Photoelectrochemical Performance Observed in Mn-Doped BiFeO3 Heterostructured Thin Films
Abstract
:1. Introduction
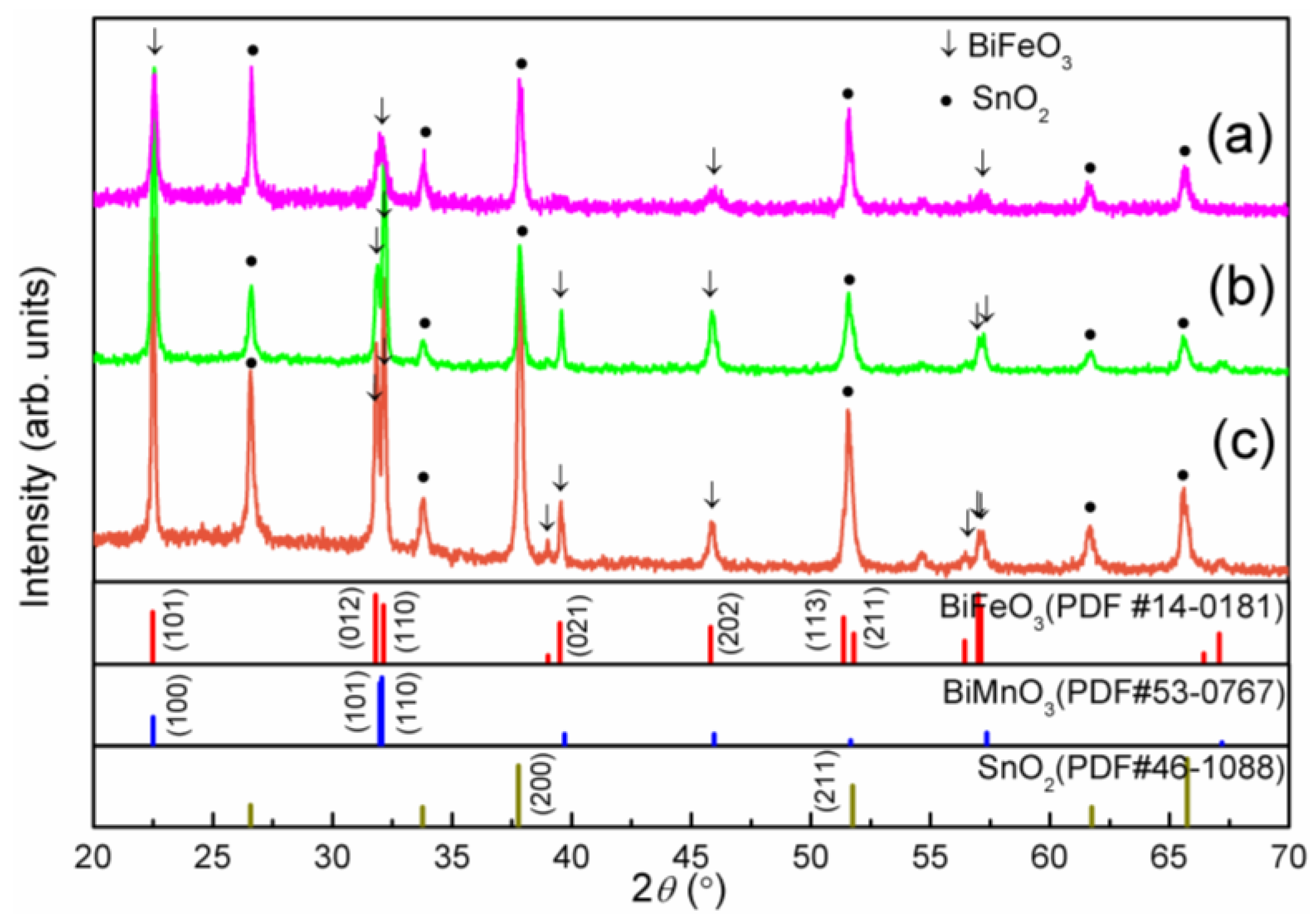
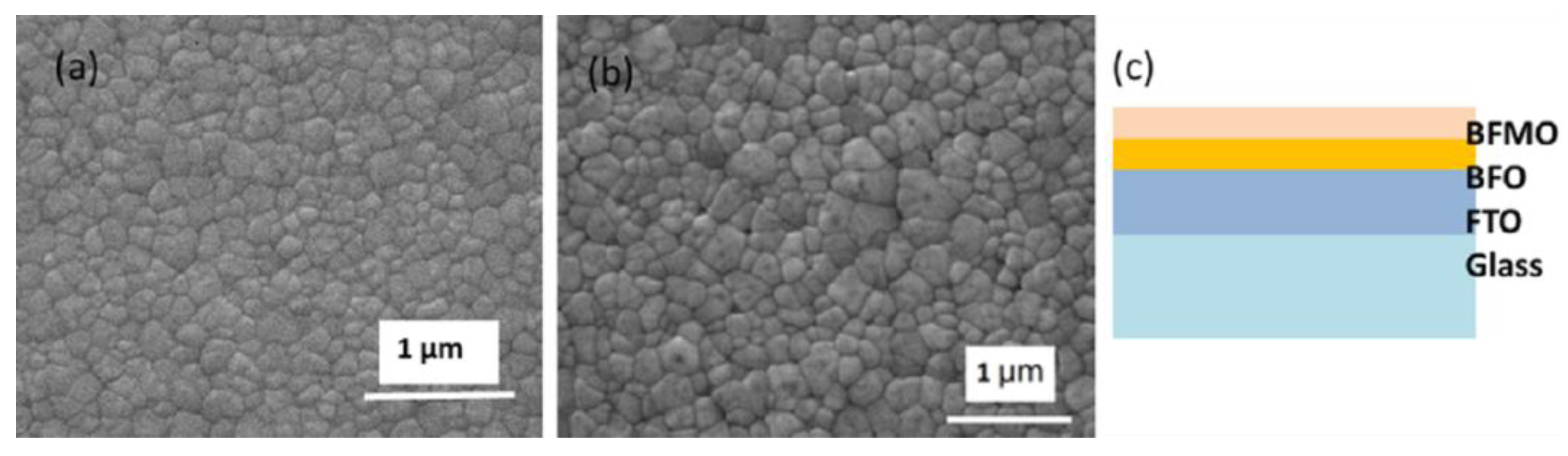
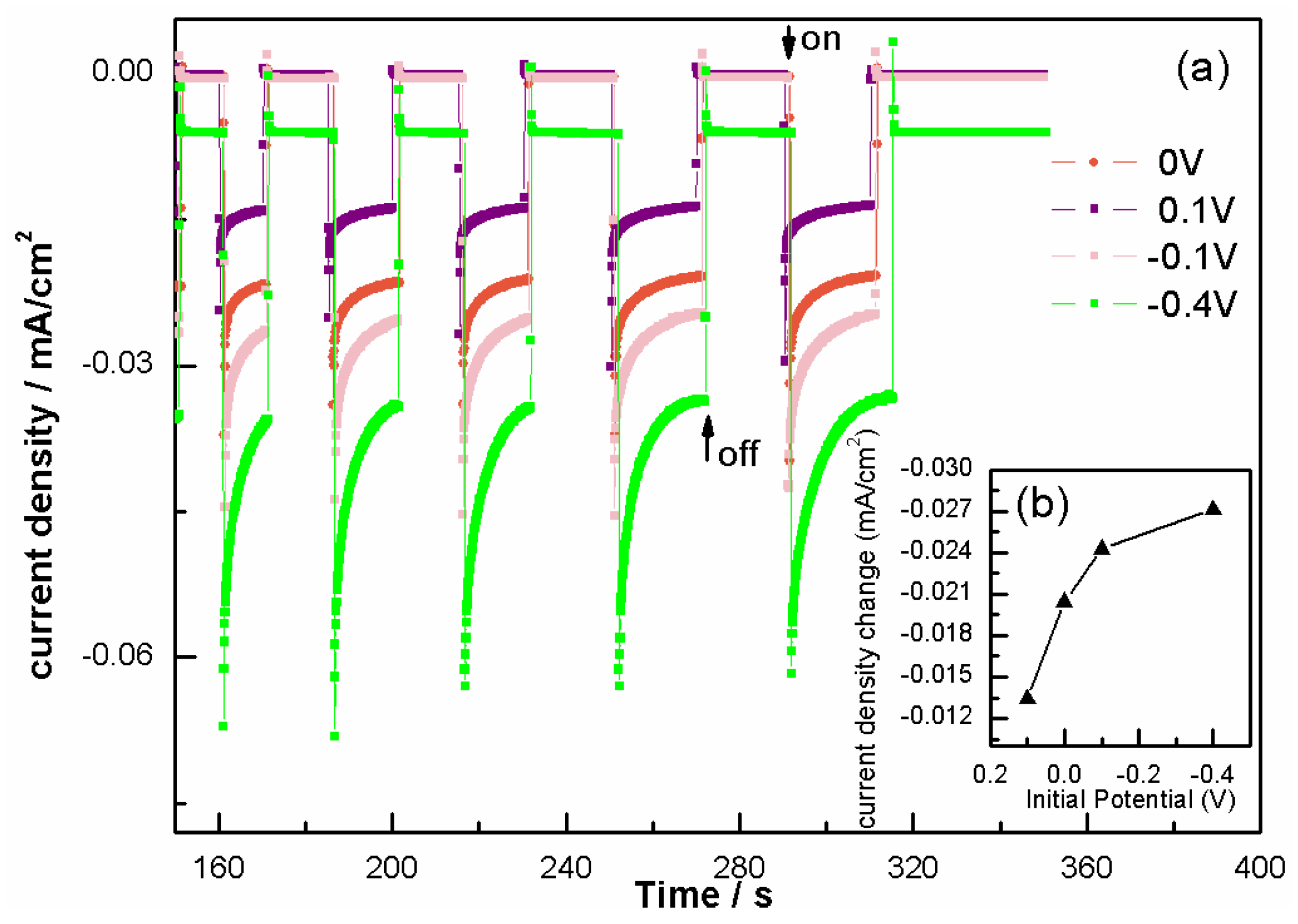
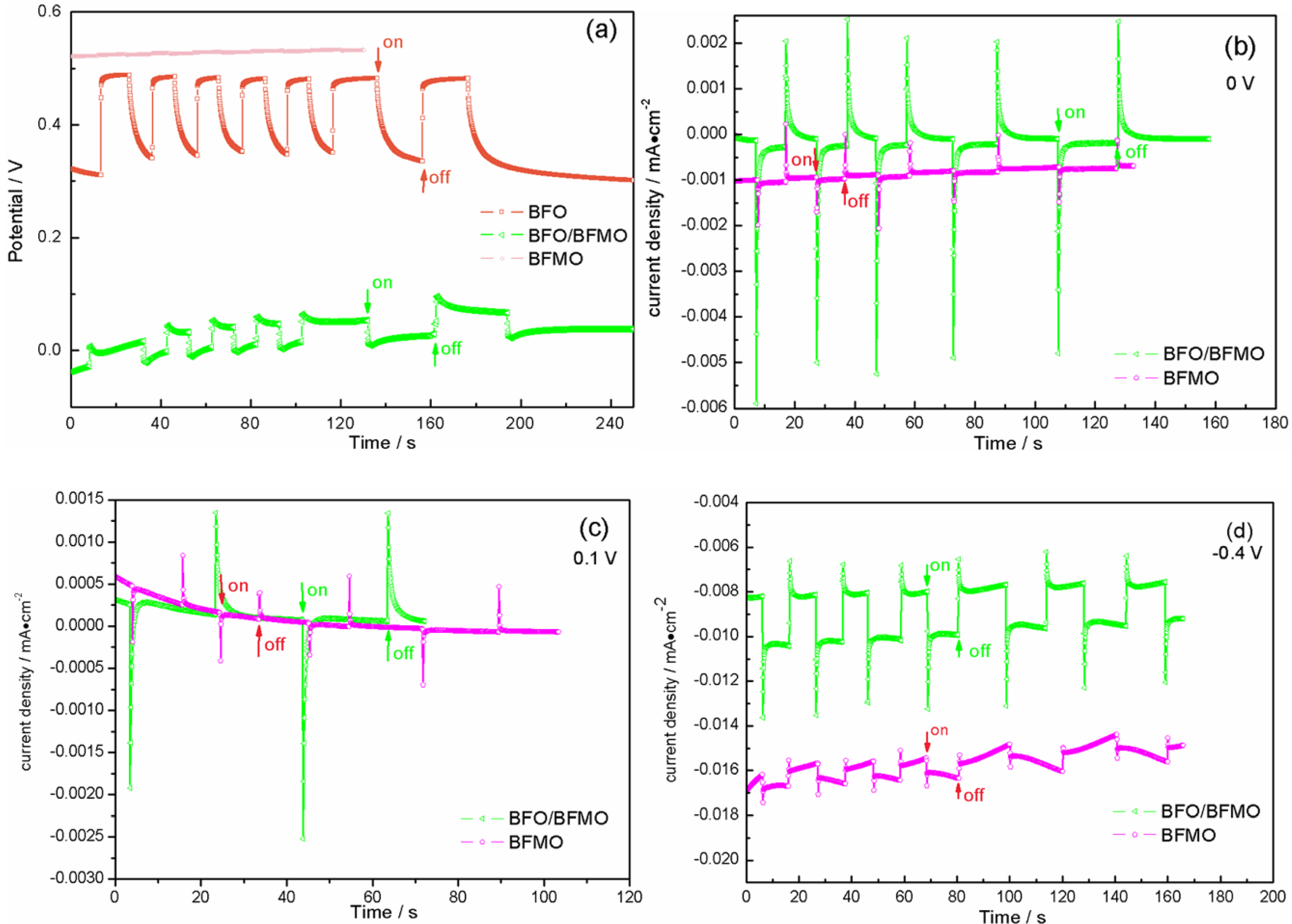
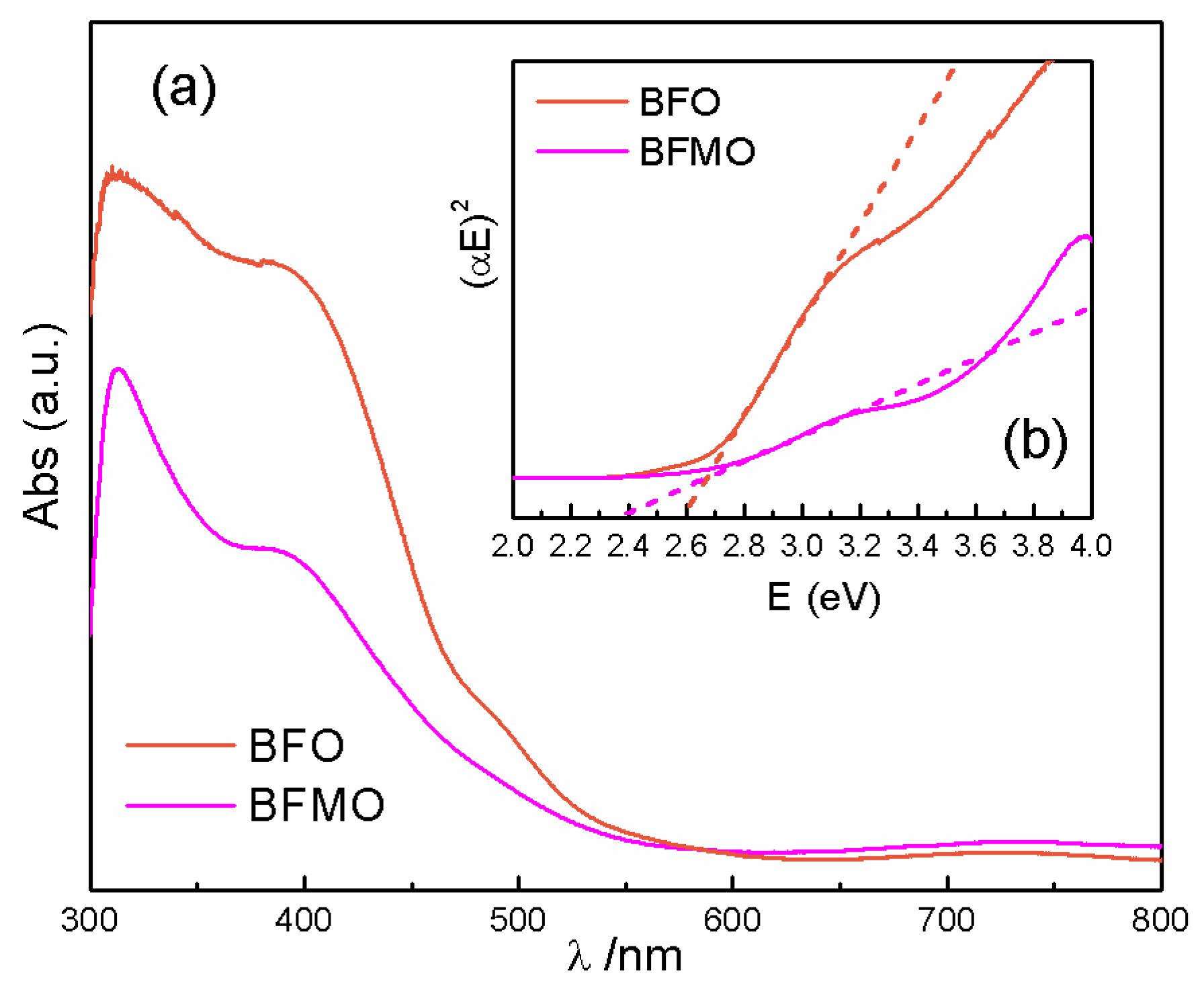
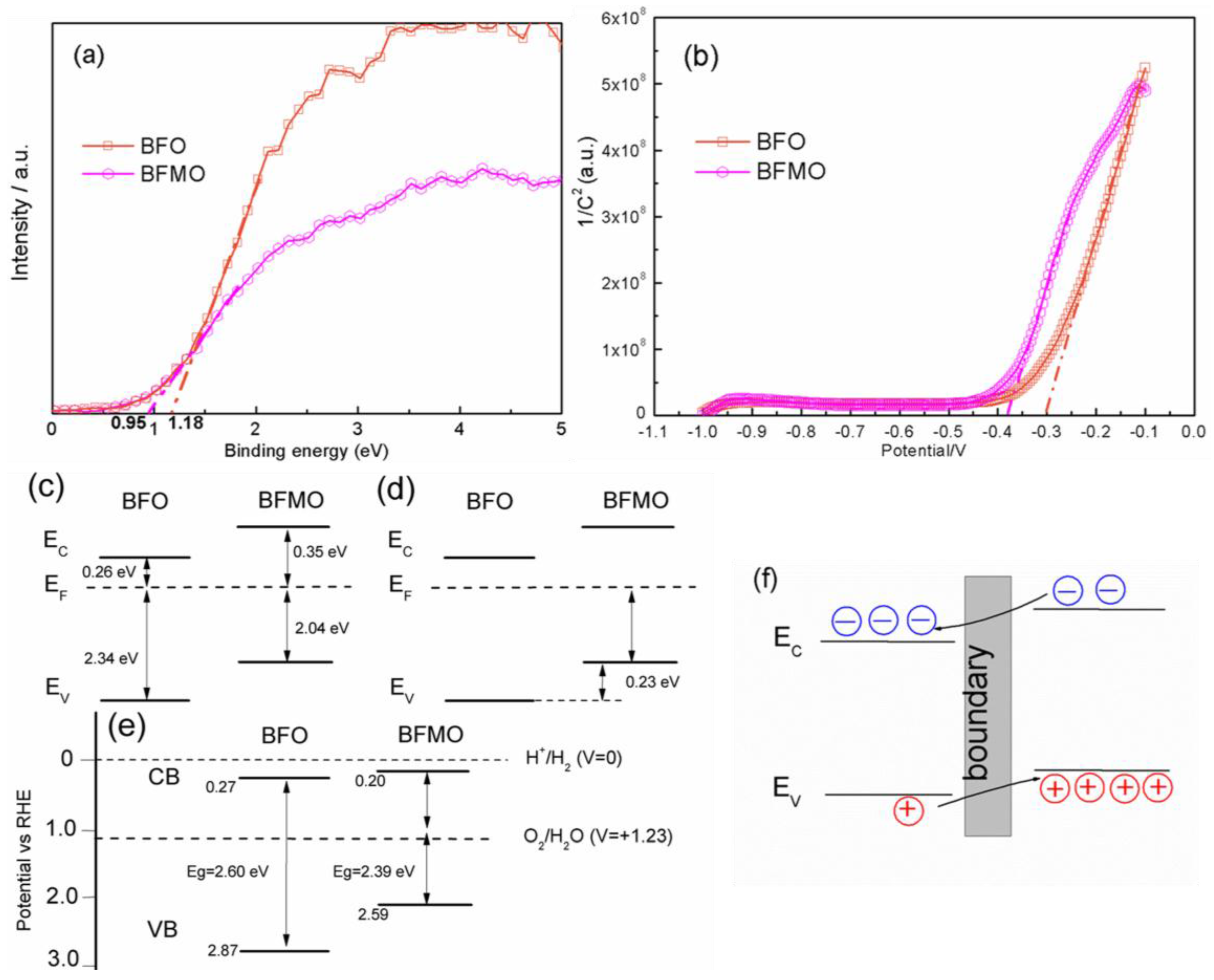
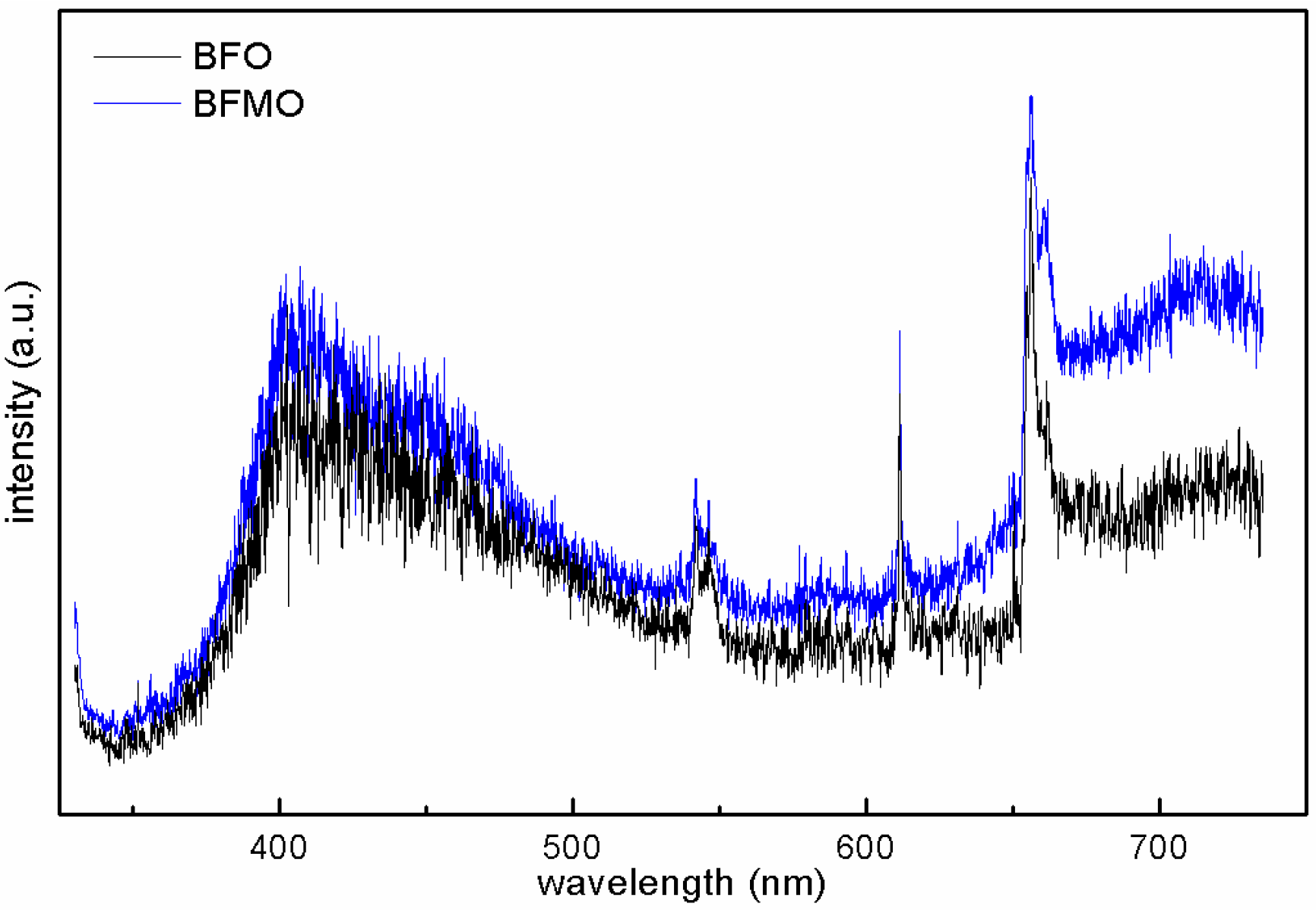
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dong, W.; Guo, Y.; Guo, B.; Liu, H.; Li, H.; Liu, H. Enhanced photovoltaic properties in polycrystalline BiFeO3 thin films with rhombohedral perovskite structure deposited on fluorine doped tin oxide substrates. Mater. Lett. 2012, 88, 140–142. [Google Scholar] [CrossRef]
- Chen, B.; Li, M.; Liu, Y.; Zuo, Z.; Zhuge, F.; Zhan, Q.F.; Li, R.W. Effect of top electrodes on photovoltaic properties of polycrystalline BiFeO3 based thin film capacitors. Nanotechnology 2011, 22, 195201. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liu, B. Photoinduced changes in the hysteresis loop and photovoltaic effect of the magnetron sputtered epitaxial BiFeO3 film. Phys. Status Solidi A 2014, 2, 1–4. [Google Scholar]
- Katiyar, R.K.; Sharma, Y.; Misra, P.; Puli, V.S.; Sahoo, S.; Kumar, A.; Scott, J.F.; Morell, G.; Weiner, B.R.; Katiyar, R.S. Studies of the switchable photovoltaic effect in co-substituted BiFeO3 thin films. Appl. Phys. Lett. 2014, 105, 172904. [Google Scholar] [CrossRef]
- Gupta, S.; Tomar, M.; Gupta, V. Ferroelectric photovoltaic properties of Ce and Mn codoped BiFeO3 thin film. J. Appl. Phys. 2014, 115, 014102. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Quesada-Cabrera, R.; Blackman, C.S.; Tang, J.; Southern, P.; Weaver, P.M.; Carmalt, C.J. A simple, low-cost CVD route to thin films of BiFeO3 for efficient water photo-oxidation. J. Mater. Chem. A 2014, 2, 2922. [Google Scholar] [CrossRef]
- Sharma, S.; Tomar, M.; Kumar, A.; Puri, N.K.; Gupta, V. Enhanced ferroelectric photovoltaic response of BiFeO3/BaTiO3 multilayered structure. J. Appl. Phys. 2015, 118, 074103. [Google Scholar] [CrossRef]
- Cao, D.; Wang, Z.; Nasori, N.; Wen, L.; Mi, Y.; Lei, Y. Switchable charge-transfer in the photoelectrochemical energy-conversion process of ferroelectric BiFeO3 photoelectrodes. Angew. Chem. Int. Ed. 2014, 126, 11207–11211. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, D.; Wen, L.; Xu, R.; Obergfell, M.; Mi, Y.; Zhan, Z.; Nasori, N.; Demsar, J.; Lei, Y. Manipulation of charge transfer and transport in plasmonic-ferroelectric hybrids for photoelectrochemical applications. Nat. Commun. 2016, 7, 10348. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.M.; Wang, H.C.; Shen, Y.; Lin, Y.-H.; Nan, C.W. Photocatalytic and magnetic behaviors of BiFeO3 thin films deposited on different substrates. J. Appl. Phys. 2014, 116, 174307. [Google Scholar] [CrossRef]
- Fan, Z.; Yao, K.; Wang, J. Photovoltaic effect in an indium-tin-oxide/ZnO/BiFeO3/Pt heterostructure. Appl. Phys. Lett. 2014, 105, 162903. [Google Scholar] [CrossRef]
- Xu, H.-M.; Wang, H.-C.; Shen, Y.; Lin, Y.-H.; Nan, C.W. Low-dimensional nanostructured photocatalysts. J. Adv. Ceram. 2015, 4, 159–182. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, H.; Lin, Y.; Wei, Y.; Nan, C.W.; Deng, X. Influence of La doping on magnetic and optical properties of bismuth ferrite nanofibers. J. Nanomater. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Vanga, P.R.; Mangalaraja, R.V.; Ashok, M. Effect of co-doping on the optical, magnetic and photocatalytic properties of the Gd modified BiFeO3. J. Mater. Sci. Mater. Electron. 2016, 27, 5699–5706. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, S.; Kumar, M.; Dwivedi, R.K. Optical Properties of Y and Ti Co-Substituted BiFeO3 Multiferroics. In Proceedings of 58th DAE Solid State Symposium, Thapar University, Patiala, India, 17–21 December 2013.
- Xu, X.S.; Ihlefeld, J.F.; Lee, J.H.; Ezekoye, O.K.; Vlahos, E.; Ramesh, R.; Gopalan, V.; Pan, X.Q.; Schlom, D.G.; Musfeldt, J.L. Tunable band gap in Bi(Fe1-xMnx)O3 films. Appl. Phys. Lett. 2010, 96, 192901. [Google Scholar] [CrossRef]
- Liu, W.; Tan, G.; Dong, G.; Yan, X.; Ye, W.; Ren, H.; Xia, A. Structure transition and multiferroic properties of Mn-doped BiFeO3 thin films. J. Mater. Sci. Mater. Electron. 2013, 25, 723–729. [Google Scholar] [CrossRef]
- Huang, J.Z.; Wang, Y.; Lin, Y.; Li, M.; Nan, C.W. Effect of Mn doping on electric and magnetic properties of BiFeO3 thin films by chemical solution deposition. J. Appl. Phys. 2009, 106, 063911. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Shen, Y.; Lin, Y.; Nan, C. Magnetic and photocatalytic behaviors of Ca Mn co-doped BiFeO3 nanofibers. Mod. Res. Catal. 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Wang, H.C.; Lin, Y.H.; Feng, Y.N.; Shen, Y. Photocatalytic behaviors observed in Ba and Mn doped BiFeO3 nanofibers. J. Electroceramics 2013, 31, 271–274. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, X.; Wang, W.; Wang, X.; Liu, C.; Xie, Q.; Li, Z.; Zhang, Z. Enhanced photoelectrochemical and photocatalytic performance of TiO2 nanorod arrays/CdS quantum dots by coating TiO2 through atomic layer deposition. Nano Energy 2015, 11, 400–408. [Google Scholar] [CrossRef]
- Schultz, A.M.; Zhang, Y.; Salvador, P.A.; Rohrer, G.S. Effect of crystal and domain orientation on the visible-light photochemical reduction of Ag on BiFeO3. ACS Appl. Mater. Interfaces 2011, 3, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Seidel, J.; Byrnes, S.J.; Shafer, P.; Yang, C.H.; Rossell, M.D.; Yu, P.; Chu, Y.H.; Scott, J.F.; Ager, J.W.; et al. Above-bandgap voltages from ferroelectric photovoltaic devices. Nat. Nanotechnol. 2010, 5, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Salvador, P.A.; Rohrer, G.S. Photocatalysts with internal electric fields. Nanoscale 2014, 6, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Yu, T.; Gao, F.; Zhang, H.T.; Liu, L.F.; Wang, Y.M.; Li, Z.S.; Zou, Z.G.; Liu, J.M. Application of weak ferromagnetic BiFeO3 films as the photoelectrode material under visible-light irradiation. Appl. Phys. Lett. 2007, 91, 022114. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781. [Google Scholar] [CrossRef]
- Sayama, K.; Nomura, A.; Arai, T.; Sugita, T.; Abe, R.; Yanagida, M.; Oi, T.; Iwasaki, Y.; Abe, Y.; Sugihara, H. Photoelectrochemical decomposition of water into H2 and O2 on porous BiVO4 thin-film electrodes under visible light and significant effect of Ag ion treatment. J. Phys. Chem. B 2006, 110, 11352–11360. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, J.; Cao, Z.; Zheng, Y.; Yao, Z.; Shao, G.; Hu, J. Fabrication of predominantly Mn4+-doped TiO2 nanoparticles under equilibrium conditions and their application as visible-light photocatalyts. Chem. Asian J. 2014, 9, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.-M.; Wang, H.; Shi, J.; Lin, Y.; Nan, C. Photoelectrochemical Performance Observed in Mn-Doped BiFeO3 Heterostructured Thin Films. Nanomaterials 2016, 6, 215. https://doi.org/10.3390/nano6110215
Xu H-M, Wang H, Shi J, Lin Y, Nan C. Photoelectrochemical Performance Observed in Mn-Doped BiFeO3 Heterostructured Thin Films. Nanomaterials. 2016; 6(11):215. https://doi.org/10.3390/nano6110215
Chicago/Turabian StyleXu, Hao-Min, Huanchun Wang, Ji Shi, Yuanhua Lin, and Cewen Nan. 2016. "Photoelectrochemical Performance Observed in Mn-Doped BiFeO3 Heterostructured Thin Films" Nanomaterials 6, no. 11: 215. https://doi.org/10.3390/nano6110215




