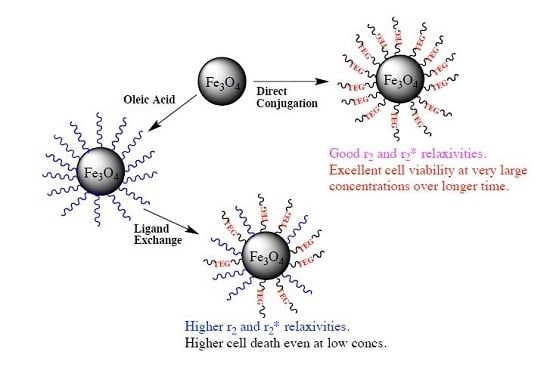
Fabricating Water Dispersible Superparamagnetic Iron Oxide Nanoparticles for Biomedical Applications through Ligand Exchange and Direct Conjugation
Abstract
:1. Introduction
2. Results and Discussion
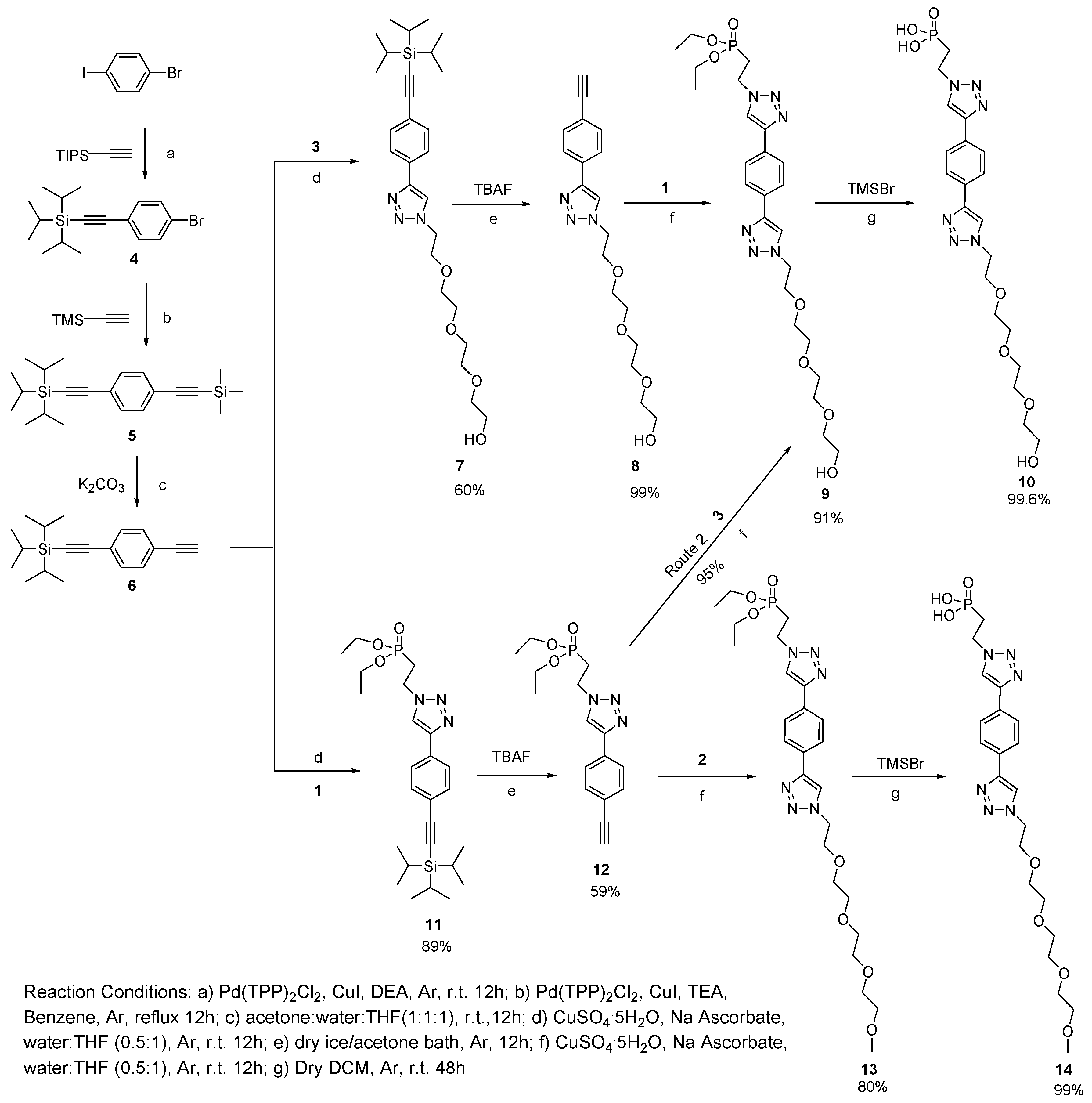
2.1. Synthesis of Ligands
2.2. Synthesis of Surface Functionalized SPIONs
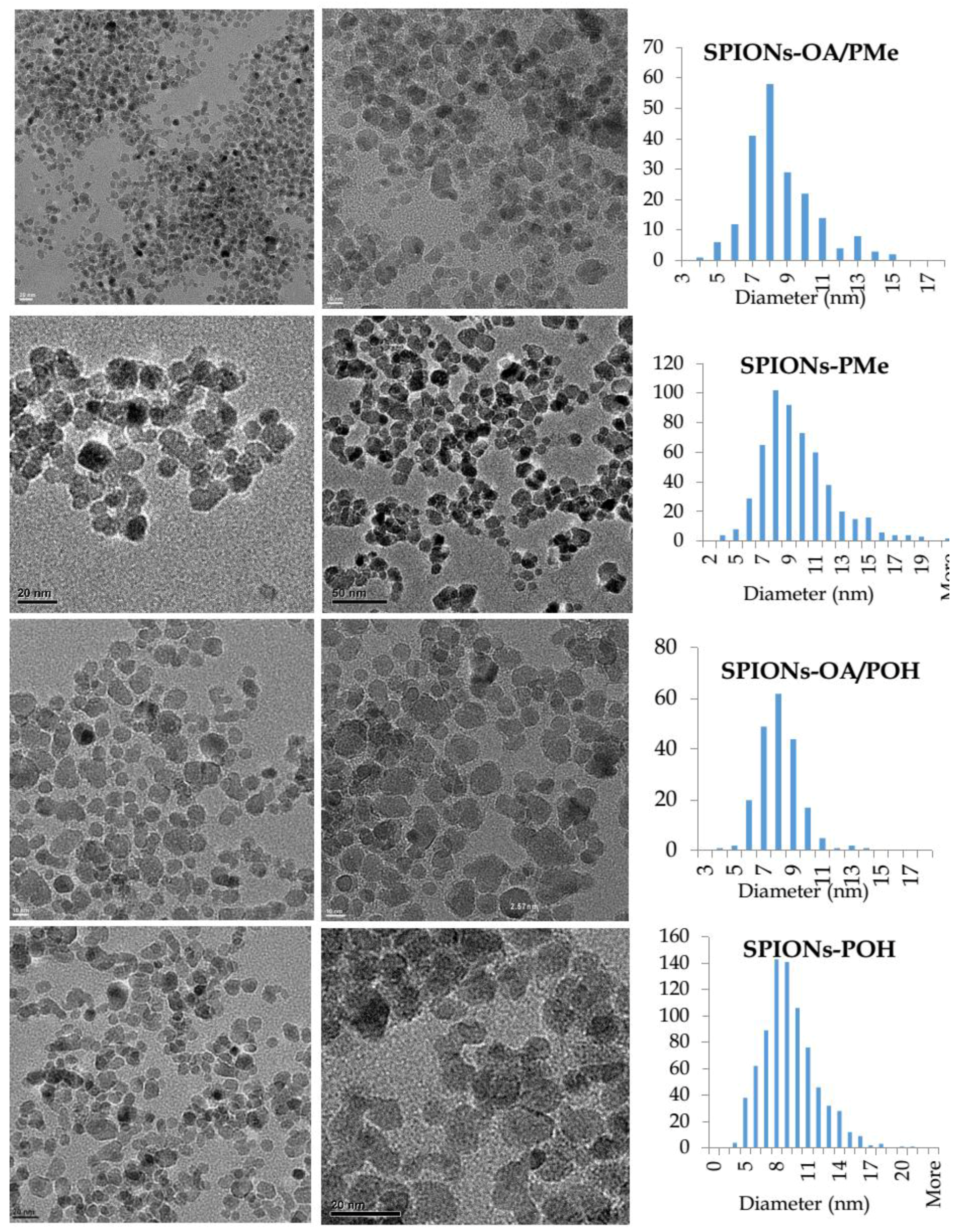
2.3. TEM, EDX, and SAED Studies of SPIONs
2.4. FT-IR Studies
2.5. Thermogravometric Analysis (TGA)
2.6. Ligand-Exchange Studies: UV-Vis
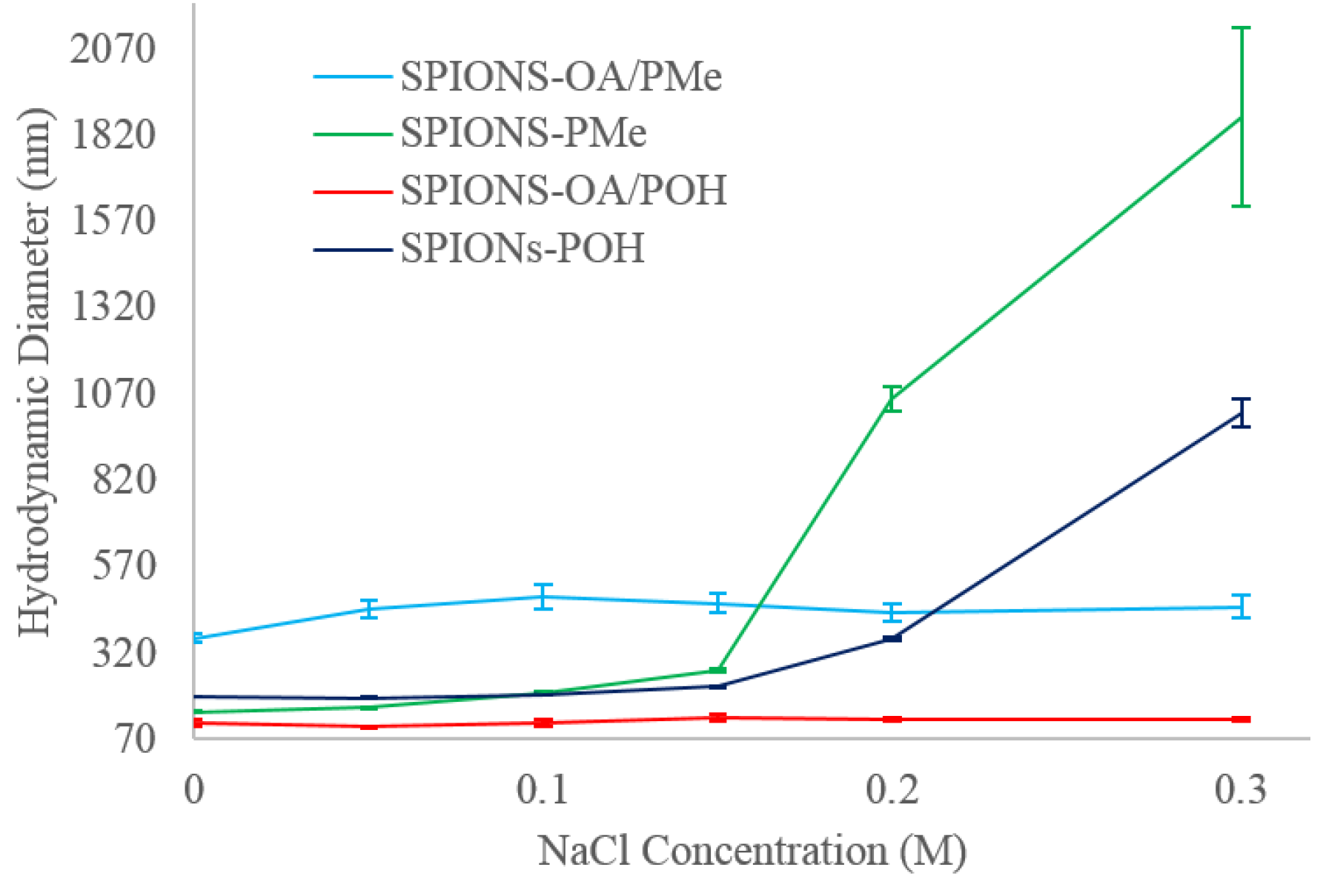
2.7. Dynamic Light Scattering
2.8. Zeta Potential
2.9. MRI Measurements
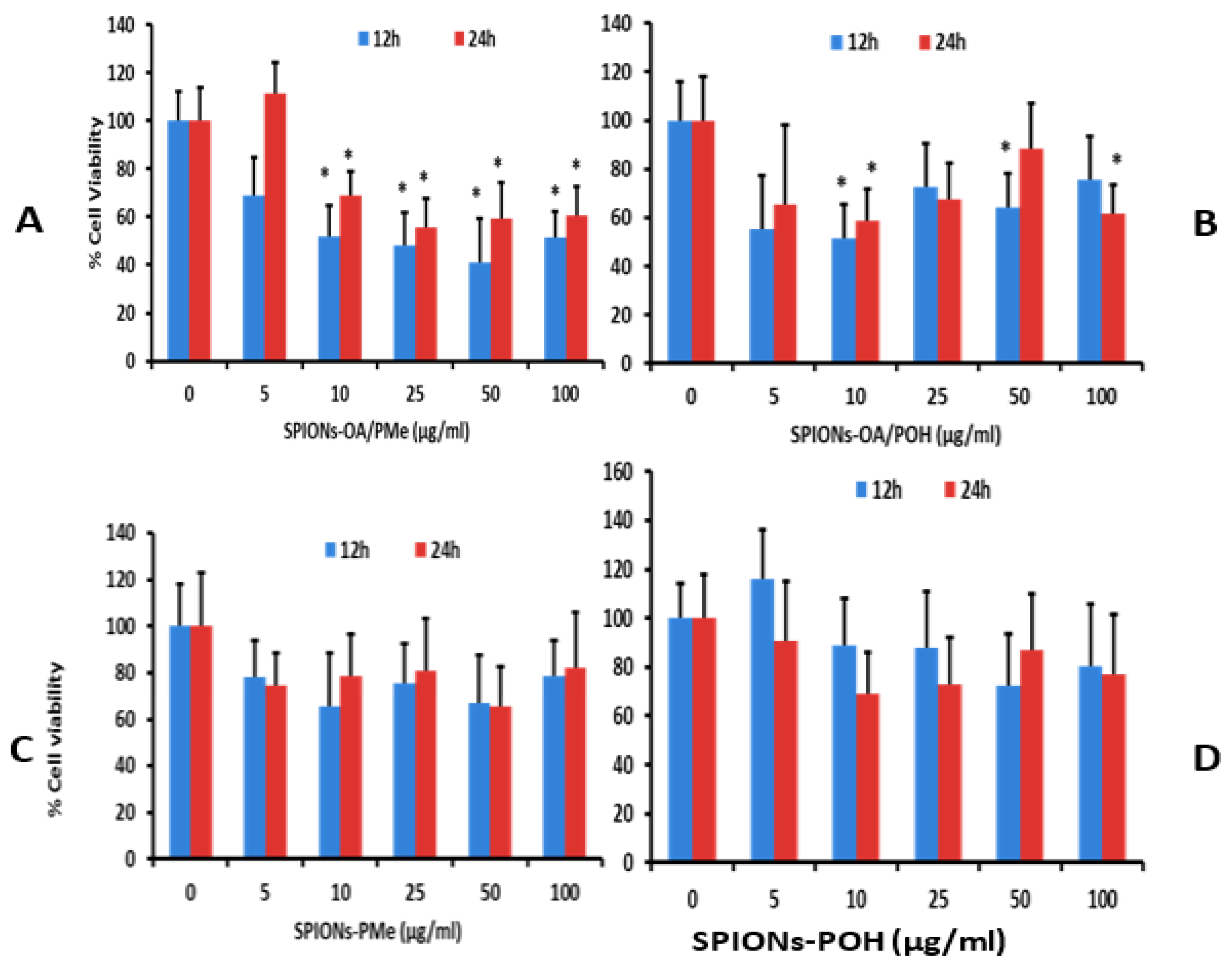
2.10. Cytotoxicity Studies
3. Materials and Methods
3.1. Synthesis of Phosphonate-TEG-OH and Phosphonate-TEG-Me
3.2. Ligand Exchange: Synthesis of SPIONs-OA/PMe and SPIONs/OA-POH
3.3. Direct Conjugation: Synthesis of SPIONs-PMe and SPIONs-POH
3.4. MRI Relaxometry Measurements
3.5. Cell Culture and Viability Studies
3.6. Cellular Uptake of SPIONs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dash, R.; Chung, J.; Chan, T.; Yamada, M.; Barral, J.; Nishimura, D.; Yang, P.C.; Simpson, P.C. A molecular MRI probe to detect treatment of cardiac apoptosis in vivo. Magn. Res. Med. 2011, 66, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, F.A.; Verjans, J.W. Non-invasive/invasive imaging: Molecular imaging of atherosclerosis: Clinical state-of-the-art. Heart 2014, 100, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Nappi, C.; Acampa, W.; Pellegrino, T.; Petretta, M.; Cuocolo, A. Beyond ultrasound: Advances in multimodality cardiac imaging. Intern. Emerg. Med. 2015, 10, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Bull, E.; Madani, S.Y.; Sheth, R.; Seifalian, A.; Green, M.; Seifalian, A.M. Stem cell tracking using iron oxide nanoparticles. Int. J. Nanomed. 2014, 9, 1641–1653. [Google Scholar]
- Cova, L.; Bigini, P.; Diana, V.; Sitia, L.; Ferrari, R.; Pesce, R.M.; Khalaf, R.; Bossolasco, P.; Ubezio, P.; Lupi, M.; et al. Biocompatible fluorescent nanoparticles for in vivo stem cell tracking. Nanotechnology 2013, 24. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xia, R.; Zhang, B.; Gao, F.B. MRI tracking stem cells transplantation for coronary heart disease. Pak. J. Med. Sci. 2014, 30, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Pouliot, P.; Avti, P.K.; Lesage, F.; Kakkar, A.K. Superparamagnetic iron oxide based nanoprobes for imaging and theranostics. Adv. Colloid Interface Sci. 2013, 199, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sahoo, S.K. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Sun, D. Superparamagnetic iron oxide nanoparticle ‘theranostics’ for multimodality tumor imaging, gene delivery, targeted drug and prodrug delivery. Expert Rev. Clin. Pharmacol. 2010, 3, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, R.A.; Hilt, J.Z. Magnetic nanoparticles in biomedicine: Synthesis, functionalization and applications. Nanomedicine 2010, 5, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Serpooshan, V.; Laurent, S. Engineered nanoparticles for biomolecular imaging. Nanoscale 2011, 3, 3007–3026. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. Eng. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.D.; Vo, T.V.; Tran, P.H.L. Design of iron oxide nanoparticles decorated oleic acid and bovine serum albumin for drug delivery. Chem. Eng. Res. Des. 2015, 94, 112–118. [Google Scholar] [CrossRef]
- Hajdu, A.; Tombacz, E.; Illes, E.; Bica, D.; Vekas, L. Magnetite Nanoparticles Stabilized Under Physiological Conditions for Biomedical Application. In Colloids for Nano- and Biotechnology; Springer-Verlag: Berlin, Heidelberg, Germany, 2008; Volume 135, pp. 29–37. [Google Scholar]
- Huang, J.; Wang, L.; Lin, R.; Wang, A.Y.; Yang, L.; Kuang, M.; Qian, W.; Mao, H. Casein-coated iron oxide nanoparticles for high MRI contrast enhancement and efficient cell targeting. ACS Appl. Mater. Interf. Sci. 2013, 5, 4632–4639. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, E.D.; Park, H.Y.; Berquo, T.S.; Pierre, V.C. Surface functionalization of magnetic iron oxide nanoparticles for MRI applications–effect of anchoring group and ligand exchange protocol. Contrast Media Mol. Imaging 2011, 6, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic particles. Philos. Trans. R. Soc. Lond A 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Basly, B.; Felder-Flesch, D.; Perriat, P.; Pourroy, G.; Begin-Colin, S. Properties and suspension stability of dendronized iron oxide nanoparticles for MRI applications. Contrast Media Mol. Imaging. 2011, 6, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Illes, E.; Tombacz, E. The role of variable surface charge and surface complexation in the adsorption of humic acid on magnetite. Colloid Surf. A 2003, 230, 99–109. [Google Scholar] [CrossRef]
- Miguel-Sancho, N.; Bomati-Miguel, O.; Colom, G.; Salvador, J.P.; Marco, M.P.; Santamaria, J. Development of stable, water dispersible, and bio-functionalizable superparamagnetic iron oxide nanoparticles. Chem. Mater. 2011, 23, 2795–2802. [Google Scholar] [CrossRef]
- Yang, H.; Hasegawa, D.; Takahashi, M.; Ogawa, T. Facile Synthesis, Phase Transfer, and Magnetic Properties of Monodisperse Magnetite Nanocubes. IEEE Transactions Magnet. 2008, 44, 3895–3898. [Google Scholar] [CrossRef]
- Peng, E.; Wang, F.; Xue, J.M. Nanostructured magnetic nanocomposites as MRI contrast agents. J. Mater. Chem. B 2015, 3, 2241–2276. [Google Scholar] [CrossRef]
- Nordmeyer, D.; Stumpf, P.; Groger, D.; Hofmann, A.; Enders, S.; Riese, S.B.; Dernedde, J.; Taupitz, M.; Rauch, U.; Haag, R.; et al. Iron oxide nanoparticles stabilized with dendritic polyglycerols as selective MRI contrast agents. Nanoscale 2014, 6, 9646–9654. [Google Scholar] [CrossRef] [PubMed]
- Ghobril, C.; Popa, G.; Parat, A.; Billotey, C.; Taleb, J.; Bonazza, P.; Begin-Colin, S.; Felder-Flesch, D. A bisphosphonate tweezers and clickable PEGylated PAMAM dendrons for the preparation of functional iron oxide nanoparticles displaying renal and hepatobiliary elimination. Chem. Commun. 2013, 49, 9158–9160. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.; Qi, B.; Witmer, M.; Kitchens, C.L.; Powell, B.A.; Mefford, O.T. Quantitative measurement of ligand exchange on iron oxides via radiolabeled oleic acid. Langmuir 2014, 30, 10918–10925. [Google Scholar] [CrossRef] [PubMed]
- Sebekova, K.; Dusinska, M.; Simon Klenovics, K.; Kollarova, R.; Boor, P.; Kebis, A.; Staruchova, M.; Vlkova, B.; Celec, P.; Hodosy, J.; et al. Comprehensive assessment of nephrotoxicity of intravenously administered sodium-oleate-coated ultra-small superparamagnetic iron oxide (USPIO) and titanium dioxide (TiO2) nanoparticles in rats. Nanotoxicology 2014, 8, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Chinchilla, R.; Najera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, A.; Parat, A.; Bordeianu, C.; Ghobril, C.; Kueny-Stotz, M.; Walter, A.; Jouhannaud, J.; Begin-Colin, S.; Felder-Flesch, D. Efficient synthesis of small-sized phosphonated dendrons: Potential organic coatings of iron oxide nanoparticles. New J. Chem. 2014, 38, 5226–5239. [Google Scholar] [CrossRef]
- Khan, A. Preparation and characterization of magnetic nanoparticles embedded in microgels. Mater. Lett. 2008, 62, 898–902. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef] [PubMed]
- Basly, B.; Felder-Flesch, D.; Perriat, P.; Billotey, C.; Taleb, J.; Pourroy, G.; Begin-Colin, S. Dendronized iron oxide nanoparticles as contrast agents for MRI. Chem. Commun. 2010, 46, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, G.; Kueny-Stotz, M.; Mamlouk-Chaouachi, H.; Ghobril, C.; Basly, B.; Bertin, A.; Miladi, I.; Billotey, C.; Pourroy, G.; Begin-Colin, S.; et al. Dendronized iron oxide nanoparticles for multimodal imaging. Biomaterials 2011, 32, 8562–8573. [Google Scholar] [CrossRef] [PubMed]
- Daou, T.J.; Pourroy, G.; Greneche, J.M.; Bertin, A.; Felder-Flesch, D.; Begin-Colin, S. Water soluble dendronized iron oxide nanoparticles. Dalton Trans. 2009, 23, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.M.; Bramble, J.P.; Rawlings, A.E.; Burnell, G.; Evans, S.D.; Staniland, S.S. Biotemplated magnetic nanoparticle arrays. Small 2012, 8, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.I.; Shivashankar, S.A. Single crystalline magnetite, maghemite, and hematite nanoparticles with rich coercivity. RSC Adv. 2014, 4, 4105–4113. [Google Scholar] [CrossRef]
- Lartigue, L.; Innocenti, C.; Kalaivani, T.; Awwad, A.; Duque, M.D.S.; Guari, Y.; Larionova, J.; Guerin, C.; Montero, J.L.G.; Barragan-Montero, V.; et al. Water-Dispersible Sugar-Coated Iron Oxide Nanoparticles. An Evaluation of their Relaxometric and Magnetic Hyperthermia Properties. J. Am. Chem. Soc. 2011, 133, 10459–10472. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.M.; Xue, Y.N.; Peng, N.; He, W.T.; Zhuo, R.X.; Huang, S.W. Dendrimer modified magnetic iron oxide nanoparticle/DNA/PEI ternary magnetoplexes: A novel strategy for magnetofection. J. Mater. Chem. 2011, 21, 13306–13315. [Google Scholar] [CrossRef]
- Yue-Jian, C.; Juan, T.; Fei, X.; Jia-Bi, Z.; Ning, G.; Yi-Hua, Z.; Ye, D.; Liang, G. Synthesis, self-assembly, and characterization of PEG-coated iron oxide nanoparticles as potential MRI contrast agent. Drug Dev. Ind. Pharm. 2010, 36, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Lee, S.H.; Xu, C.; Xie, J.; Lee, J.H.; Wu, B.; Koh, A.L.; Wang, X.; Sinclair, R.; Wang, S.X. Synthesis and characterization of PVP-coated large core iron oxide nanoparticles as an MRI contrast agent. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, K.; Kanayama, N.; Asai, K.; Kishimoto, M.; Ohara, Y.; Akashi, Y.; Yamada, K.; Hashimoto, S.; Oda, T.; Ohkohchi, N.; et al. Preparation of highly dispersible and tumor-accumulative, iron oxide nanoparticles Multi-point anchoring of PEG-b-poly(4-vinylbenzylphosphonate) improves performance significantly. Colloid Surf. B 2011, 88, 771–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Gao, W.; Chen, Z.; Fan, H.; Li, M.; Deng, F. Tumor selectivity of stealth multi-functionalized superparamagnetic iron oxide nanoparticles. Int. J. Pharm. 2011, 404, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J. Colloid Stability. In Colloid Science; Blackwell Publishing Ltd.: Oxford, UK, 2009; pp. 36–49. [Google Scholar]
- Qiao, R.; Yang, C.H.; Gao, M.Y. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Balan, V.; Petrache, I.A.; Popa, M.I.; Butnaru, M.; Barbu, E.; Tsibouklis, J.; Verestiuc, L. Biotinylated chitosan-based SPIONs with potential in blood-contacting applications. J. Nanopart. Res. 2012, 14, 730–734. [Google Scholar] [CrossRef]
- Tombacz, E.; Toth, I.Y.; Nesztor, D.; Illes, E.; Hajdu, A.; Szekeres, M.; Vekas, L. Adsorption of organic acids on magnetite nanoparticles, pH-dependent colloidal stability and salt tolerance. Colloid Surf. A 2013, 435, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Corot, C.; Port, M.; Guilbert, I.; Robert, P.; Raynal, I.; Robic, C.; Raynaud, J.-S.; Prigent, P.; Dencausse, A.; Idee, J.-M. Superparamagnetic Contrast Agents. In Molecular and Cellular MR Imaging; CRC Press: Boca Raton, Florida, USA, 2007; pp. 59–83. [Google Scholar]
- Kalber, T.L.; Smith, C.J.; Howe, F.A.; Griffiths, J.R.; Ryan, A.J.; Waterton, J.C.; Robinson, S.P. A longitudinal study of R2* and R2 magnetic resonance imaging relaxation rate measurements in Murine liver after a single administration of 3 different iron oxide based contrast agents. Invest. Radiol. 2005, 40, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Chapon, C.; Franconi, F.; Lemaire, L.; Marescaux, L.; Legras, P.; Saint-Andre, J.P.; Denizot, B.; Le Jeune, J.J. High field magnetic resonance imaging evaluation of superparamagnetic iron oxide nanoparticles in a permanent rat myocardial infraction. Invest. Radiol. 2003, 38, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Schwarz, S.; Ahlemeyer, B.; Grzeschik, S.; Klumpp, S.; Krieglstein, J. Oleic acid causes apoptosis and dephosphorylates Ba. Neurochem. Int. 2005, 46, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Voicu, R.; Boukherroub, R.; Bartzoka, V.; Ward, T.; Wojtyk, J.T.C.; Wayner, D.D.M. Formation, characterization, and chemistry of undecanoic acid-terminated silicon surfaces: Patterning and immobilization of DNA. Langmuir 2004, 20, 11713–11720. [Google Scholar] [CrossRef] [PubMed]
- Kitto, H.J.; Schwartz, E.; Nijemeisland, M.; Koepf, M.; Cornelissen, J.J.L.M.; Rowan, A.E.; Nolte, R.J.M. Post-modification of helical dipeptido polyisocyanides using the ‘click’ reaction. J. Mater. Chem. 2008, 18, 5615–5624. [Google Scholar] [CrossRef]
- Yu, X.; Yang, X.; Horte, S.; Kizhakkedathu, J.N.; Brooks, D.E. A pH and thermosensitive choline phosphate-based delivery platform targeted to the acidic tumor microenvironment. Biomaterials 2014, 35, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Brunet, E.; Juanes, O.; Jimenez, L.; Rodriguez-Ubis, J.C. Click-chemistry-based bis-triazolylpyridine diphosphonate ligand for the sensitized luminescence of lanthanides in the solid state within the layers of γ-zirconium phosphate. Tetrahedron Lett. 2009, 50, 5361–5363. [Google Scholar] [CrossRef]
- Turp, D.; Wagner, M.; Enkelmann, V.; Mullen, K. Synthesis of nanometer-sized, rigid, and hydrophobic anions. Angew. Chem. Int. Ed. Eng. 2011, 50, 4962–4965. [Google Scholar] [CrossRef] [PubMed]
- Florian, A.; Mayoral, M.J.; Stepanenko, V.; Fernandez, G. Alternated stacks of nonpolar oligo(p-phenyleneethynylene)-BODIPY systems. Chem. Eur. J. 2012, 18, 14957–14961. [Google Scholar] [CrossRef] [PubMed]
- Nierengarten, J.F.; Zhang, S.; Gegout, A.; Urbani, M.; Armaroli, N.; Marconi, G.; Rio, Y. Synthesis and optical properties of isomeric branched π-conjugated systems. J. Org. Chem. 2005, 70, 7550–7557. [Google Scholar] [CrossRef] [PubMed]
- He, X.H.; Wu, X.M.; Cai, X.; Lin, S.L.; Xie, M.R.; Zhu, X.Y.; Yan, D.Y. Functionalization of magnetic nanoparticles with dendritic–linear–brush-like triblock copolymers and their drug release properties. Langmuir 2012, 28, 11938–11947. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Huang, J.; Yan, H.; Liu, K. Size-controlled preparation of magnetite nanoparticles in the presence of graft copolymers. J. Mater. Chem. 2006, 16, 298–303. [Google Scholar] [CrossRef]

| Nanoparticles | DH (nm) | r2 (mM−1·s−1) | r1 (mM−1·s−1) | r2* (mM−1·s−1) | r2/r1 |
|---|---|---|---|---|---|
| SPIONs-OA/PMe | 363 | 707 (663, 751) | 0.857 (0.771, 0.944) | 565 (525, 606) | 825 (728, 923) |
| SPIONs-OA/POH | 116 | 687 (496, 878) | 1.06 (0.959, 1.17) | 1280 (708, 1850) | 648 (458, 840) |
| SPIONs-PMe | 149 | 87(81, 93) | 0.159 (0.145, 0.173) | 358 (181, 534) | 546 (484, 608) |
| SPIONs-POH | 193 | 306 (270, 343) | 0.641 (0.529, 0.753) | 639 (566, 712) | 477 (377, 579) |
| Ferumoxides (Endorem®/Feridex®) | 120–180 | 132 | 1.8 | 111.9–134.3 * | 76.7 |
| Ferucarbotran A (Resovist, SHU55A) | 65 | 205 | 1.6 | - | 128.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, T.; Avti, P.K.; Pouliot, P.; Maafi, F.; Tardif, J.-C.; Rhéaume, É.; Lesage, F.; Kakkar, A. Fabricating Water Dispersible Superparamagnetic Iron Oxide Nanoparticles for Biomedical Applications through Ligand Exchange and Direct Conjugation. Nanomaterials 2016, 6, 100. https://doi.org/10.3390/nano6060100
Lam T, Avti PK, Pouliot P, Maafi F, Tardif J-C, Rhéaume É, Lesage F, Kakkar A. Fabricating Water Dispersible Superparamagnetic Iron Oxide Nanoparticles for Biomedical Applications through Ligand Exchange and Direct Conjugation. Nanomaterials. 2016; 6(6):100. https://doi.org/10.3390/nano6060100
Chicago/Turabian StyleLam, Tina, Pramod K. Avti, Philippe Pouliot, Foued Maafi, Jean-Claude Tardif, Éric Rhéaume, Frédéric Lesage, and Ashok Kakkar. 2016. "Fabricating Water Dispersible Superparamagnetic Iron Oxide Nanoparticles for Biomedical Applications through Ligand Exchange and Direct Conjugation" Nanomaterials 6, no. 6: 100. https://doi.org/10.3390/nano6060100