. J. Mater. Chem. 2012, 22, 14438–14442. [Google Scholar] [CrossRef]
- Choi, S.-H.; Ankonina, G.; Youn, D.-Y.; Oh, S.-G.; Hong, J.-M.; Rothschild, A.; Kim, I.-D. Hollow ZnO nanofibers fabricated using electrospun polymer templates and their electronic transport properties. ACS Nano 2009, 3, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Zheng, X.; Chen, R.; Chen, X.; Chen, L.; Chen, Z. Silver nanoparticles filling in TiO2 hollow nanofibers by coaxial electrospinning. Acta Phys. Chim. Sin. 2008, 24, 1790–1797. [Google Scholar] [CrossRef]
- Nagamine, S.; Ochi, S.; Ohshima, M. Fabrication of TiO2 hollow fibers with surface nanostructure. Mater. Res. Bull. 2011, 46, 2328–2332. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Cui, Q.; Liu, G.; Yu, W. Fabrication of LaNiO3 porous hollow nanofibers via an electrospinning technique. Mod. Appl. Sci. 2008, 3, 75. [Google Scholar] [CrossRef]
- Yao, P.-J.; Wang, J.; Qiao, Q.; Du, H.-Y. Direct fabrication of La0.7Sr0.3FeO3 nanofibers with tunable hollow structures by electrospinning and their gas sensing properties. J. Mater. Sci. 2015, 50, 1338–1349. [Google Scholar] [CrossRef]
- Gu, Y.; Jian, F. Hollow LiNi0.8Co0.1Nn0.1O2–MgO coaxial fibers: Sol–gel method combined with Co-electrospun preparation and electrochemical properties. J. Phys. Chem. C 2008, 112, 20176–20180. [Google Scholar] [CrossRef]
- Mohanapriya, P.; Pradeepkumar, R.; Victor Jaya, N.; Natarajan, T. Magnetic and optical properties of electrospun hollow nanofibers of SnO2 doped with Ce-ion. Appl. Phys. Lett. 2014, 105, 022406. [Google Scholar] [CrossRef]
- Li, D.; Dong, X.; Yu, W.; Wang, J.; Liu, G. Synthesis and upconversion luminescence properties of YF3: Yb3+/Er3+ hollow nanofibers derived from Y2O3: Yb3+/Er3+ hollow nanofibers. J. Nanopart. Res. 2013, 15, 1704. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow carbon nanofiber-encapsulated sulfur cathodes for high specific capacity rechargeable lithium batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Dong, X.; Wei, Q.; Cai, Y.; Lu, K. Formation mechanism of porous hollow SnO2 nanofibers prepared by one-step electrospinning. Express Polym. Lett. 2012, 6, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.; Lin, T. Fiber generators in needleless electrospinning. J. Nanomater. 2012, 2012, 12. [Google Scholar] [CrossRef]
- Shadi, L.; Karimi, M.; Ramazani, S.; Entezami, A.A. Preparation of electrospun nanofibers of star-shaped polycaprolactone and its blends with polyaniline. J. Mater. Sci. 2014, 49, 4844–4854. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, H.; Zhao, Y.; Zhu, Z.; Fong, H. Electrospun polyimide nanofibers and their applications. Prog. Polym. Sci. 2016, 61, 67–103. [Google Scholar] [CrossRef]
- Rodríguez, K.; Gatenholm, P.; Renneckar, S. Electrospinning cellulosic nanofibers for biomedical applications: Structure and in vitro biocompatibility. Cellulose 2012, 19, 1583–1598. [Google Scholar] [CrossRef]
- Mokhena, T.; Jacobs, V.; Luyt, A. A review on electrospun bio-based polymers for water treatment. Express Polym. Lett. 2015, 9, 839–880. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun porous cellulose acetate fibers from volatile solvent mixture. Mater. Lett. 2011, 65, 2291–2294. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Zhou, M.; Du, W. Improved acetone sensing properties of zno hollow nanofibers by single capillary electrospinning. Sens. Actuators B Chem. 2011, 160, 753–759. [Google Scholar] [CrossRef]
- Yu, W.; Ma, Q.; Li, X.; Dong, X.; Wang, J.; Liu, G. One-pot coaxial electrospinning fabrication and properties of magnetic-luminescent bifunctional flexible hollow nanofibers. Mater. Lett. 2014, 120, 126–129. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Q.; Yang, M.; Dong, X.; Yang, Y.; Wang, J.; Yu, W.; Liu, G. Flexible hollow nanofibers: Novel one-pot electrospinning construction, structure and tunable luminescence–electricity–magnetism trifunctionality. Chem. Eng. J. 2016, 284, 831–840. [Google Scholar] [CrossRef]
- Chang, W.; Xu, F.; Mu, X.; Ji, L.; Ma, G.; Nie, J. Fabrication of nanostructured hollow TiO2 nanofibers with enhanced photocatalytic activity by coaxial electrospinning. Mater. Res. Bull. 2013, 48, 2661–2668. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Z.; Nakata, K.; Nishimoto, S.; Murakami, T.; Zhao, Y.; Jiang, L.; Fujishima, A. Multichannel TiO 2 hollow fibers with enhanced photocatalytic activity. J. Mater. Chem. 2010, 20, 5095–5099. [Google Scholar] [CrossRef]
- Kalra, V.; Lee, J.H.; Park, J.H.; Marquez, M.; Joo, Y.L. Confined assembly of asymmetric block-copolymer nanofibers via multiaxial jet electrospinning. Small 2009, 5, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Chen, W.; Li, Z.; Zhang, H.; Li, T. Rapid and sensitive ethanol sensor based on hollow Au/V2O5 nanotubes via emulsion-electrospinning route. Mater. Res. Bull. 2015, 65, 157–162. [Google Scholar] [CrossRef]
- Wang, T.; Wei, J.; Shi, H.; Zhou, M.; Zhang, Y.; Chen, Q.; Zhang, Z. Preparation of electrospun Ag/TiO2 nanotubes with enhanced photocatalytic activity based on water/oil phase separation. Physica E 2017, 86, 103–110. [Google Scholar] [CrossRef]
- Qi, R.; Guo, R.; Shen, M.; Cao, X.; Zhang, L.; Xu, J.; Yu, J.; Shi, X. Electrospun poly (lactic-co-glycolic acid)/halloysite nanotube composite nanofibers for drug encapsulation and sustained release. J. Mater. Chem. 2010, 20, 10622–10629. [Google Scholar] [CrossRef]
- Zander, N.E.; Strawhecker, K.E.; Orlicki, J.A.; Rawlett, A.M.; Beebe, T.P. Coaxial electrospun poly(methyl methacrylate)–polyacrylonitrile nanofibers: Atomic force microscopy and compositional characterization. J. Phys. Chem. B 2011, 115, 12441–12447. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhu, Y.; Ma, J.; Yang, S.; Gong, J.; Xu, J. Preparation of continuous porous alumina nanofibers with hollow structure by single capillary electrospinning. Colloids Surf. A 2013, 436, 489–494. [Google Scholar] [CrossRef]
- Hou, H.; Shang, M.; Wang, L.; Li, W.; Tang, B.; Yang, W. Efficient photocatalytic activities of TiO2 hollow fibers with mixed phases and mesoporous walls. Sci. Rep. 2015, 5, 15228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, C.; Zhao, Y.; Tang, G.; Yuan, X. Electrospinning of ultrafine core/shell fibers for biomedical applications. Sci. China Chem. 2010, 53, 1246–1254. [Google Scholar] [CrossRef]
- Huang, J.; You, T. Electrospun nanofibers: From rational design, fabrication to electrochemical sensing applications. In Advances in Nanofibers; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Zhou, F.-L.; Gong, R.-H.; Porat, I. Three-jet electrospinning using a flat spinneret. J. Mater. Sci. 2009, 44, 5501–5508. [Google Scholar] [CrossRef]
- Nurwaha, D.; Han, W.; Wang, X. Investigation of a new needleless electrospinning method for the production of nanofibers. J. Eng. Fibers Fabr. 2013, 8, 42–49. [Google Scholar]
- Forward, K.M.; Rutledge, G.C. Free surface electrospinning from a wire electrode. Chem. Eng. J. 2012, 183, 492–503. [Google Scholar] [CrossRef]
- Tijing, L.D.; Choi, W.; Jiang, Z.; Amarjargal, A.; Park, C.-H.; Pant, H.R.; Im, I.-T.; Kim, C.S. Two-nozzle electrospinning of (MWNT/PU)/PU nanofibrous composite mat with improved mechanical and thermal properties. Curr. Appl. Phys. 2013, 13, 1247–1255. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Zhang, A.; Hu, J.; Wang, X.; Yang, Z. Electrospun nanofibers for cancer diagnosis and therapy. Biomater. Sci. 2016, 4, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Song, J.-C.; Yoon, K.-B. Controlled wall thickness and porosity of polymeric hollow nanofibers by coaxial electrospinning. Macromol. Res. 2010, 18, 571–576. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. Core–shell structured peo-chitosan nanofibers by coaxial electrospinning. Biomacromolecules 2012, 13, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Chattopadhyay, S.; Kim, J.J.; Kim, S.-J.; Tuller, H.L.; Rutledge, G.C.; Kim, I.-D. Coaxial electrospinning of WO3 nanotubes functionalized with bio-inspired pd catalysts and their superior hydrogen sensing performance. Nanoscale 2016, 8, 9159–9166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Choi, S.-W.; Kim, S.S. Micro-and nano-scale hollow TiO2 fibers by coaxial electrospinning: Preparation and gas sensing. J. Solid State Chem. 2011, 184, 3008–3013. [Google Scholar] [CrossRef]
- Lee, B.-S.; Son, S.-B.; Park, K.-M.; Lee, G.; Oh, K.H.; Lee, S.-H.; Yu, W.-R. Effect of pores in hollow carbon nanofibers on their negative electrode properties for a lithium rechargeable battery. ACS Appl. Mater. Interfaces 2012, 4, 6702–6710. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Yang, H.-S.; Jung, H.; Mah, S.K.; Kwon, S.; Park, J.-H.; Lee, K.H.; Yu, W.-R.; Doo, S.-G. Facile method to improve initial reversible capacity of hollow carbon nanofiber anodes. Eur. Polym. J. 2015, 70, 392–399. [Google Scholar] [CrossRef]
- Kaerkitcha, N.; Chuangchote, S.; Sagawa, T. Control of physical properties of carbon nanofibers obtained from coaxial electrospinning of pmma and pan with adjustable inner/outer nozzle-ends. Nanoscale Res. Lett. 2016, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Yang, M.; Yang, H.; Xu, Z. Tailoring the morphologies of PVDF nanofibers by interfacial diffusion during coaxial electrospinning. Mater. Des. 2016, 109, 264–269. [Google Scholar] [CrossRef]
- Lee, B.-S.; Jeon, S.-Y.; Park, H.; Lee, G.; Yang, H.-S.; Yu, W.-R. New electrospinning nozzle to reduce jet instability and its application to manufacture of multi-layered nanofibers. Sci. Rep. 2013, 4, 6758. [Google Scholar] [CrossRef] [PubMed]
- Loscertales, I.G.; Barrero, A.; Márquez, M.; Spretz, R.; Velarde-Ortiz, R.; Larsen, G. Electrically forced coaxial nanojets for one-step hollow nanofiber design. J. Am. Chem. Soc. 2004, 126, 5376–5377. [Google Scholar] [CrossRef] [PubMed]
- Na, H.; Chen, P.; Wong, S.-C.; Hague, S.; Li, Q. Fabrication of PVDF/PVA microtubules by coaxial electrospinning. Polymer 2012, 53, 2736–2743. [Google Scholar] [CrossRef]
- Qian, W.; Yu, D.-G.; Li, Y.; Liao, Y.-Z.; Wang, X.; Wang, L. Dual drug release electrospun core-shell nanofibers with tunable dose in the second phase. Int. J. Mol. Sci. 2014, 15, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, Q.; Peng, M.; Wang, X.; Yang, J. Preparation and drug delivery study of electrospun hollow pes ultrafine fibers with a multilayer wall. Colloid Polym. Sci. 2014, 292, 1339–1345. [Google Scholar] [CrossRef]
- Li, X.H.; Shao, C.L.; Liu, Y.C. A simple method for controllable preparation of polymer nanotubes via a single capillary electrospinning. Langmuir 2007, 23, 10920–10923. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, N.; Di, J.; Zhao, Y.; Song, Y.; Jiang, L. Nanowire-in-microtube structured core/shell fibers via multifluidic coaxial electrospinning. Langmuir 2010, 26, 11291–11296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, X.; Jiang, L.; Qin, J. Flexible generation of gradient electrospinning nanofibers using a microfluidic assisted approach. Langmuir 2012, 28, 10026–10032. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.K.; Kang, E.; Khademhosseini, A.; Lee, S.H. Micro/nanometer-scale fiber with highly ordered structures by mimicking the spinning process of silkworm. Adv. Mater. 2013, 25, 3071–3078. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, Y.; Marquez, M.; Thorsen, T. Multijet electrospinning of conducting nanofibers from microfluidic manifolds. J. Appl. Polym. Sci. 2007, 106, 3171–3178. [Google Scholar] [CrossRef]
- Srivastava, Y.; Loscertales, I.; Marquez, M.; Thorsen, T. Electrospinning of hollow and core/sheath nanofibers using a microfluidic manifold. Microfluid. Nanofluid. 2008, 4, 245–250. [Google Scholar] [CrossRef]
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.B.; Arnold, L. Recent advances in nanofibre fabrication techniques. Text. Res. J. 2012, 82, 129–147. [Google Scholar] [CrossRef]
- Liu, W.; Ni, C.; Chase, D.B.; Rabolt, J.F. Preparation of multilayer biodegradable nanofibers by triaxial electrospinning. ACS Macro Lett. 2013, 2, 466–468. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, X.-Y.; Wang, X.; Yang, J.-H.; Bligh, S.; Williams, G.R. Nanofibers fabricated using triaxial electrospinning as zero order drug delivery systems. ACS Appl. Mater. Interfaces 2015, 7, 18891–18897. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Steckl, A.J. Triaxial electrospun nanofiber membranes for controlled dual release of functional molecules. ACS Appl. Mater. Interfaces 2013, 5, 8241–8245. [Google Scholar] [CrossRef] [PubMed]
- Khalf, A.; Singarapu, K.; Madihally, S.V. Influence of solvent characteristics in triaxial electrospun fiber formation. React. Funct. Polym. 2015, 90, 36–46. [Google Scholar] [CrossRef]
- Zanjani, J.S.M.; Okan, B.S.; Letofsky-Papst, I.; Yildiz, M.; Menceloglu, Y.Z. Rational design and direct fabrication of multi-walled hollow electrospun fibers with controllable structure and surface properties. Eur. Polym. J. 2015, 62, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Zanjani, J.S.M.; Saner Okan, B.; Menceloglu, Y.Z.; Yildiz, M. Design and fabrication of multi-walled hollow nanofibers by triaxial electrospinning as reinforcing agents in nanocomposites. J. Reinf. Plast. Compos. 2015, 34, 1273–1286. [Google Scholar] [CrossRef]
- Tang, S.; Zeng, Y.; Wang, X. Splashing needleless electrospinning of nanofibers. Polym. Eng. Sci. 2010, 50, 2252–2257. [Google Scholar] [CrossRef]
- Zhou, F.L.; Gong, R.H.; Porat, I. Needle and needleless electrospinning for nanofibers. J. Appl. Polym. Sci. 2010, 115, 2591–2598. [Google Scholar] [CrossRef]
- Vonch, J.; Yarin, A.; Megaridis, C. Electrospinning: A study in the formation of nanofibers. J. Undergrad. Res. 2007, 1. [Google Scholar] [CrossRef]
- Samanta, A.; Nandan, B.; Srivastava, R.K. Morphology of electrospun fibers derived from high internal phase emulsions. J. Colloid Interface Sci. 2016, 471, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Shi, H.; Zhou, M.; Song, D.; Zhang, Y.; Pan, X.; Zhou, J.; Wang, T. Effect of oil on the morphology and photocatalysis of emulsion electrospun titanium dioxide nanomaterials. Appl. Catal. 2015, 499, 101–108. [Google Scholar] [CrossRef]
- Elahi, F.; Lu, W.; Guoping, G.; Khan, F. Core-shell fibers for biomedical applications—A review. Bioeng. Biomed. Sci. J. 2013, 3, 1–14. [Google Scholar] [CrossRef]
- Samanta, A.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Electrospun composite matrices of poly (ε-caprolactone)-montmorillonite made using tenside free Pickering emulsions. Mater. Sci. Eng. 2016, 69, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Angeles, M.; Cheng, H.L.; Velankar, S.S. Emulsion electrospinning: Composite fibers from drop breakup during electrospinning. Polym. Adv. Technol. 2008, 19, 728–733. [Google Scholar] [CrossRef]
- Choi, K.-I.; Lee, S.H.; Park, J.-Y.; Choi, D.-Y.; Hwang, C.-H.; Lee, I.-H.; Chang, M.H. Fabrication and characterization of hollow TiO2 fibers by microemulsion electrospinning for photocatalytic reactions. Mater. Lett. 2013, 112, 113–116. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, D.; Song, G.; Nie, J.; Chen, B.; Ma, G. Fabrication of core–shell nanofibers by single capillary electrospinning combined with vapor induced phase separation. New J. Chem. 2013, 37, 2917–2924. [Google Scholar] [CrossRef]
- Pal, J.; Singh, S.; Sharma, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Emulsion electrospun composite matrices of poly (ε-caprolactone)-hydroxyapatite: Strategy for hydroxyapatite confinement and retention on fiber surface. Mater. Lett. 2016, 167, 288–296. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Tong, S.; Li, X.; Song, W. Three-dimensional network films of electrospun copper oxide nanofibers for glucose determination. Biosens. Bioelectron. 2009, 25, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, E.; Taghavinia, N.; Zakeeruddin, S.M.; Grätzel, M.; Moser, J.-E. Enhanced electron collection efficiency in dye-sensitized solar cells based on nanostructured TiO2 hollow fibers. Nano Lett. 2010, 10, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S. Novel hollow mesoporous 1D TiO2 nanofibers as photovoltaic and photocatalytic materials. Nanoscale 2012, 4, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-K.; Hwang, I.-S.; Kim, S.-J.; Park, J.-S.; Park, S.-S.; Jeong, U.; Kang, Y.C.; Lee, J.-H. Design of selective gas sensors using electrospun pd-doped SnO2 hollow nanofibers. Sens. Actuators B Chem. 2010, 150, 191–199. [Google Scholar] [CrossRef]
- Cho, N.G.; Yang, D.J.; Jin, M.-J.; Kim, H.-G.; Tuller, H.L.; Kim, I.-D. Highly sensitive SnO2 hollow nanofiber-based NO2 gas sensors. Sens. Actuators B 2011, 160, 1468–1472. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, W.; Zhang, Y.; Zhu, L.; Liu, Y.; Fan, X.; Cao, X. Preparation of TiO2 hollow nanofibers by electrospining combined with sol-gel process. CrystEngComm 2010, 12, 2256–2260. [Google Scholar] [CrossRef]
- Peng, X.; Santulli, A.C.; Sutter, E.; Wong, S.S. Fabrication and enhanced photocatalytic activity of inorganic core–shell nanofibers produced by coaxial electrospinning. Chem. Sci. 2012, 3, 1262–1272. [Google Scholar] [CrossRef]
- He, G.; Cai, Y.; Zhao, Y.; Wang, X.; Lai, C.; Xi, M.; Zhu, Z.; Fong, H. Electrospun anatase-phase TiO2 nanofibers with different morphological structures and specific surface areas. J. Colloid Interface Sci 2013, 398, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Yu, Y.; Mu, X.; van Aken, P.A.; Maier, J. Multichannel hollow TiO2 nanofibers fabricated by single-nozzle electrospinning and their application for fast lithium storage. Electrochem. Commun. 2013, 28, 54–57. [Google Scholar] [CrossRef]
- Lee, S.; Ha, J.; Choi, J.; Song, T.; Lee, J.W.; Paik, U. 3D cross-linked nanoweb architecture of binder-free Tio2 electrodes for lithium ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 11525–11529. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-Y.; Lee, D.; Lee, Y.-S. Cnt-embedded hollow TiO2 nanofibers with high adsorption and photocatalytic activity under uv irradiation. J. Alloys Compd. 2015, 622, 651–656. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Pant, H.R.; Mousa, H.M.; Lee, J.; Kim, H.J.; Park, C.H.; Kim, C.S. Synthesis of high porous electrospun hollow TiO2 nanofibers for bone tissue engineering application. J. Ind. Eng. Chem. 2016, 35, 75–82. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, J.; Ye, W.; Yu, C.; Chang, Y. Preparation of Pt/TiO2 hollow nanofibers with highly visible light photocatalytic activity. Appl. Surf. Sci. 2017, 392, 472–480. [Google Scholar] [CrossRef]
- Cheng, Y.; Zou, B.; Yang, J.; Wang, C.; Liu, Y.; Fan, X.; Zhu, L.; Wang, Y.; Ma, H.; Cao, X. Fabrication of CoFe2O4 hollow fibers by direct annealing of the electrospun composite fibers and their magnetic properties. CrystEngComm 2011, 13, 2268–2272. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, Y.; Yan, X.; Sun, D.; Zhu, F.; Xue, Q. Magnetic and electrochemical properties of CuFe2O4 hollow fibers fabricated by simple electrospinning and direct annealing. CrystEngComm 2012, 14, 5879–5885. [Google Scholar] [CrossRef]
- Kim, K.N.; Jung, H.-R.; Lee, W.-J. Hollow cobalt ferrite–polyaniline nanofibers as magnetically separable visible-light photocatalyst for photodegradation of methyl orange. J. Photochem. Photobiol. A 2016, 321, 257–265. [Google Scholar] [CrossRef]
- Cheng, Y.; Zou, B.; Wang, C.; Liu, Y.; Fan, X.; Zhu, L.; Wang, Y.; Ma, H.; Cao, X. Formation mechanism of Fe2O3 hollow fibers by direct annealing of the electrospun composite fibers and their magnetic, electrochemical properties. CrystEngComm 2011, 13, 2863–2870. [Google Scholar] [CrossRef]
- Zhan, S.; Qiu, M.; Yang, S.; Zhu, D.; Yu, H.; Li, Y. Facile preparation of MnO2 doped Fe2O3 hollow nanofibers for low temperature SCR of NO with NH3. J. Mater. Chem. A 2014, 2, 20486–20493. [Google Scholar] [CrossRef]
- Wei, B.-B.; Wu, Y.-B.; Yu, F.-Y.; Zhou, Y.-N. Preparation and electrochemical properties of carbon-coated LiFePO4 hollow nanofibers. Int. J. Miner. Metall. Mater. 2016, 23, 474–480. [Google Scholar] [CrossRef]
- Xiang, H.; Long, Y.; Yu, X.; Zhang, X.; Zhao, N.; Xu, J. A novel and facile method to prepare porous hollow CuO and Cu nanofibers based on electrospinning. CrystEngComm 2011, 13, 4856–4860. [Google Scholar] [CrossRef]
- Park, J.; Seo, S.; Kang, Y.-C.; Koh, S.-W. Tuning the oxidation states and crystallinity of copper oxide nanofibers by calcination. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. 2014, 32, 04E104. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, Y.; Zhou, M. Toluene sensing properties of SnO2–ZnO hollow nanofibers fabricated from single capillary electrospinning. Solid State Commun. 2011, 151, 895–899. [Google Scholar] [CrossRef]
- Rani, R.; Sharma, S. Preparation and characterization of SnO2 nanofibers via electrospinning. Adv. Nanopart. 2016, 5, 53. [Google Scholar] [CrossRef]
- Mohanapriya, P.; Sathish, C.I.; Pradeepkumar, R.; Segawa, H.; Yamaura, K.; Watanabe, K.; Natarajan, T.; Jaya, N.V. Optical and magnetic studies of electrospun Mn-doped SnO2 hollow nanofiber dilute magnetic semiconductor. J. Nanosci. Nanotechnol. 2013, 13, 5391–5400. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, J.; Xiong, Z.; Zhao, B.; Liu, P. Fabrication of porous hollow γ-Al2O3 nanofibers by facile electrospinning and its application for water remediation. Microporous Mesoporous Mater. 2015, 215, 133–142. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, B.; Xu, S.; Yang, J.; Lu, Y. Fabrication and electrochemical properties of porous vn hollow nanofibers. J. Alloys Compd. 2015, 651, 785–792. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, D.; Wu, J.; Zhang, Q.; Xin, Y.; Bao, N. One–step preparation of CNTs/InVO4 hollow nanofibers by electrospinning and its photocatalytic performance under visible light. Appl. Surf. Sci. 2015, 353, 1260–1268. [Google Scholar] [CrossRef]
- Park, H.; Jung, H.; Zhang, M.; Chang, C.H.; Ndifor-Angwafor, N.G.; Choa, Y.; Myung, N.V. Branched tellurium hollow nanofibers by galvanic displacement reaction and their sensing performance toward nitrogen dioxide. Nanoscale 2013, 5, 3058–3062. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Wang, J.; Dong, X.; Yu, W.; Liu, G.; Zhang, F.; Wang, L. Coaxial electrospinning fabrication and electrochemical properties of LiFePO4/C/Ag composite hollow nanofibers. J. Mater. Sci. 2013, 24, 4718–4724. [Google Scholar] [CrossRef]
- Dong, G.; Xiao, X.; Peng, M.; Ma, Z.; Ye, S.; Chen, D.; Qin, H.; Deng, G.; Liang, Q.; Qiu, J. Synthesis and optical properties of chromium-doped spinel hollow nanofibers by single-nozzle electrospinning. RSC Adv. 2012, 2, 2773–2782. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Dong, X.; Yu, W.; Liu, G. Fabrication and luminescence properties of YF3: Eu3+ hollow nanofibers via coaxial electrospinning combined with fluorination technique. J. Mater. Sci. 2013, 48, 5930–5937. [Google Scholar] [CrossRef]
- Batool, S.S.; Imran, Z.; Rafiq, M.A.; Hasan, M.M.; Willander, M. Investigation of dielectric relaxation behavior of electrospun titanium dioxide nanofibers using temperature dependent impedance spectroscopy. Ceram. Int. 2013, 39, 1775–1783. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zheng, W.; Wang, W.; Huang, H.; Wang, C.; MacDiarmid, A.G.; Wei, Y. Highly sensitive and stable humidity nanosensors based on licl doped TiO2 electrospun nanofibers. J. Am. Chem. Soc. 2008, 130, 5036–5037. [Google Scholar] [CrossRef] [PubMed]
- Jamil, H.; Batool, S.S.; Imran, Z.; Usman, M.; Rafiq, M.; Willander, M.; Hassan, M. Electrospun titanium dioxide nanofiber humidity sensors with high sensitivity. Ceram. Int. 2012, 38, 2437–2441. [Google Scholar] [CrossRef]
- Homaeigohar, S.S.; Mahdavi, H.; Elbahri, M. Extraordinarily water permeable sol–gel formed nanocomposite nanofibrous membranes. J. Colloid Interface Sci. 2012, 366, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tran, T.; Lu, W.; Wu, J. Electrospun silicon/carbon/titanium oxide composite nanofibers for lithium ion batteries. J. Power Sources 2014, 258, 39–45. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Wang, Y.; Zhao, L.; Jiang, Q. Adsorption capability for congo red on nanocrystalline MFe2O4 (M= Mn, Fe, Co, Ni) spinel ferrites. Chem. Eng. J. 2012, 181, 72–79. [Google Scholar] [CrossRef]
- Nilmoung, S.; Kidkhunthod, P.; Pinitsoontorn, S.; Rujirawat, S.; Yimnirun, R.; Maensiri, S. Fabrication, structure, and magnetic properties of electrospun carbon/cobalt ferrite (C/COFe2O4) composite nanofibers. Appl. Phys. A 2015, 119, 141–154. [Google Scholar] [CrossRef]
- Patange, S.; Shirsath, S.E.; Jadhav, S.; Hogade, V.; Kamble, S.; Jadhav, K. Elastic properties of nanocrystalline aluminum substituted nickel ferrites prepared by co-precipitation method. J. Mol. Struct. 2013, 1038, 40–44. [Google Scholar] [CrossRef]
- Khalil, L.; Eid, C.; Bechelany, M.; Abboud, N.; Khoury, A.; Miele, P. Design of CoFe2O4/Co3O4 nanofibers with tunable morphology by electrospinning. Mater. Lett. 2015, 140, 27–30. [Google Scholar] [CrossRef]
- Ammar, S.; Helfen, A.; Jouini, N.; Fievet, F.; Rosenman, I.; Villain, F.; Molinie, P.; Danot, M. Magnetic properties of ultrafine cobalt ferrite particles synthesized by hydrolysis in a polyol medium. J. Mater. Chem. 2001, 11, 186–192. [Google Scholar] [CrossRef]
- Andrade, P.; Silva, V.; Maciel, J.; Santillan, M.; Moreno, N.; Valladares, L.D.L.S.; Bustamante, A.; Pereira, S.; Silva, M.; Aguiar, J.A. Preparation and characterization of cobalt ferrite nanoparticles coated with fucan and oleic acid. Hyperfine Interact. 2014, 224, 217–225. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Sangsanoh, P.; Supaphol, P. Poly (3-hydroxybutyrate)/magnetite composite nanofibers obtained via combined electrospinning and ammonia gas-enhancing in situ co-precipitation: Preparation and potential use in biomedical applications. Chiang Mai J. Sci. 2014, 41, 676–690. [Google Scholar]
- Zhang, L.; Wu, H.B.; Lou, X.W.D. Iron-oxide-based advanced anode materials for lithium-ion batteries. Adv. Energy Mater. 2014, 4, 1300958. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Fernández-Bertólez, N.; Kiliç, G.; Costa, C.; Costa, S.; Fraga, S.; Bessa, M.J.; Pásaro, E.; Teixeira, J.P.; Laffon, B. Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J. Trace Elem. Med. Biol. 2016, 38, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Binitha, G.; Soumya, M.; Madhavan, A.A.; Praveen, P.; Balakrishnan, A.; Subramanian, K.; Reddy, M.; Nair, S.V.; Nair, A.S.; Sivakumar, N. Electrospun α-Fe2O3 nanostructures for supercapacitor applications. J. Mater. Chem. A 2013, 1, 11698–11704. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Q.; Dong, X.; Yu, W.; Wang, J.; Liu, G. A novel strategy to directly fabricate flexible hollow nanofibers with tunable luminescence–electricity–magnetism trifunctionality using one-pot electrospinning. Phys. Chem. Chem. Phys. 2015, 17, 22977–22984. [Google Scholar] [CrossRef] [PubMed]
- Duo, S.; Li, Y.; Zhang, H.; Liu, T.; Wu, K.; Li, Z. A facile salicylic acid assisted hydrothermal synthesis of different flower-like ZnO hierarchical architectures with optical and concentration-dependent photocatalytic properties. Mater. Charact. 2016, 114, 185–196. [Google Scholar] [CrossRef]
- Li, C.; Cheng, Z.; Gao, J.; Han, Q.; Ye, M.; Zhang, J.; Huang, R.; Qu, L. Oxidation degree of graphene reflected by morphology-tailored zno growth. Carbon 2016, 107, 583–592. [Google Scholar] [CrossRef]
- Hong, K.-S.; Kim, J.W.; Bae, J.-S.; Hong, T.E.; Jeong, E.D.; Jin, J.S.; Ha, M.G.; Kim, J.-P. Structure, chemical bonding states, and optical properties of the hetero-structured ZnO/CuO prepared by using the hydrothermal and the electrospinning methods. Physica B 2017, 504, 103–108. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, G.; Chen, M.; Ma, X.; Yang, J. Fabrication of electrospun zno nanofiber-modified electrode for the determination of trace Cd (ii). Sens. Actuators B Chem. 2016, 234, 84–91. [Google Scholar] [CrossRef]
- An, S.; Joshi, B.N.; Lee, M.W.; Kim, N.Y.; Yoon, S.S. Electrospun graphene-ZnO nanofiber mats for photocatalysis applications. Appl. Surf. Sci. 2014, 294, 24–28. [Google Scholar] [CrossRef]
- Senthil, T.; Anandhan, S. Structure–property relationship of sol–gel electrospun zno nanofibers developed for ammonia gas sensing. J. Colloid Interface Sci. 2014, 432, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, Y.; Zhou, M. Formaldehyde sensing properties of ZnO-based hollow nanofibers. Sens. Rev. 2014, 34, 327–334. [Google Scholar] [CrossRef]
- Zhao, J.-G.; Zhang, W.-Y.; Xie, E.-Q.; An, X.-Y.; Fu, J.-C.; Liu, Z.-J.; Liu, Z.-L.; Zhang, Y.-J.; Chen, Y.-F.; Zhang, C.-Y. Room-temperature ferromagnetism in SnO2 nanofibers and nanotubes prepared by electrospinning. Nano 2014, 9, 1450026. [Google Scholar] [CrossRef]
- Asokan, K.; Park, J.Y.; Choi, S.; Chang, C.; Kim, S.S. Stabilization of the anatase phase of Ti1−xSnxO2 (x < 0.5) nanofibers. Nano Res. 2010, 3, 256–263. [Google Scholar]
- Zhao, Y.; He, X.-L.; Li, J.-P.; Jia, J.; Gao, X.-G. Enhanced gas sensing properties of aligned porous SnO2 nanofibers. Chin. Phys. Lett. 2012, 29, 070701. [Google Scholar] [CrossRef]
- Chandraiah, M.; Sahoo, B.; Panda, P.K. Preparation and characterization of SnO2 nanofibers by electrospinning. Trans. Indian Ceram. Soc. 2014, 73, 266–269. [Google Scholar] [CrossRef]
- Balu, R.; Singaravelu, S.; Nagiah, N. Bioceramic nanofibres by electrospinning. Fibers 2014, 2, 221–239. [Google Scholar] [CrossRef]
- Lala, N.L.; Jose, R.; Yusoff, M.M.; Ramakrishna, S. Continuous tubular nanofibers of vanadium pentoxide by electrospinning for energy storage devices. J. Nanopart. Res. 2012, 14, 1201. [Google Scholar] [CrossRef]




| Method | Precursors | Parameters | Ensuing Hollow Nanofibers | Reference |
|---|---|---|---|---|
| Single-spinneret electrospinning | Zn(AC)2·2H2O in water/Poly(vinylpyrrolidon)(PVP) in DMF | Voltage = 17 kV Distance = 20 cm | ZnO | [44] |
| Coaxial electrospinning | Fe3O4 nanoparticles/DMF/Chloroform (CHCl3)/PVP/Eu(BA)3phen powders | Voltage = 11 kV Distance = 12 cm Flow rate = 1 mL·h−1 | Fe3O4/Eu(BA)3phen/PVP | [45] |
| Coaxial electrospinning | PVP/Tb4O7, BA, phen/FeCl3·6H2O/FeSO4·7H2O/NH4NO3, polyethyleneglycol (PEG)/ammonia/oleic acid (OA)/aniline (ANI), (IS)-(+)-camphor-10 sulfonic acid (CSA)/ammonium persulfate (APS)/ethanol (CHCl3)/DMF/nitric acid/water | Voltage = 13 Kv Flow rate = 0.0167 mL·min−1 | Tb(BA)3phen/PANI/Fe3O4/PVP (BA = benzoic acid, phen = phenanthroline, PANI = polyaniline, PVP = polyvinylpyrrolidone) | [46] |
| Coaxial electrospinning | Titanium butoxide (TBT, Ti(OBu)4)/PVP | Voltage = 4–30 kV Distance = 50 cm | TiO2 | [47] |
| Microfluidic approach electrospinning | PVP/tetrabutyl titanate (Ti(OC4H9)4/ethanol/paraffin oil | Voltage = 20–30 kV Distance = 15–25 cm Flow rates of inner jet (paraffin oil) = 1.0 mL·h−1 Flow rates of outer jet (PVP/Ti(OiPr)4) = 6–12 mL·h−1 | TiO2 | [48] |
| Triaxial electrospinning | tetraethyl orthosilicate (TEOS)/ethanol/water/HCl (shell and innermost layers)+poly(styrene-b-isoprene)(middle layer) | Voltage = 20 kV Distance = 11.5 cm Flow rates of inner jet = 0.02 mL·min−1 Flow rates of outer jet = 0.03 mL·min−1 | SiO2/PS-b-PI/SiO2 | [49] |
| Emulsion electrospinning | PVP/VO(acac)2/HAuCl4·3H2O-DMF solution and PS-DMF solution | Voltage = 5–30 kV Distance = 7 cm Flow rate = 2 mL·h−1 | Au/V2O5 | [50] |
| Emulsion electrospinning | tetrabutyl titanate (C16H36O4Ti)/ethanol/acetic acid + PVP/AgNO3/DMF/ethanol + mechanical pump oil | Voltage = 16 kV Distance = 15 cm Flow rate = 2 mL·h−1 | Ag/TiO2 | [51] |
| Hollow Nanofiber | Precursors | Electrospinning Conditions | Annealing Conditions | Reference |
|---|---|---|---|---|
| TiO2 | PVP/Tetra-butyl titanate (TBT)/ethanol/acetic acid | V = 30 kV D = 15 cm | T = 500 °C t = 4 h HR = 2 °C·min−1 | [105] |
| PVP/tetrabutyl titanate (Ti(OC4H9)4)/ethanol/paraffin oil | V = 20–30 kV D = 15–25 cm FR (outer) = 6–12 mL·h−1 FR (inner) = 1 mL·h−1 | T = 500 °C t = 8 h | [48] | |
| Titanium isopropoxide/PVP/acetic acid/ethanol | V = 30 kV D = 20 cm FR = 0.1 mL·h−1 | T = 600 °C t = 2 h | [66] | |
| SnO2/TiO2 | Titanium isopropoxide/PVP/acetic acid/ethanol | V = 5 kV FR (outer) = 1 mL·h−1 FR (inner) = 0.1 mL·h−1 | T = 500 °C t = 2 h | [106] |
| TiO2 | Titaniumisopropoxide(TiP)/poly (methylmethacrylate)(PMMA)/hexadecyl trimethylammoniumbromide/paraffin oil/methylene chloride/ethanol/acetic acid | V = 18 kV D = 15 cm FR = 100 μL·min−1 | T = 500 °C | [97] |
| Titanium butoxide (TBT, I(OBu)4)/PVP/ethylene glycol (EG)/ethanol/acetic acid | V = 0–50 kV D = 50 cm FR = 100 μL·min−1 | T = 550 °C t = 3 h HR = 2 °C·min−1 | [47] | |
| Titanium (IV) N-butoxide (TNBT)/PVP/ethanol/paraffin oil | V = 15 kV FR (outer) = 0.8 mL·h−1 FR (inner) = 0.6 mL·h−1 | T = 500 °C t = 6 h | [107] | |
| polyacrylonitrile (PAN)/PVP/dimethylformamide (DMF)/tetrabutyl titanate (Ti(OC4H9)4) | T = 500 °C t = 5 h | [108] | ||
| BaTiO3 | Barium acetate/acetic acid/Titanium (IV)-isopropoxide/PVP/ethanol | V = 12 kV D = 15 cm FR = 0.3 μL·s−1 | T = 500, 700, 950 °C t = 1 h HR = 2.5 °C·min−1 | [109] |
| Carbon nanotube (CNT)-TiO2 | PAN/Multiwalled CNTs (MWCNTs)/DMF/titanium tetra-isopropoxide (TTIP)/isopropyl alcohol | V = 18 kV D = 10 cm FR = 1 mL·h−1 | T = 550 °C t = 1 h | [110] |
| TiO2 | Butyl titanate (TBOT)/diiso-propyl azodiformate (DIPA)/paraffin oil/ethyl alcohol/acetic acid/deionized water | V = 18 kV D = 20 cm FR = 1 mL·h−1 | T = 500 °C t = 3 h HR = 1 °C·min−1 | [55] |
| Polyvinyl acetate (PVAc)/titanium isopropoxide (TIP)/DMF/calcium carbonate (CaCO3)/hydrochloric acid (HCl) | V = 17 kV D = 18 cm FR = 1 mL·h−1 | T = 500 °C t = 3 h | [111] | |
| Pt/TiO2 | Tetrabutyl titanate (Ti(OC4H9)4,TBOT)/ethanol/hexachloro-platinic acid (H2PtCl6·6H2O)/PVP/Nitric acid(HNO3) | V = 25 kV D = 25 cm FR = 1.3 ± 0.02 mL·h−1 | T = 350–500 °C t = 4 h | [112] |
| CoFe2O4 | PVP/Fe(NO3)3·9H2O/Co(NO3)2·6H2O/ethanol/water | V = 30 kV D = 15 cm FR = 1.3 ± 0.02 mL·h−1 | T = 500–600–700 °C t= 4 h HR = 3 °C·min−1 | [113] |
| CuFe2O4 | PVP/Fe(NO3)3·9H2O/Cu(NO3)2·3H2O/ethanol/water | V = 15 kV D = 15 cm | T = 500 °C t = 2 h HR = 0.5 °C·min−1 | [114] |
| CoFe2O4–PANI | Cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O/iron(III) nitrate enneahydrate (Fe(NO3)3/ethanol/PVP/ammonium peroxodisulfate | V = 20 kV D = 17 cm FR = 0.5 mL·h−1 | T = 550 °C t = 2 h HR = 5 °C·min−1 | [115] |
| SrFe12O19 | Strontium nitrate (Sr(NO3)2)/Ferric nitrate (Fe(NO3)3·9H2O)/PVP/DMF | V = 15 kV D = 15 cm FR = 0.5 mL·h−1 | T = 600–650–700–750 °C t = 3 h HR = 1 °C·min−1 | [26] |
| Fe2O3 | PVP/Fe(NO3)3·9H2O/water/ethanol | V = 30 kV D = 15 cm | T = 500 °C t = 4 h HR = 1–7 °C·min−1 | [116] |
| MnO2-doped Fe2O3 | Citric acid/ferric citrate/deionized water/manganese acetate | V = 15 kV D = 10 cm | T = 400 °C t = 4 h HR = 0.5 °C·min−1 | [117] |
| Fe3O4/Eu (BA)3phen/PVP | Fe3O4 nanoparticles/DMF/CHCl3/PVP/Eu (BA)3phen powders | V = 11 kV D = 12 cm | [45] | |
| Tb(BA)3phen/PANI/Fe3O4/PVP | Benzoic acid (BA)/phenan-throline (phen)/polyaniline (PANI)/PVP/sulfonic acid/ammonium persulfate/ethanol/CHCl3/DMF/nitric acid/deionized water/Tb4O7 | V = 13 kV D = 14 cm FR = 0.0167 mL·min−1 | [46] | |
| Carbon-coated LiFePO4 | Lithium dihydrogen phosphate (LiH2PO4)/iron nitrate 9-hydrate ((Fe(NO3)3·9H2O)/ferrous sulfate 7-hydrate (FeSO4·7H2O)/DMF/PMMA | V = 16 kV D = 15 cm FR (outer) = 0.2 mL·h−1 FR (inner) = 0.4 mL·h−1 | T = 750 °C t = 3 h HR = 2 °C·min−1 | [118] |
| CuO | PVP/copper acetate (Cu(CH3COO)2)/ethanol | V = 10 kV D = 13 cm FR = 0.02 mL·min−1 | T = 500 °C t = 2 h HR = 6.7 °C·min−1 | [119] |
| CuO | Copper (II) sulfate pentahydrate (CuSO4·5H2O)/PVP/water | V = 16.8 kV FR = 6 μL·min−1 | T = 673 and 873 K t = 5 h | [120] |
| SnO2-ZnO | Zn(AC)2·2H2O/SnCl2·2H2O/PVP/DMF/ethanol/ethyl acetate | V = 19 kV D = 20 cm FR = 0.7 mL·h−1 | T = 600 °C t = 3 h | [121] |
| SnO2 | Stannic chloride pentahydrate (SnCl4·5H2O)/ethanol/DMF/PVP | Electric field = 1.25 kV/cm D = 18 cm FR = 0.2 mL·h−1 | T = 550–650°C t = 4 h | [122] |
| Mn-Doped SnO2 | SnCl2·2H2O/DMF/ethanol/PVP/Mn(CH3COO)2·4H2O | V = 25 kV D = 18 cm FR = 1mL·h−1 | T = 600 °C t = 3 h | [123] |
| Cerium-doped SnO2 | SnCl2·2H2O/DMF/ethanol/PVP/Ce(NO3)3·6H2O | V = 25 kV D = 18 cm | T = 600 °C t = 5 h HR = 5 °C·min−1 | [34] |
| Al2O3 | Aluminum nitrate (Al(NO3)3)/PAN/DMF | V = 20 kV D = 20 cm FR = 1 mL·h−1 | T = 500–1000–1300 °C HR = 5 °C·min−1 | [54] |
| γ-Al2O3 | Aluminum nitrate (Al(NO3)3)/PAN/DMF | V = 20 kV D = 20 cm FR = 1 mL·h−1 | T = 800 °C t = 2 h HR = 5 °C·min−1 | [124] |
| Au/V2O5 | Vanadyl acetylacetonate (VO(acac)2)/gold(III) chloride trihydrate (HAuCl4·3H2O)/PVP/polystyrene (PS) | V = 20 kV D = 20 cm FR = 2 mL·h−1 | T1 = 330 °C t1 = 2 h HR1 =5 °C·min−1 T2 = 330–430 °C t2 = 30 min HR2 = 2°C·min−1 | [50] |
| Vanadium nitride (VN) | Oxalic acid dihydrate (C2H2O4·2H2O)/ethanol/PVP/ammonium metavanadate (NH4VO3) | V = 15 kV D = 15 cm | T = 400–600–800 °C t = 1 h HR = 2 °C·min−1 | [125] |
| CNTs/InVO4 | Multi-walled carbon nanotubes/In(NO3)3·4.5H2O/C10H14O5V/PVP/ethanol | V = 21 kV D = 15 cm | T = 550 °C t = 2 h | [126] |
| Te | Ni acetate/PVP/HTeO2+ | [127] | ||
| LiFePO4/C/Ag | Fe(NO3)3·9H2O/AgNO3/H3PO4/LiOH·H2O/DMF/PVP | V = 13 kV D = 16 cm | T = 700 °C t = 10 h HR = 1 °C·min−1 | [128] |
| Chromium-doped spinel | Zn(NO3)2·6H2O/Mg(NO3)2·6H2O/Al(NO3)3·9H2O/Cr(NO3)3·9H2O/ethanol/deionized water/PVP | V = 20 kV D = 12 cm FR = 1.5 mL·h−1 | T = 1000–1100–1200 °C t = 5 h HR = 200 °C·h−1 | [129] |
| YF3:Eu3+ | Yttrium oxide (Y2O3)/europium oxide (Eu2O3)/DMF/ammonium hydrogen fluoride (NH4HF2)/Nitric acid (HNO3)/ethyl alcohol | V = 13 kV D = 16 cm | T1 = 700 °C t1 = 8 h HR1 = 1 °C·min−1 T2 = 200 °C HR2 = 1 °C·min−1 | [130] |
| YF3:Yb3+/Er3+ | Yttrium oxide (Y2O3)/erbium oxide (Er2O3)/PVP/DMF/ammonium hydrogen fluoride (NH4HF2)/Nitric acid (HNO3)/ | V = 16 kV D = 18 cm | T1 = 700 °C t1 = 8 h HR1 = 1 °C·min−1 T2 = 200 °C HR2 = 1 °C·min−1 | [35] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homaeigohar, S.; Davoudpour, Y.; Habibi, Y.; Elbahri, M. The Electrospun Ceramic Hollow Nanofibers. Nanomaterials 2017, 7, 383. https://doi.org/10.3390/nano7110383
Homaeigohar S, Davoudpour Y, Habibi Y, Elbahri M. The Electrospun Ceramic Hollow Nanofibers. Nanomaterials. 2017; 7(11):383. https://doi.org/10.3390/nano7110383
Chicago/Turabian StyleHomaeigohar, Shahin, Yalda Davoudpour, Youssef Habibi, and Mady Elbahri. 2017. "The Electrospun Ceramic Hollow Nanofibers" Nanomaterials 7, no. 11: 383. https://doi.org/10.3390/nano7110383
APA StyleHomaeigohar, S., Davoudpour, Y., Habibi, Y., & Elbahri, M. (2017). The Electrospun Ceramic Hollow Nanofibers. Nanomaterials, 7(11), 383. https://doi.org/10.3390/nano7110383








