On the Mass Fractal Character of Si-Based Structural Networks in Amorphous Polymer Derived Ceramics
Abstract
:1. Introduction
2. Experimental Methods
2.1. Synthesis
2.2. 29Si NMR SLR Spectroscopy
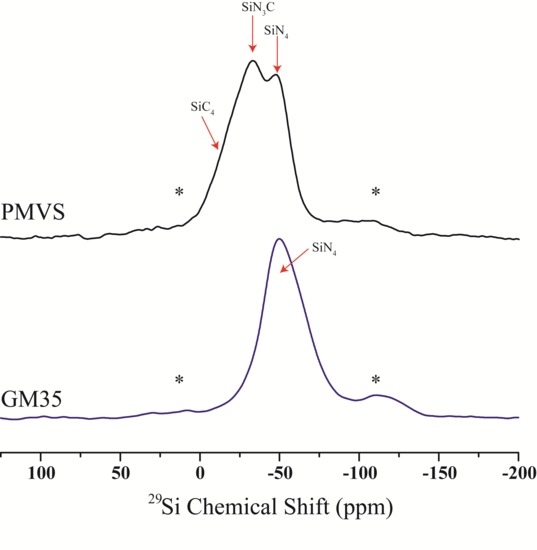
3. Results
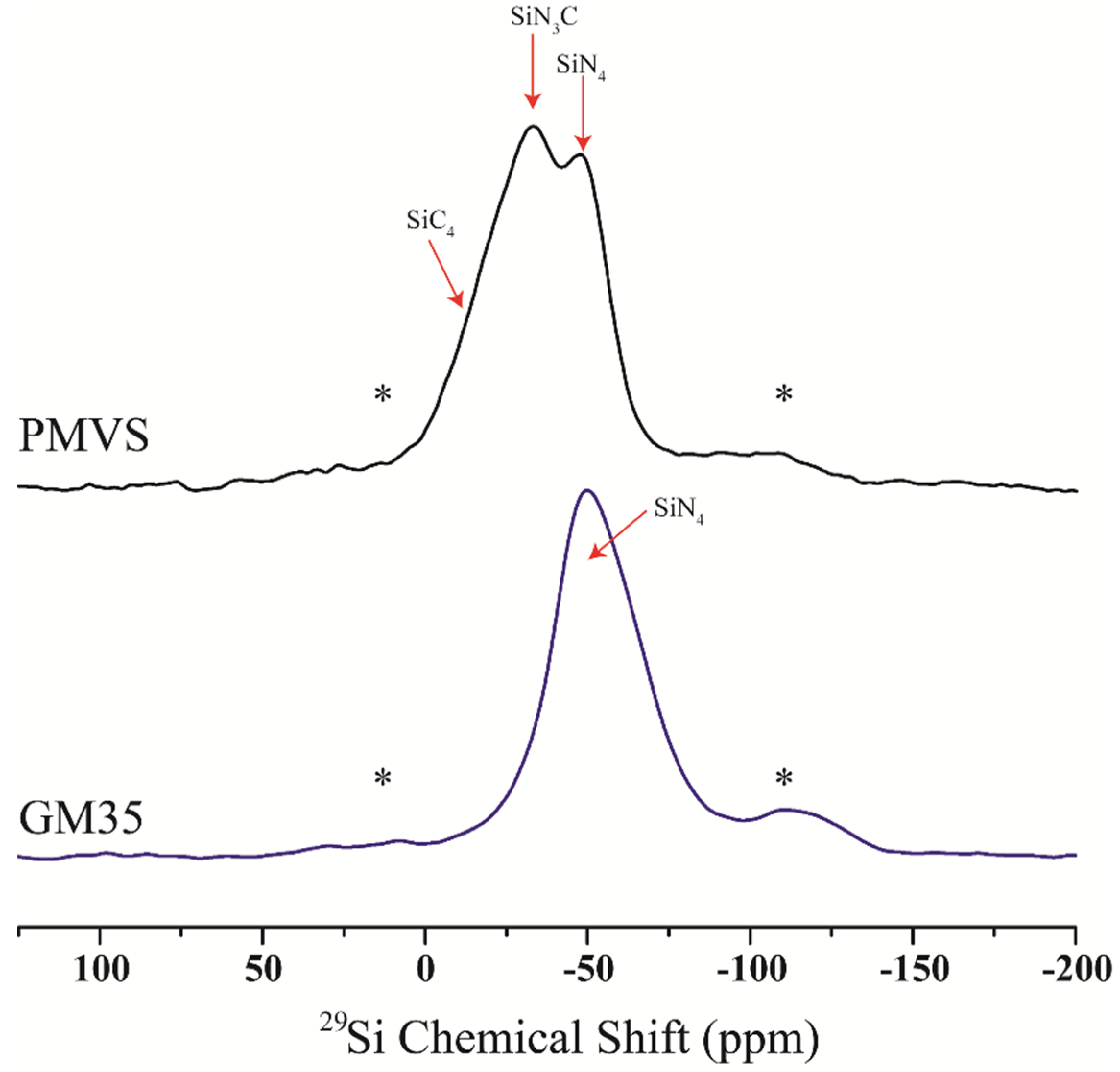
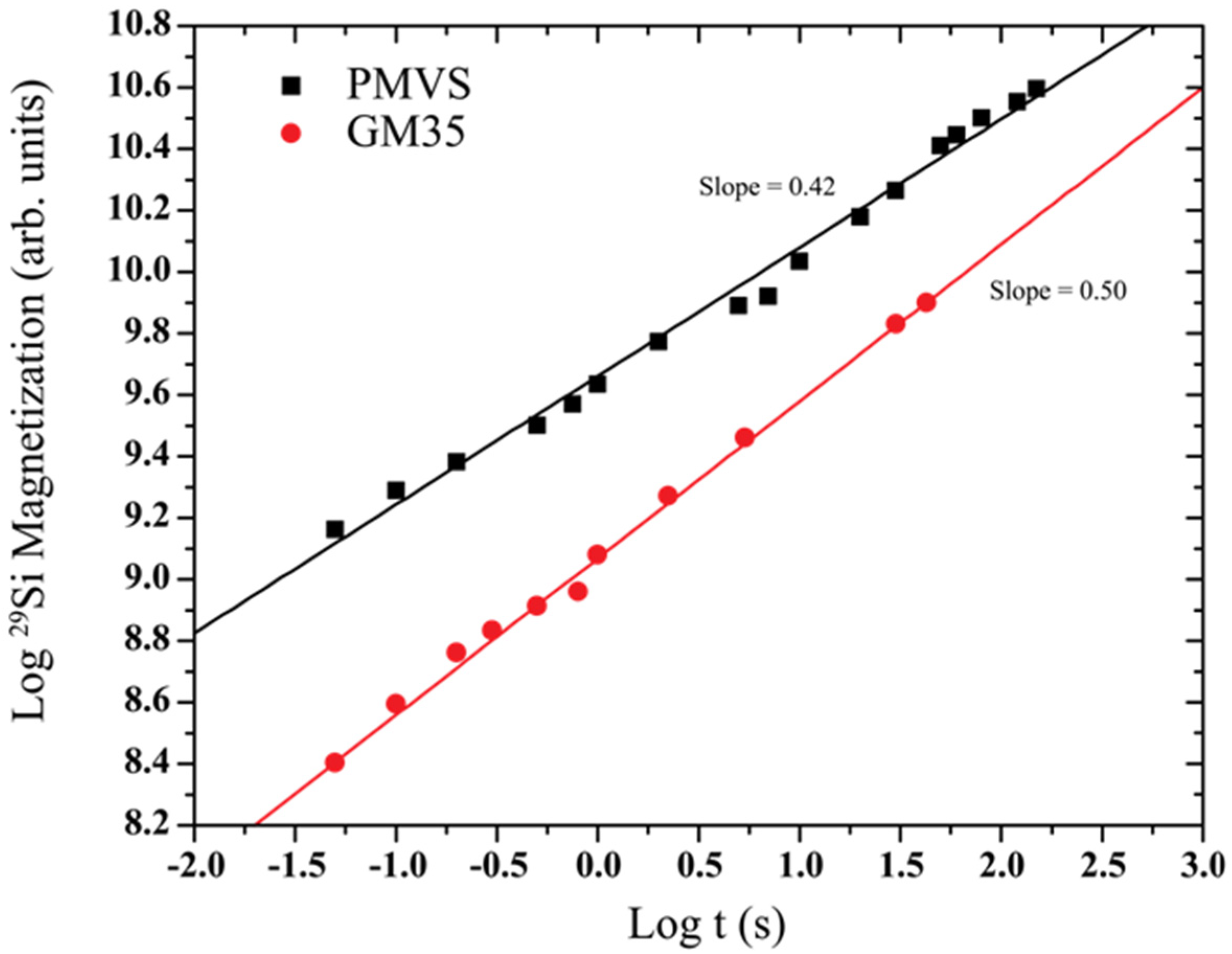
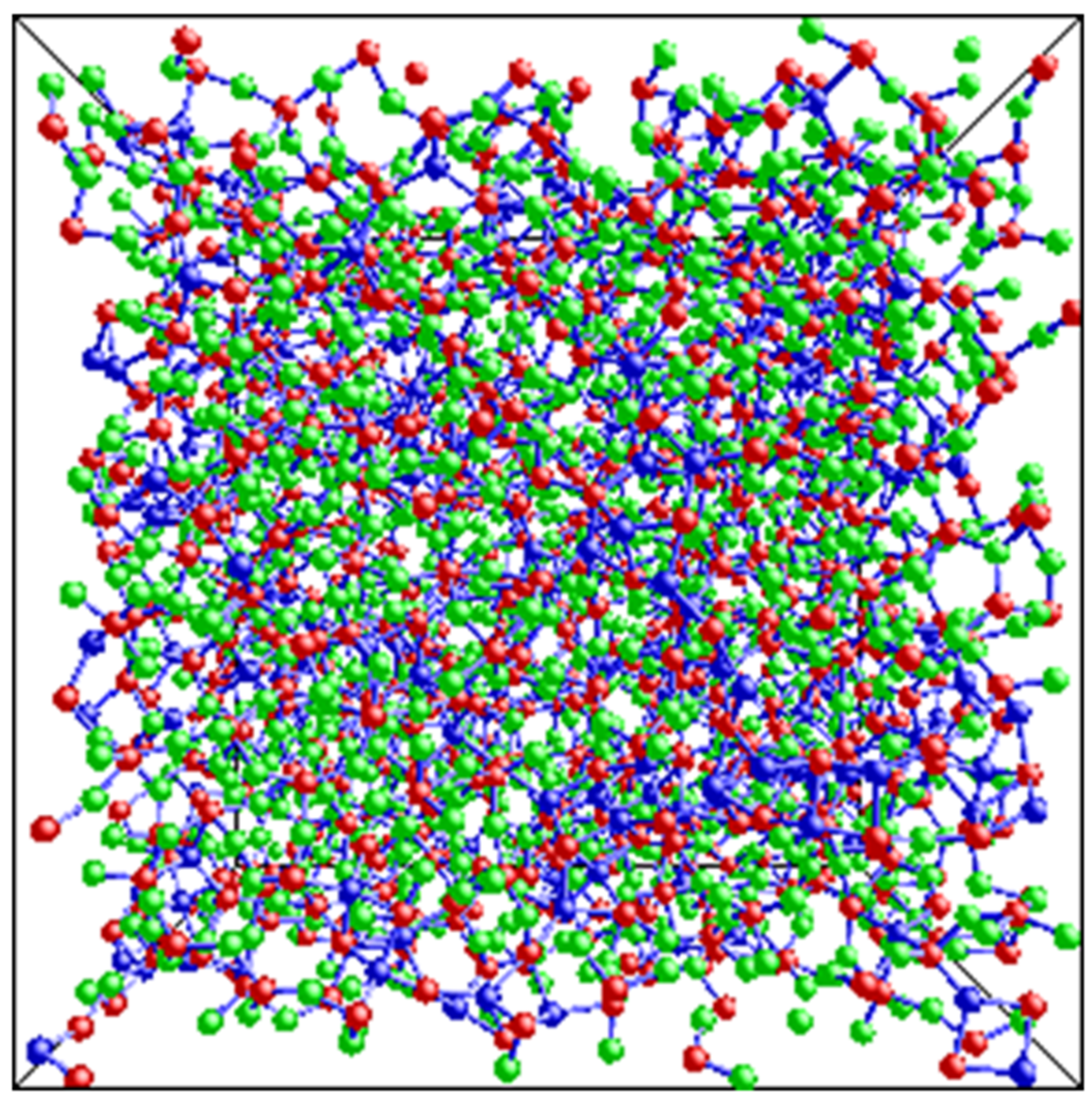
4. Discussion
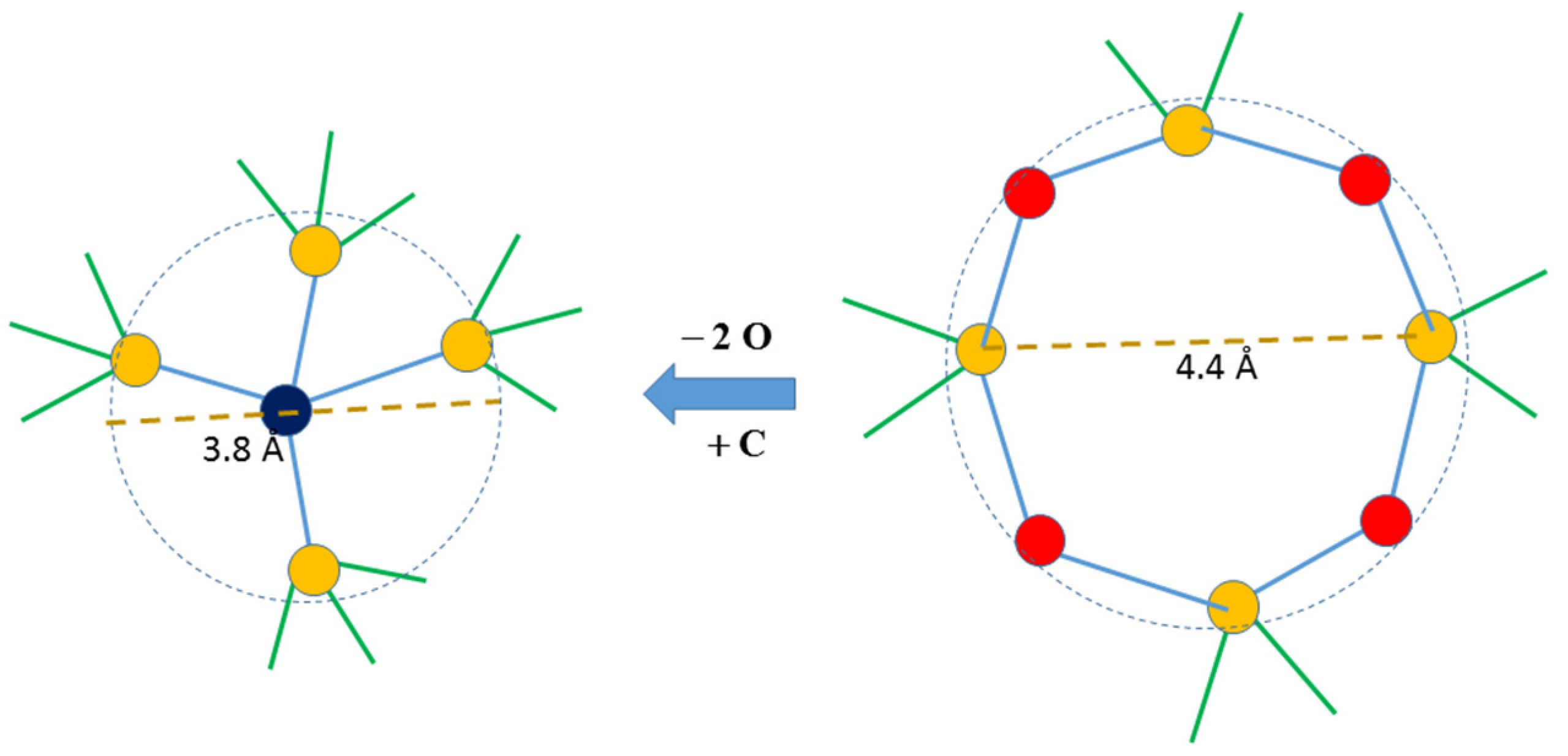
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mera, G.; Navrotsky, A.; Sen, S.; Kleebe, H.-J.; Riedel, R. Polymer-derived SiCN and SiOC ceramics–structure and energetics at the nanoscale. J. Mater. Chem. A 2013, 1, 3826–3836. [Google Scholar] [CrossRef]
- Mera, G.; Riedel, R. Polymer-Derived Ceramics: From Nanostructure to Applications; Colombo, P., Riedel, R., Sorarù, G.D., Kleebe, H., Eds.; DEStech Publications Inc.: Lancaster, PA, USA, 2009; pp. 51–89. [Google Scholar]
- Widgeon, S.J.; Sen, S.; Mera, G.; Ionescu, E.; Riedel, R.; Navrotsky, A. 29Si and13C solid-state NMR spectroscopic study of nanometer-scale structure and mass fractal characteristics of amorphous polymerderived silicon oxycarbide ceramics. Chem. Mater. 2010, 22, 6221–6228. [Google Scholar] [CrossRef]
- Widgeon, S.; Mera, G.; Gao, Y.; Stoyanov, E.; Sen, S.; Navrotsky, A.; Riedel, R. Nanostructure and energetics of carbon-rich SiCN ceramics derived from polysilylcarbodiimides: Role of the nanodomain interfaces. Chem. Mater. 2012, 24, 1181–1191. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-based polymer-derived ceramics: Synthesis, properties and applications—A review. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef]
- Gregori, G.; Turquat, C.; Kleebe, H.J.; Sorarù, G.D. Characterisation of SiCN/SiCO glasses via SEM and TEM. Key Eng. Mater. 2002, 206–213, 2061–2064. [Google Scholar] [CrossRef]
- Trimmel, G.; Badheka, R.; Babonneau, F.; Latournerie, J.; Dempsey, P.; Bahloul-Houlier, D.; Parmentier, J.; Soraru, G.D. Solid state NMR and TG/MS study on the transformation of methyl groups during pyrolysis of preceramic precursors to SiCO glasses. J. Sol-Gel Sci. Technol. 2003, 26, 279–283. [Google Scholar] [CrossRef]
- Mera, G.; Riedel, R.; Poli, F.; Müller, K. Carbon-rich SiCN ceramics derived from phenyl-containing poly(silylcarbodiimides). J. Eur. Ceram. Soc. 2009, 29, 2873–2883. [Google Scholar] [CrossRef]
- Li, Y.-L.; Kroke, E.; Riedel, R.; Fasel, C.; Gervais, C.; Babonneau, F. Thermal cross-linking and pyrolytic conversion of poly(ureamethylvinyl)silazanes to silicon-based ceramics. Appl. Organomet. Chem. 2001, 15, 820–832. [Google Scholar] [CrossRef]
- Kleebe, H.; Turquat, C.; Soraru, G.D. Phase Separation in an SiCO glass studied by transmission electron microscopy and electron energy-loss spectroscopy. J. Am. Ceram. Soc. 2001, 84, 1073–1080. [Google Scholar] [CrossRef]
- Saha, A.; Raj, R.; Williamson, D.L.; Kleebe, H.-J. Characterization of nanodomains in polymer-derived SiCN ceramics employing multiple techniques. J. Am. Ceram. Soc. 2004, 88, 232–234. [Google Scholar] [CrossRef]
- Brequel, H.; Parmentier, J.; Walter, S.; Badheka, R.; Trimmel, G.; Masse, S.; Latournerie, J.; Dempsey, P.; Turquat, C.; Desmartin-Chomel, A.; et al. Systematic structural characterization of the high-temperature behavior of nearly stoichiometric silicon oxycarbide glasses. Chem. Mater. 2004, 16, 2585–2598. [Google Scholar] [CrossRef]
- Turquat, C.; Kleebe, H.; Gregori, G.; Walter, S.; Soraru, G.D. Transmission electron microscopy and electron energy-loss spectroscopy study of nonstoichiometric silicon-carbon-oxygen glasses. J. Am. Ceram. Soc. 2001, 84, 2189–2196. [Google Scholar] [CrossRef]
- Saha, A.; Raj, R.; Williamson, D.L. A model for the nanodomains in polymer-derived SiCO. J. Am. Ceram. Soc. 2006, 89, 2188–2195. [Google Scholar]
- Kleebe, H.-J.; Blum, Y.D. SiOC ceramic with high excess free carbon. J. Eur. Ceram. Soc. 2008, 28, 1037–1042. [Google Scholar] [CrossRef]
- Gao, Y.; Mera, G.; Nguyen, H.; Morita, K.; Kleebe, H.-J.; Riedel, R. Processing route dramatically influencing the nanostructure of carbon-rich SiCN and SiBCN polymer-derived ceramics. Part I: Low temperature thermal transformation. J. Eur. Ceram. Soc. 2012, 32, 1857–1866. [Google Scholar]
- Mera, G.; Tamayo, A.; Nguyen, H.; Sen, S.; Riedel, R. Nanodomain structure of carbon-rich SiCN polymer-derived ceramics. J. Am. Ceram. Soc. 2010, 93, 1169–1175. [Google Scholar] [CrossRef]
- Bai, W.; Widgeon, S.; Sen, S. Structure and topological characteristics of amorphous silicon oxycarbide networks: Results from reverse Monte Carlo simulations. J. Non-Cryst. Solids 2014, 386, 29–33. [Google Scholar] [CrossRef]
- Pantano, C.G.; Singh, A.T.; Zhang, H. Silicon oxycarbide glasses. J. Sol-gel Sci. Technol. 1999, 14, 7–25. [Google Scholar] [CrossRef]
- Bois, L.; Maquet, J.; Babonneau, F.; Bahloul, D. Structural characterization of sol-gel derived oxycarbide glasses. 2. Study of the thermal stability of the silicon oxycarbide phase. Chem. Mater. 1995, 7, 975–981. [Google Scholar] [CrossRef]
- Blum, Y.D.; MacQueen, D.B.; Kleebe, H.J. Synthesis and characterization of carbon-enriched silicon oxycarbides. J. Eur. Ceram. Soc. 2005, 25, 143–149. [Google Scholar] [CrossRef]
- Tavakoli, A.H.; Golczewsky, J.A.; Bill, J.; Navrotsky, A. Effect of boron on the thermodynamic stability of amorphous polymer-derived Si–(B–)C–N ceramics. Acta Mater. 2012, 60, 4514–4522. [Google Scholar] [CrossRef]
- Bodart, J.R.; Bork, V.P.; Cull, T.; Fedders, P.A.; Leopold, D.J.; Norberg, R.E. Recovery of nuclear magnetization under extreme inhomogeneous broadening. Phys. Rev. B 1996, 54, 15291–15298. [Google Scholar] [CrossRef]
- Devreux, F.; Boilot, J.O.; Chaput, F.; Sapoval, B. NMR determination of the fractal dimension in silica aerogels. Phys. Rev. Lett. 1990, 65, 614–617. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.I.; Hudson, T.S. New high-density packings of similarly sized binary spheres. J. Phys. Chem. C 2011, 115, 19037–19040. [Google Scholar] [CrossRef]
- Sen, S. Spectroscopic observation of fractal packing of oxygen in variably modified glassy tetrahedral networks. J. Phys. Chem. Lett. 2014, 5, 555–559. [Google Scholar] [CrossRef]
- First, E.L.; Gounaris, C.E.; Wei, J.; Floudas, C.A. Computational characterization of zeolite porous networks: An automated approach. Phys. Chem. Chem. Phys. 2011, 13, 17339–17358. [Google Scholar] [CrossRef] [PubMed]
- Enright, M.B.; Leitner, D.M. Mass fractal dimension and the compactness of proteins. Phys. Rev. E 2005, 71. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, S.; Widgeon, S. On the Mass Fractal Character of Si-Based Structural Networks in Amorphous Polymer Derived Ceramics. Nanomaterials 2015, 5, 366-375. https://doi.org/10.3390/nano5010366
Sen S, Widgeon S. On the Mass Fractal Character of Si-Based Structural Networks in Amorphous Polymer Derived Ceramics. Nanomaterials. 2015; 5(1):366-375. https://doi.org/10.3390/nano5010366
Chicago/Turabian StyleSen, Sabyasachi, and Scarlett Widgeon. 2015. "On the Mass Fractal Character of Si-Based Structural Networks in Amorphous Polymer Derived Ceramics" Nanomaterials 5, no. 1: 366-375. https://doi.org/10.3390/nano5010366