Spectroscopic and Electrochemical Studies of Imogolite and Fe-Modified Imogolite Nanotubes
Abstract
:1. Introduction
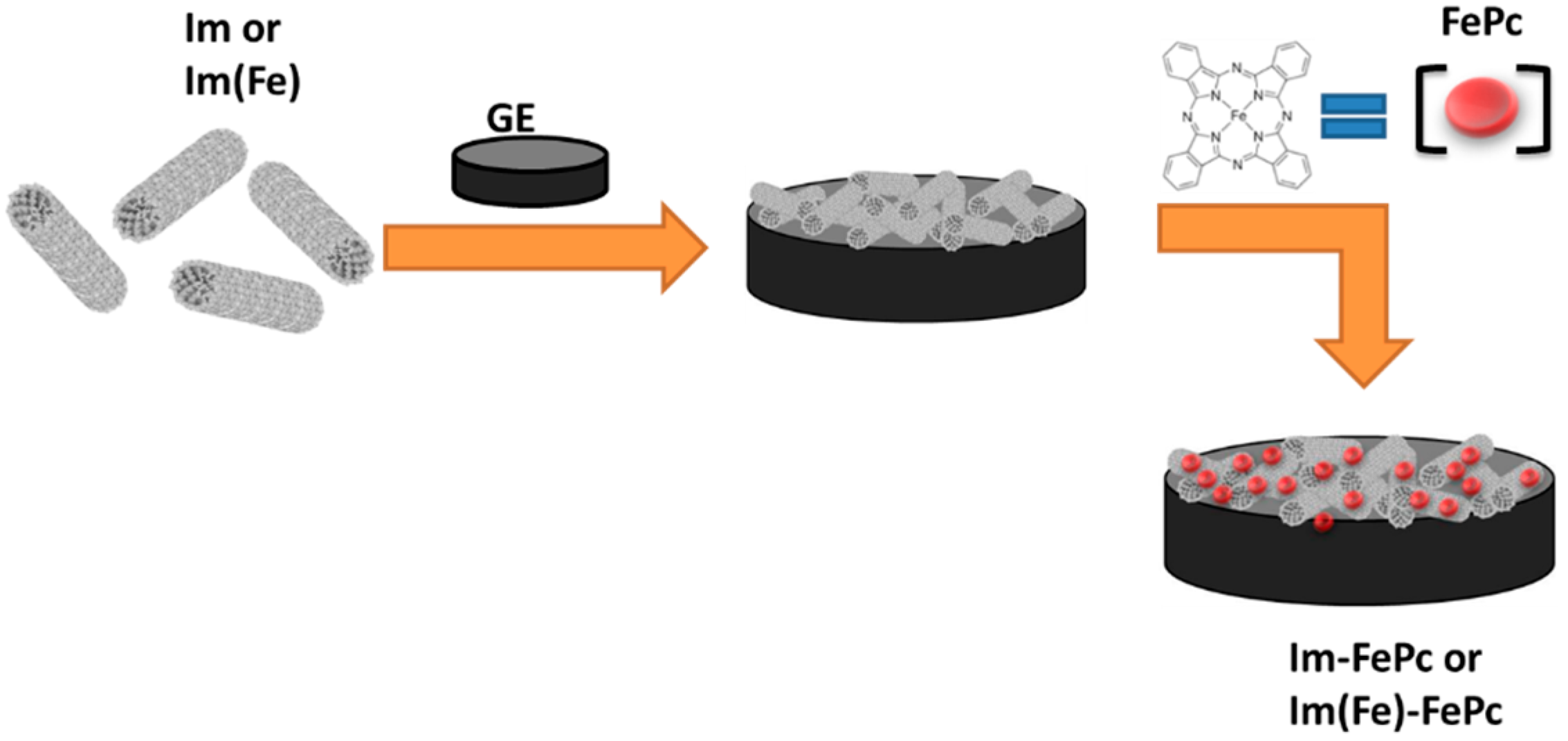
2. Materials and Methods
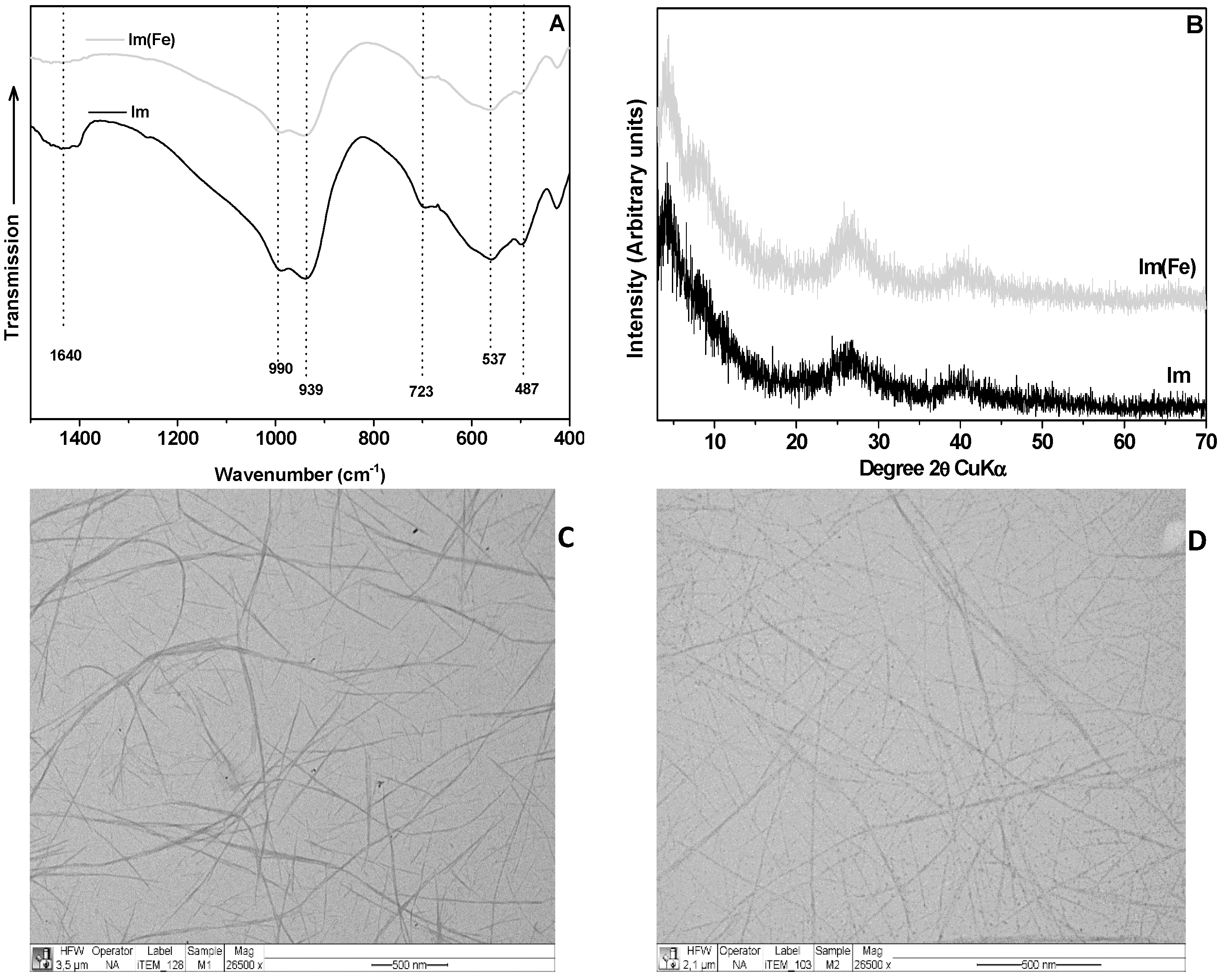
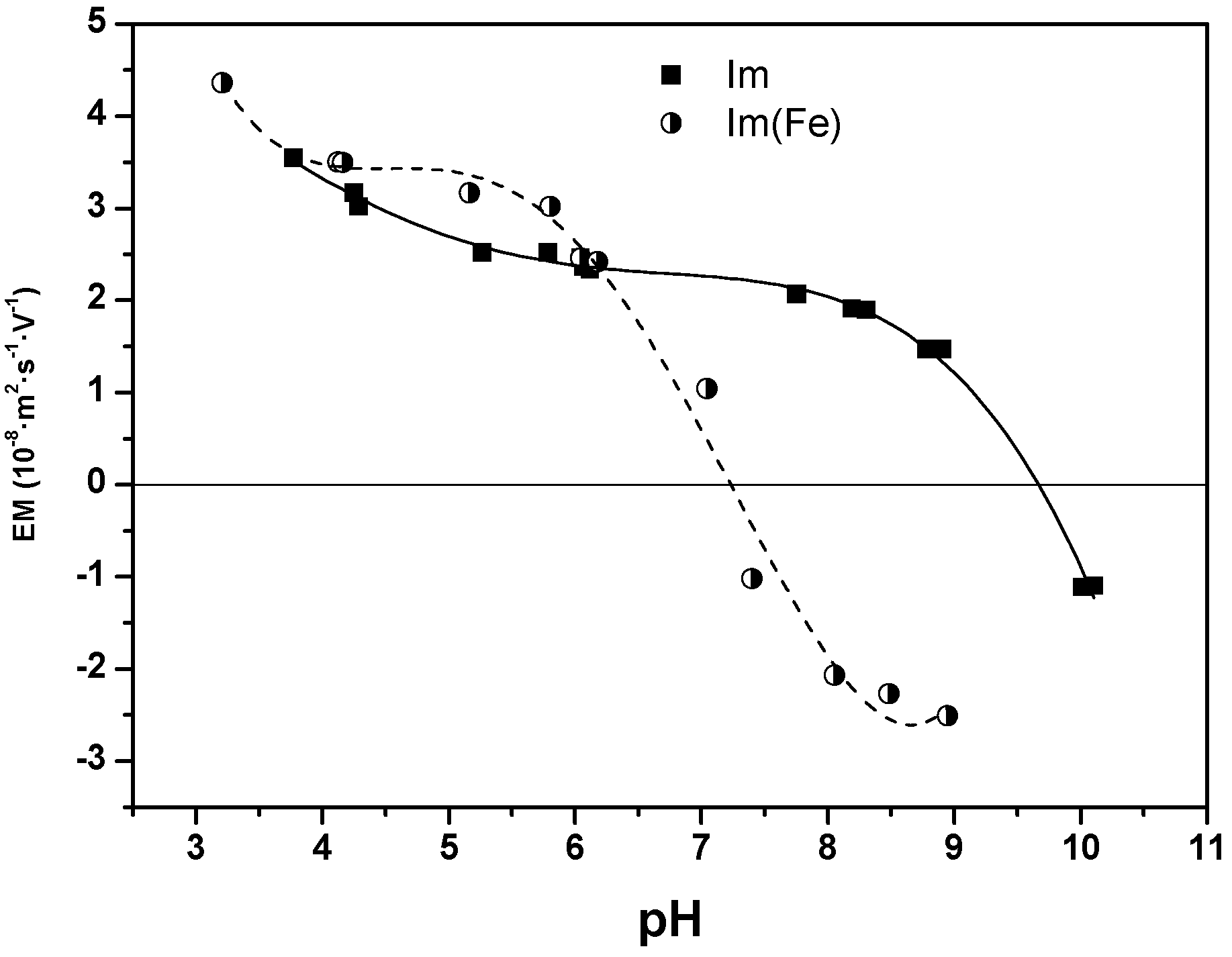
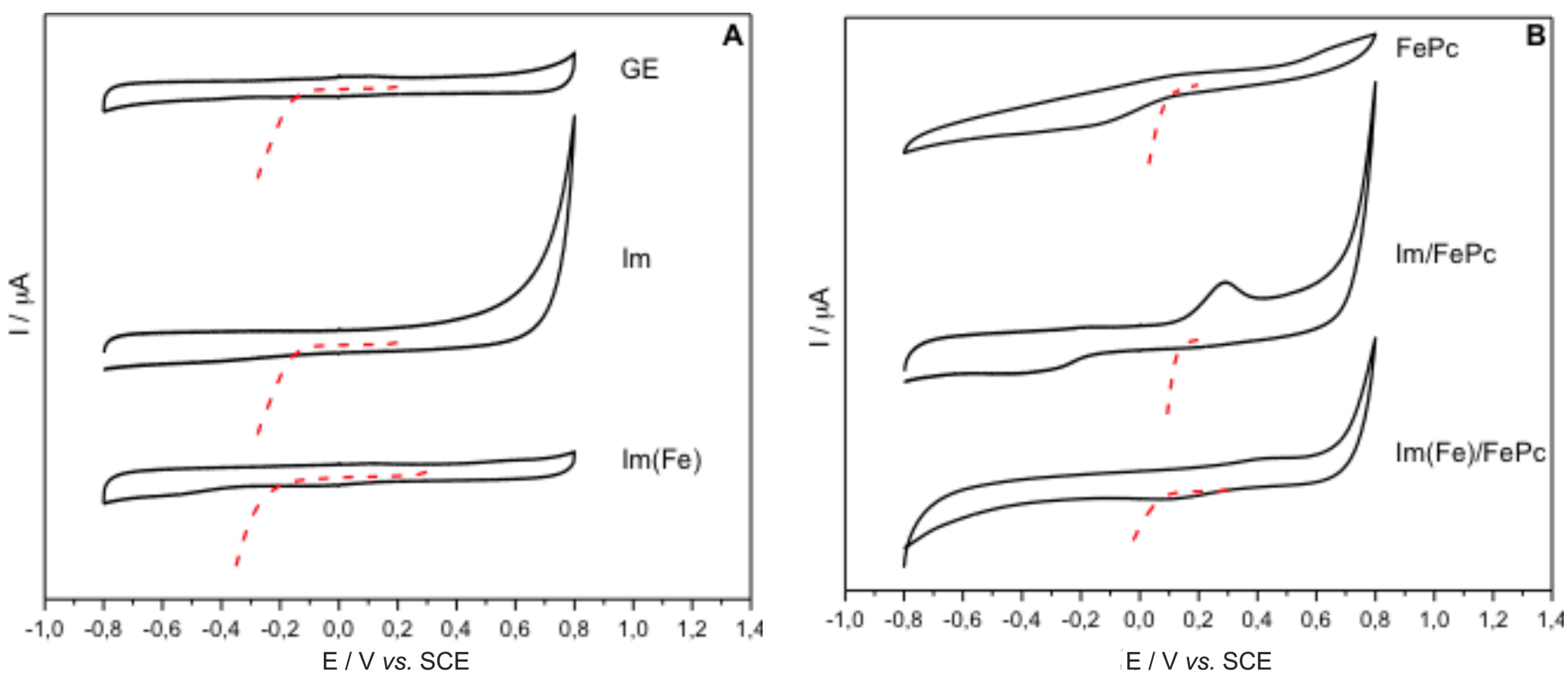
3. Discussion and Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andrews, R.; Jacques, D.; Qian, D.L.; Rantell, T. Multiwall carbon nanotubes: Synthesis and application. Acc. Chem. Res. 2002, 35, 1008–1017. [Google Scholar] [CrossRef]
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. TrAC-Trend Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Tasca, F.; Gorton, L.; Wagner, J.B.; Nöll, G. Increasing amperometric biosensor sensitivity by length fractionated single-walled carbon nanotubes. Biosens. Bioelectron. 2008, 24, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Tasca, F.; Harreither, W.; Ludwig, R.; Gooding, J.J.; Gorton, L. Cellobiose Dehydrogenase Aryl Diazoniunn Modified Single Walled Carbon Nanotubes: Enhanced Direct Electron Transfer through a Positively Charged Surface. Anal. Chem. 2011, 83, 3042–3049. [Google Scholar] [CrossRef] [PubMed]
- Paradise, M.; Goswami, T. Carbon nanotubes—Production and industrial applications. Mater. Des. 2007, 28, 1477–1489. [Google Scholar] [CrossRef]
- Bursill, L.A.; Peng, J.L.; Bourgeois, L.N. Imogolite: An aluminosilicate nanotube material. Philos. Mag. A 2000, 80, 105–117. [Google Scholar] [CrossRef]
- Tamura, K.; Kawamura, K. Molecular dynamics modeling of tubular aluminum silicate: Imogolite. J. Phys. Chem. B 2002, 106, 271–278. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bartlow, V.A.; Nair, S. Phenomenology of the growth of single-walled aluminosilicate and aluminogermanate nanotubes of precise dimensions. Chem. Mater. 2005, 17, 4900–4909. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Escudey, M.; Molina, M.; Garcia-Gonzalez, M.T. Use of isoelectric point and pH to evaluate the synthesis of a nanotubular aluminosilicate. J. Non-Cryst. Solids. 2011, 357, 1750–1756. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Escudey, M.; Pizarro, C.; Denardin, J.C.; García-González, M.T.; Fabris, J.D.; Charlet, L. Preparation and characterization of a single-walled aluminosilicate nanotube-iron oxide composite: Its applications to removal of aqueous arsenate. Mater. Res. Bull. 2014, 51, 145–152. [Google Scholar] [CrossRef]
- Ookawa, M.; Takata, Y.; Suzuki, M.; Inukai, K.; Maekawa, T.; Yamaguchi, T. Oxidation of aromatic hydrocarbons with H2O2 catalyzed by a nano-scale tubular aluminosilicate, Fe-containing imogolite. Res. Chem. Intermed. 2008, 34, 679–685. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kim, K.; Nair, S. Short, highly ordered, single-walled mixed-oxide nanotubes assemble from amorphous nanoparticles. J. Am. Chem. Soc. 2007, 129, 6820–6826. [Google Scholar] [CrossRef] [PubMed]
- Konduri, S.; Mukherjee, S.; Nair, S. Controlling nanotube dimensions: Correlation between composition, diameter, and internal energy of single-walled mixed oxide nanotubes. ACS Nano 2007, 1, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Yucelen, G.I.; Choudhury, R.P.; Vyalikh, A.; Scheler, U.; Beckham, H.W.; Nair, S. Formation of Single-Walled Aluminosilicate Nanotubes from Molecular Precursors and Curved Nanoscale Intermediates. J. Am. Chem. Soc. 2011, 133, 5397–5412. [Google Scholar] [CrossRef] [PubMed]
- Yucelen, G.I.; Kang, D.Y.; Guerrero-Ferreira, R.C.; Wright, E.R.; Beckham, H.W.; Nair, S. Shaping Single-Walled Metal Oxide Nanotubes from Precursors of Controlled Curvature. Nano Lett. 2012, 12, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Zang, J.; Jones, C.W.; Nair, S. Single-Walled Aluminosilicate Nanotubes with Organic-Modified Interiors. J. Phys. Chem. 2011, 115, 7676–7685. [Google Scholar] [CrossRef]
- Yamamoto, K.; Otsuka, H.; Takahara, A.; Wada, S.I. Preparation of a novel (polymer/inorganic nanofiber) composite through surface modification of natural aluminosilicate nanofiber. J. Adhes. 2002, 78, 591–602. [Google Scholar] [CrossRef]
- Yamamoto, K.; Otsuka, H.; Wada, S.I.; Sohn, D.; Takahara, A. Preparation and properties of poly(methyl methacrylate)/imogolite hybrid via surface modification using phosphoric acid ester. Polymer 2005, 46, 12386–12392. [Google Scholar] [CrossRef]
- Yamamoto, K.; Otsuka, H.; Takahara, A. Preparation of novel polymer hybrids from imogolite nanofiber. Polym. J. 2007, 39, 1–15. [Google Scholar] [CrossRef]
- Guimaraes, L.; Enyashin, A.N.; Frenzel, J.; Heine, T.; Duarte, H.A.; Seifert, G. Imogolite nanotubes: Stability, electronic, and mechanical properties. ACS Nano 2007, 1, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Otsuka, H.; Wada, S.; Takahara, A. Surface modification of aluminosilicate nanofiber “imogolite”. Chem. Lett. 2001, 30, 1162–1173. [Google Scholar] [CrossRef]
- Yah, W.O.; Yamamoto, K.; Jiravanichanun, N.; Otsuka, H.; Takahara, A. Imogolite Reinforced Nanocomposites: Multifaceted Green Materials. Materials 2010, 3, 1709–1745. [Google Scholar] [CrossRef]
- Yamamoto, K.; Otsuka, H.; Wada, S.I.; Sohn, D.; Takahara, A. Transparent polymer nanohybrid prepared by in situ synthesis of aluminosilicate nanofibers in poly(vinyl alcohol) solution. Soft Matter 2005, 1, 372–377. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kuroda, K. Layer-by-layer assembly of imogolite nanotubes and polyelectrolytes into core-shell particles and their conversion to hierarchically porous spheres. Sci. Technol. Adv. Mat. 2008, 9. [Google Scholar] [CrossRef]
- Ben Liew, K.; Daud, W.R.W.; Ghasemi, M.; Leong, J.X.; Lim, W.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrog. Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Bonelli, B.; Bottero, I.; Ballarini, N.; Passeri, S.; Cavani, F.; Garrone, E. IR spectroscopic and catalytic characterization of the acidity of imogolite-based systems. J. Catal. 2009, 264, 15–30. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Escudey, M.; Molina, M.; García-González, M. Kinetic and Surface Study of Single-Walled Aluminosilicate Nanotubes and Their Precursors. Nanomaterials 2013, 3, 126–140. [Google Scholar] [CrossRef]
- Cradwick, P.D.G.; Farmer, V.C.; Russell, J.D.; Masson, C.R.; Wada, K.; Yoshinaga, N. Imogolite, a Hydrated Aluminium Silicate of Tubular Structure. Nature-Phys. Sci. 1972, 240, 187–199. [Google Scholar] [CrossRef]
- Levard, C.; Masion, A.; Rose, J.; Doelsch, E.; Borschneck, D.; Dominici, C.; Ziarelli, F.; Bottero, J.Y. Synthesis of Imogolite Fibers from Decimolar Concentration at Low Temperature and Ambient Pressure: A Promising Route for Inexpensive Nanotubes. J. Am. Chem. Soc. 2009, 131, 17080–17089. [Google Scholar] [CrossRef] [PubMed]
- Shafia, E.; Esposito, S.; Manzoli, M.; Chiesa, M.; Tiberto, P.; Barrera, G.; Menard, G.; Allia, P. Al/Fe isomorphic substitution versus Fe2O3 clusters formation in Fe-doped aluminosilicate nanotubes (imogolite). J. Nanopart Res. 2015, 17, 1–14. [Google Scholar] [CrossRef]
- Avellan, A.; Levard, C.; Kumar, N.; Rose, J.; Olivi, L.; Thill, A.; Chaurand, P.; Borschneck, D.; Maison, A. Structural incorporation of iron into Ge-imogolite nanotubes: A promising step for innovative nanomaterials. RSC Adv. 2014, 4, 49827–49830. [Google Scholar] [CrossRef]
- Favero, G.; Fusco, G.; Mazzei, F.; Tasca, F.; Antiochia, R. Electrochemical Characterization of Graphene and MWCNTs Screen-Printed Electrodes Modified with AuNPs for Laccase Biosensor Development. Nanomaterials 2015, 5, 1995–2006. [Google Scholar] [CrossRef] [Green Version]
- Tasca, F.; Gorton, L.; Harreither, W.; Haltrich, D.; Ludwig, R.; Noll, G. Highly Efficient and Versatile Anodes for Biofuel Cells Based on Cellobiose Dehydrogenase from Myriococcum thermophilum. J. Phys. Chem. C 2008, 112, 13668–13673. [Google Scholar] [CrossRef]
- Tasca, F.; Gorton, L.; Harreither, W.; Haltrich, D.; Ludwig, R.; Noll, G. Direct electron transfer at cellobiose dehydrogenase modified anodes for biofuel cells. J. Phys. Chem. C 2008, 112, 9956–9961. [Google Scholar] [CrossRef]
- Imamura, S.; Kokubu, T.; Yamashita, T.; Okamoto, Y.; Kajiwara, K.; Kanai, H. Shape-selective copper-loaded imogolite catalyst. J. Catal. 1996, 160, 137–139. [Google Scholar] [CrossRef]
- Tasca, F.; Recio, F.J.; Venegas, R.; Geraldo, D.A.; Sancy, M.; Zagal, J.H. Linear versus volcano correlations for the electrocatalytic oxidation of hydrazine on graphite electrodes modified with MN4 macrocyclic complexes. Electrochim. Acta 2014, 140, 314–319. [Google Scholar] [CrossRef]
- Javier Recio, F.; Canete, P.; Tasca, F.; Linares-Flores, C.; Zagal, J.H. Tuning the Fe(II)/(I) formal potential of the FeN4 catalysts adsorbed on graphite electrodes to the reversible potential of the reaction for maximum activity: Hydrazine oxidation. Electrochem. Commun. 2013, 30, 34–37. [Google Scholar] [CrossRef]
- Zagal, J.H.; Gulppi, M.A.; Cardenas-Jiron, G. Metal-centered redox chemistry of substituted cobalt phthalocyanines adsorbed on graphite and correlations with MO calculations and Hammett parameters. Electrocatalytic reduction of a disulfide. Polyhedron 2000, 19, 2255–2260. [Google Scholar] [CrossRef]
- Bedioui, F.; Griveau, S.; Nyokong, T.; Appleby, A.J.; Caro, C.A.; Gulppi, M.; Ochoa, G.; Zagal, J.H. Tuning the redox properties of metalloporphyrin- and metallophthalocyanine-based molecular electrodes for the highest electrocatalytic activity in the oxidation of thiols. PCCP 2007, 9, 3383–3396. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, C.; Arancibia-Miranda, N.; Acuña-Rougier, C.; Escudey, M.; Tasca, F. Spectroscopic and Electrochemical Studies of Imogolite and Fe-Modified Imogolite Nanotubes. Nanomaterials 2016, 6, 28. https://doi.org/10.3390/nano6020028
Castro C, Arancibia-Miranda N, Acuña-Rougier C, Escudey M, Tasca F. Spectroscopic and Electrochemical Studies of Imogolite and Fe-Modified Imogolite Nanotubes. Nanomaterials. 2016; 6(2):28. https://doi.org/10.3390/nano6020028
Chicago/Turabian StyleCastro, Carmen, Nicolas Arancibia-Miranda, Cristina Acuña-Rougier, Mauricio Escudey, and Federico Tasca. 2016. "Spectroscopic and Electrochemical Studies of Imogolite and Fe-Modified Imogolite Nanotubes" Nanomaterials 6, no. 2: 28. https://doi.org/10.3390/nano6020028
APA StyleCastro, C., Arancibia-Miranda, N., Acuña-Rougier, C., Escudey, M., & Tasca, F. (2016). Spectroscopic and Electrochemical Studies of Imogolite and Fe-Modified Imogolite Nanotubes. Nanomaterials, 6(2), 28. https://doi.org/10.3390/nano6020028







