X-ray Absorption Spectroscopy Characterization of a Li/S Cell
Abstract
:1. Introduction
2. Results and Discussion
2.1. Introduction of X-Ray Absorption Tools
2.2. Application of S K-Edge XAS to the Characterization of the Sample Synthesis Process

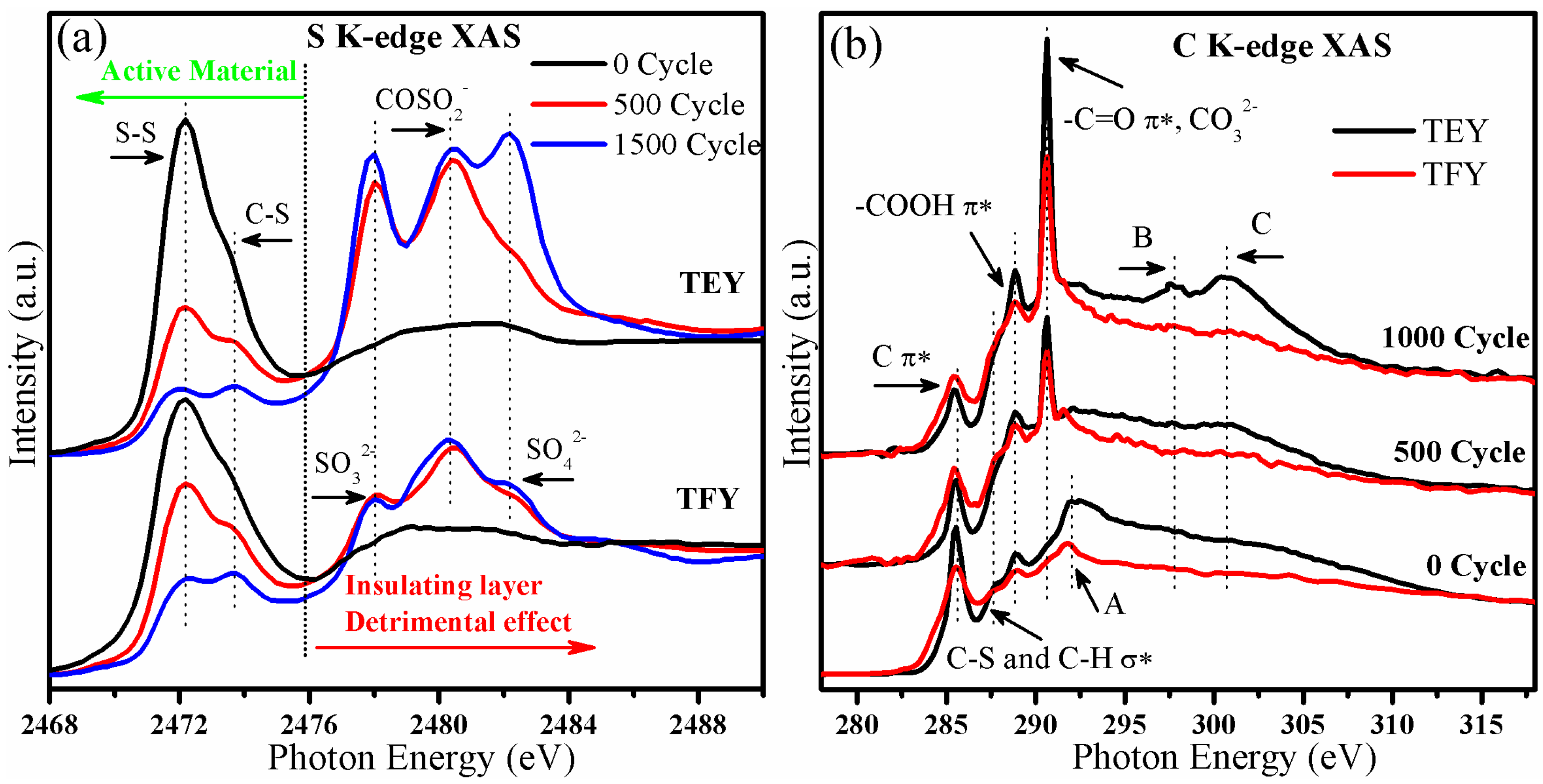
2.3. Application of S K-Edge Spectroscopy to the Characterization of Cycled Cathode Materials
2.4. In-situ/in-Operando Experimental Setup
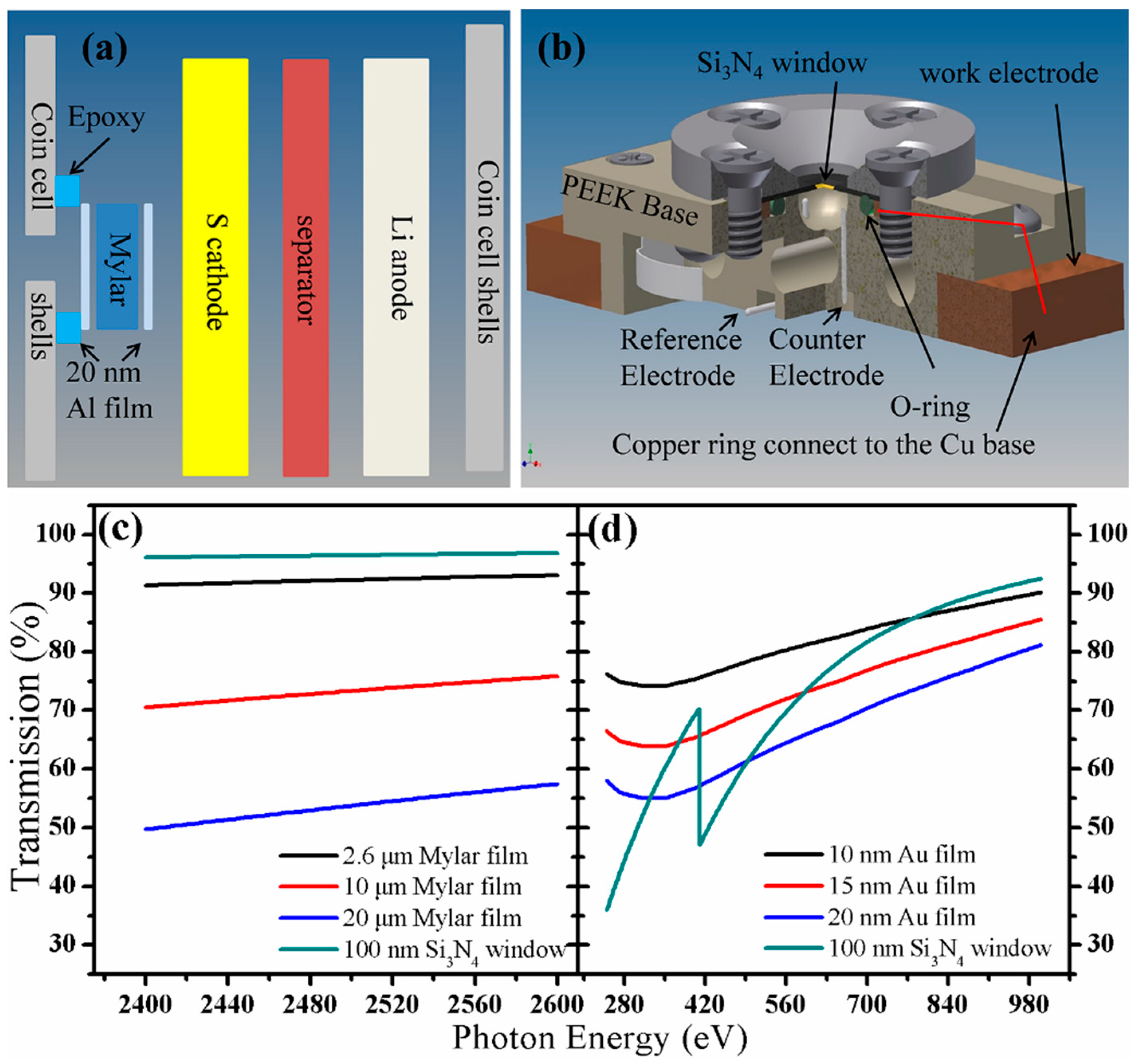
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Qiu, Y.; Li, W.; Zhao, W.; Li, G.; Hou, Y.; Liu, M.; Zhou, L.; Ye, F.; Li, H.; Wei, Z.; et al. High-rate, ultralong cycle-life lithium/sulfur batteries enabled by nitrogen-doped graphene. Nano Lett. 2014, 14, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-K.; Zhang, Y.; Cairns, E.J. A long-life, high-rate lithium/sulfur cell: A multifaceted approach to enhancing cell performance. Nano Lett. 2013, 13, 5891–5899. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Nazar, L.F. Advances in Li-S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable lithium–sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and prospects of lithium–sulfur batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous hollow Carbon@Sulfur composites for high-power lithium–sulfur batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lee, J.T.; Magasinski, A.; Kim, H.; Yushin, G. Solution-based processing of graphene–Li2S composite cathodes for lithium-ion and lithium–sulfur batteries. Part. Part. Syst. Charact. 2014, 31, 639–644. [Google Scholar] [CrossRef]
- Feng, X.; Song, M.-K.; Stolte, W.C.; Gardenghi, D.; Zhang, D.; Sun, X.; Zhu, J.; Cairns, E.J.; Guo, J. Understanding the degradation mechanism of rechargeable lithium/sulfur cells: A comprehensive study of the sulfur-graphene oxide cathode after discharge-charge cycling. Phys. Chem. Chem. Phys. 2014, 16, 16931–16940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, L.; Glans, P.-A.; Zhang, Y.; Zhu, J.; Guo, J. Electronic structure and chemical bonding of a graphene oxide-sulfur nanocomposite for use in superior performance lithium-sulfur cells. Phys.Chem. Chem. Phys. 2012, 14, 13670–13675. [Google Scholar] [CrossRef] [PubMed]
- Jalilehvand, F. Sulfur: Not a “silent” element any more. Chem. Soc. Rev. 2006, 35, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Vairavamurthy, A. Using X-ray absorption to probe sulfur oxidation states in complex molecules. Spectrochim. Acta A 1998, 54, 2009–2017. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Glans, P.-A.; Chuang, C.-H.; Kapilashrami, M.; Guo, J. Perspectives of in situ/operando resonant inelastic X-ray scattering in catalytic energy materials science. J. Electron Spectrosc. Relat. Phenom. 2015, 200, 282–292. [Google Scholar] [CrossRef]
- De Groot, F. High-resolution X-ray emission and X-ray absorption spectroscopy. Chem. Rev. 2001, 101, 1779–1808. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, M. A review on the structural studies of batteries and host materials by X-ray absorption spectroscopy. ISRN Mater. Sci. 2013, 2013, 1–22. [Google Scholar] [CrossRef]
- Frazer, B.H.; Gilbert, B.; Sonderegger, B.R.; de Stasio, G. The probing depth of total electron yield in the sub-keV range: TEY-XAS and X-PEEM. Surf. Sci. 2003, 537, 161–167. [Google Scholar] [CrossRef]
- Lin, Z.; Nan, C.; Ye, Y.; Guo, J.; Zhu, J.; Cairns, E.J. High-performance lithium/sulfur cells with a bi-functionally immobilized sulfur cathode. Nano Energy 2014, 9, 408–416. [Google Scholar] [CrossRef]
- Ai, G.; Dai, Y.; Ye, Y.; Mao, W.; Wang, Z.; Zhao, H.; Chen, Y.; Zhu, J.; Fu, Y.; Battaglia, V.; et al. Investigation of surface effects through the application of the functional binders in lithium sulfur batteries. Nano Energy 2015, 16, 28–37. [Google Scholar] [CrossRef]
- Pascal, T.A.; Wujcik, K.H.; Velasco-Velez, J.; Wu, C.; Teran, A.A.; Kapilashrami, M.; Cabana, J.; Guo, J.; Salmeron, M.; Balsara, N.; et al. X-ray absorption spectra of dissolved polysulfides in lithium–sulfur batteries from first-principles. J. Phys. Chem. Lett. 2014, 5, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Wujcik, K.H.; Pascal, T.A.; Pemmaraju, C.D.; Devaux, D.; Stolte, W.C.; Balsara, N.P.; Prendergast, D. Characterization of polysulfide radicals present in an ether-based electrolyte of a lithium–sulfur battery during initial discharge using in situ X-ray absorption spectroscopy experiments and first-principles calculations. Adv. Energy Mater. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Patel, M.U.M.; Arčon, I.; Aquilanti, G.; Stievano, L.; Mali, G.; Dominko, R. X-ray absorption near-edge structure and nuclear magnetic resonance study of the lithium–sulfur battery and its components. ChemPhysChem 2014, 15, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, H.; Uesugi, M.; Naraoka, H.; Ito, M.; Kilcoyne, A.; Sandford, S.; Kitajima, F.; Mita, H.; Takano, Y.; Yada, T.; et al. X-ray absorption near edge structure spectroscopic study of Hayabusa category 3 carbonaceous particles. Earth Planet Sp. 2014, 66, 1–8. [Google Scholar] [CrossRef]
- Kikuma, J.; Tonner, B.P. XANES spectra of a variety of widely used organic polymers at the C K-edge. J. Electron Spectrosc. Relat. Phenom. 1996, 82, 53–60. [Google Scholar] [CrossRef]
- Kebukawa, Y.; Zolensky, M.E.; Kilcoyne, A.L.D.; Rahman, Z.; Jenniskens, P.; Cody, G.D. Diamond xenolith and matrix organic matter in the Sutter’s Mill meteorite measured by C-XANES. Meteorit. Planet. Sci. 2014, 49, 2095–2103. [Google Scholar] [CrossRef]
- Brandes, J.A.; Wirick, S.; Jacobsen, C. Carbon K-edge spectra of carbonate minerals. J. Synchrotron Radiat. 2010, 17, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yi, F.; Li, Z.; Zhu, Y.; Xu, Y.; Luo, C.; Yang, J.; Wang, C. Copper-stabilized sulfur-microporous carbon cathodes for Li–S batteries. Adv. Funct. Mater. 2014, 24, 4156–4163. [Google Scholar] [CrossRef]
- Evers, S.; Yim, T.; Nazar, L.F. Understanding the nature of absorption/adsorption in nanoporous polysulfide sorbents for the Li–S battery. J. Phys. Chem. C 2012, 116, 19653–19658. [Google Scholar] [CrossRef]
- Song, M.-S.; Han, S.-C.; Kim, H.-S.; Kim, J.-H.; Kim, K.-T.; Kang, Y.-M.; Ahn, H.-J.; Dou, S.X.; Lee, J.-Y. Effects of nanosized adsorbing material on electrochemical properties of sulfur cathodes for Li/S secondary batteries. J. Electrochem. Soc. 2004, 151, A791–A795. [Google Scholar] [CrossRef]
- Lowe, M.A.; Gao, J.; Abruna, H.D. Mechanistic insights into operational lithium-sulfur batteries by in situ X-ray diffraction and absorption spectroscopy. RSC Adv. 2014, 4, 18347–18353. [Google Scholar] [CrossRef]
- Cuisinier, M.; Cabelguen, P.-E.; Evers, S.; He, G.; Kolbeck, M.; Garsuch, A.; Bolin, T.; Balasubramanian, M.; Nazar, L.F. Sulfur speciation in Li–S batteries determined by operando X-ray absorption spectroscopy. J. Phys. Chem. Lett. 2013, 4, 3227–3232. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Liu, G.; Srinivasan, V.; Liu, Z.; Hussain, Z.; Yang, W. Distinct charge dynamics in battery electrodes revealed by in situ and operando soft X-ray spectroscopy. Nat. Commun. 2013, 4, 1–8. [Google Scholar]
- Takada, K. Progress and prospective of solid-state lithium batteries. Acta Mater. 2013, 61, 759–770. [Google Scholar] [CrossRef]
- Arthur, T.S.; Glans, P.-A.; Matsui, M.; Zhang, R.; Ma, B.; Guo, J. Mg deposition observed by in situ electrochemical Mg K-edge X-ray absorption spectroscopy. Electrochem. Commun. 2012, 24, 43–46. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Kawase, A.; Song, M.-K.; Feng, B.; Liu, Y.-S.; Marcus, M.A.; Feng, J.; Cairns, E.J.; Guo, J.; Zhu, J. X-ray Absorption Spectroscopy Characterization of a Li/S Cell. Nanomaterials 2016, 6, 14. https://doi.org/10.3390/nano6010014
Ye Y, Kawase A, Song M-K, Feng B, Liu Y-S, Marcus MA, Feng J, Cairns EJ, Guo J, Zhu J. X-ray Absorption Spectroscopy Characterization of a Li/S Cell. Nanomaterials. 2016; 6(1):14. https://doi.org/10.3390/nano6010014
Chicago/Turabian StyleYe, Yifan, Ayako Kawase, Min-Kyu Song, Bingmei Feng, Yi-Sheng Liu, Matthew A. Marcus, Jun Feng, Elton J. Cairns, Jinghua Guo, and Junfa Zhu. 2016. "X-ray Absorption Spectroscopy Characterization of a Li/S Cell" Nanomaterials 6, no. 1: 14. https://doi.org/10.3390/nano6010014





