LDH/MXene Synergistic Carrier Separation Effects to Improve the Photoelectric Catalytic Activities of Bi2WO6 Nanosheet Arrays
Abstract
:1. Introduction
2. Results and Discussion
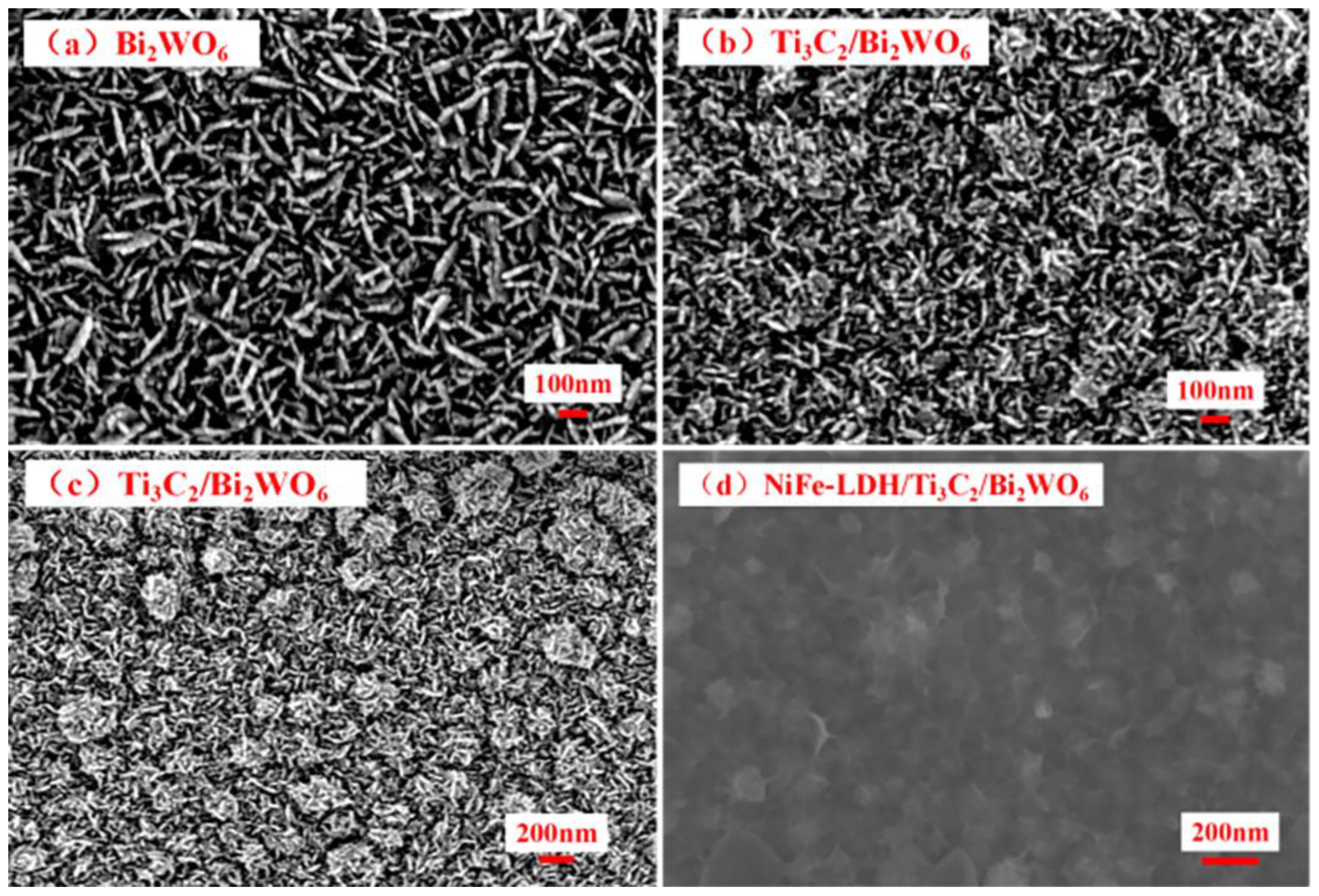
2.1. Catalyst Characterization
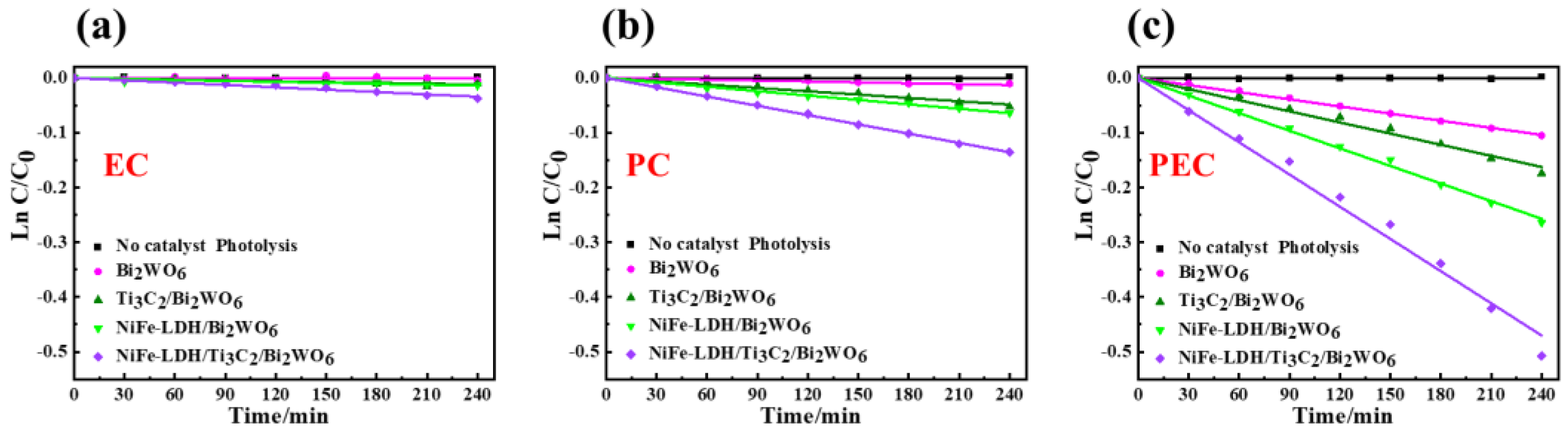
2.2. Enhanced PEC Degradation Activity
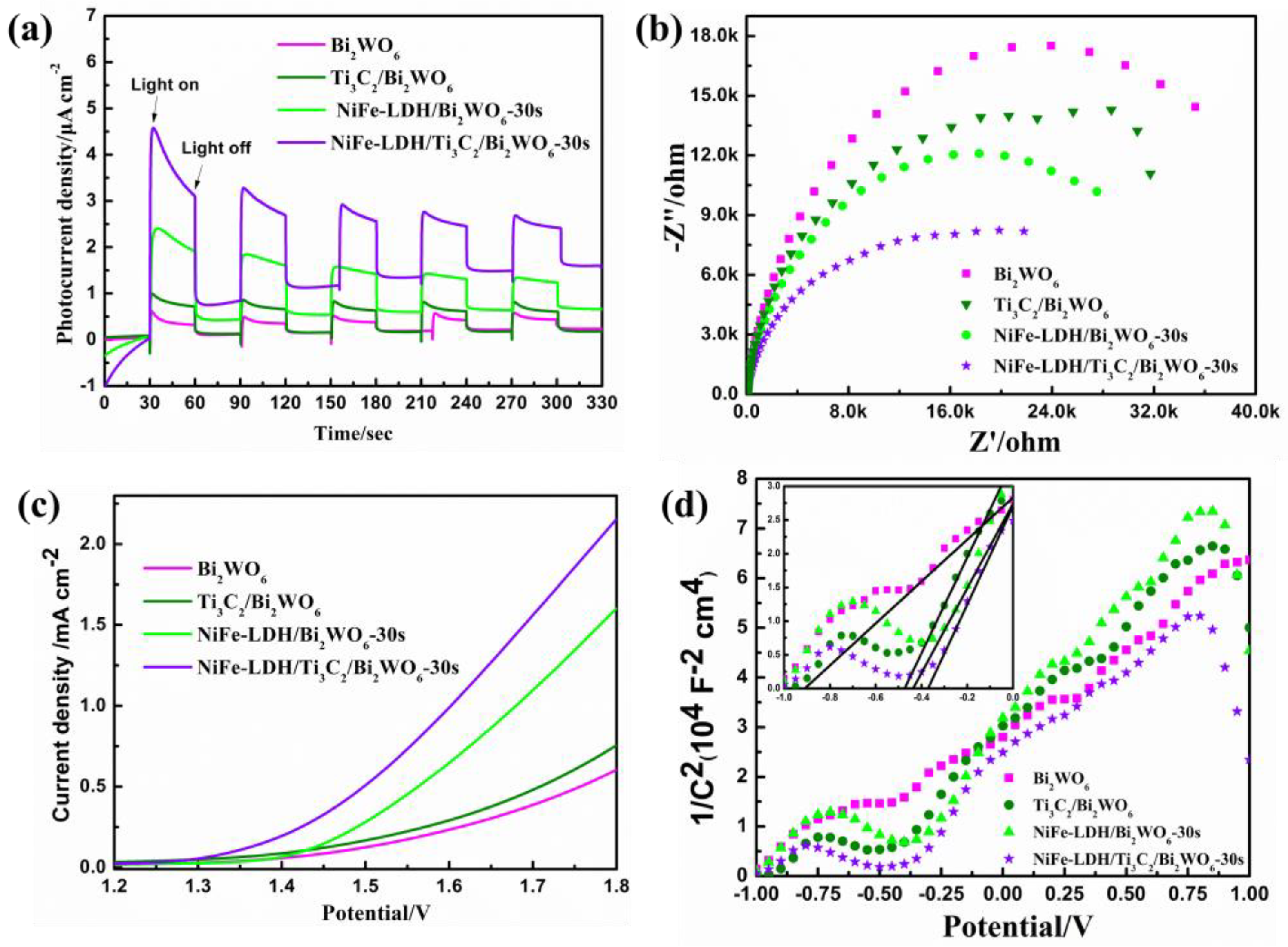
2.3. Enhancement Mechanism of Photoelectrochemical (PEC) Activity
3. Conclusions
4. Materials and Methods
4.1. Preparation of Bi2WO6 NAs Photoelectrode
4.2. Preparation of Ti3C2
4.3. Preparation of NiFe-LDH
4.4. Preparation of Ti3C2/Bi2WO6 NAs Photoelectrode
4.5. Preparation of NiFe-LDH/Bi2WO6 NAs Photoelectrode
4.6. Preparation of NiFe-LDH/Ti3C2/Bi2WO6 NAs Photoelectrode
4.7. Characterization Techniques
4.8. Degradation Activity Test
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, G.; Yang, S.; Du, B.; Luo, J.; Lin, X.; Li, X. Sono-photo hybrid process for the synergistic degradation of levofloxacin by FeVO4/BiVO4: Mechanisms and kinetics. Environ. Res. 2022, 204, 112032. [Google Scholar] [CrossRef]
- Shen, R.; He, K.; Zhang, A.; Li, N.; Ng, Y.H.; Zhang, P.; Hu, J.; Li, X. In-situ construction of metallic Ni3C@Ni core-shell cocatalysts over g-C3N4 nanosheets for shell-thickness-dependent photocatalytic H2 production. Appl. Catal. B-Environ. 2021, 291, 120104. [Google Scholar] [CrossRef]
- Fei, T.; Yu, L.; Liu, Z.; Song, Y.; Xu, F.; Mo, Z.; Liu, C.; Deng, J.; Ji, H.; Cheng, M.; et al. Graphene quantum dots modified flower like Bi2WO6 for enhanced photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2019, 557, 498–505. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Yin, W.; Shang, M.; Wang, L.; Sun, S. Inducing photocatalysis by visible light beyond the absorption edge: Effect of upconversion agent on the photocatalytic activity of Bi2WO6. Appl. Catal. B-Environ. 2010, 101, 68–73. [Google Scholar] [CrossRef]
- Ren, G.M.; Han, H.T.; Wang, Y.X.; Liu, S.T.; Zhao, J.Y.; Meng, X.C.; Li, Z.Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Bessy, T.C.; Bindhu, M.R.; Johnson, J.; Chen, S.-M.; Chen, T.-W.; Almaary, K.S. UV light assisted photocatalytic degradation of textile waste water by Mg0.8-xZnxFe2O4 synthesized by combustion method and in-vitro antimicrobial activities. Environ. Res. 2022, 204, 111917. [Google Scholar] [CrossRef]
- Wu, S.J.; Sun, J.G.; Li, Q.; Hood, Z.D.; Yang, S.; Su, T.M.; Peng, R.; Wu, Z.; Sun, W.W.; Kent, P.R.C.; et al. Effects of Surface Terminations of 2D Bi2WO6 on Photocatalytic Hydrogen Evolution from Water Splitting. Acs Appl. Mater. Interfaces 2020, 12, 20067–20074. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Sharma, S.; Sood, S.; Umar, A.; Bhinder, S.S.; Kansan, S.K. Visible Light Driven Photo-Catalytic Degradation of Fluoroquinolone Antibiotic Drug Using Bi2WO6 Spheres Composed of Fluffy Nanosheets. Nanosci. Nanotechnol. Lett. 2016, 8, 660–666. [Google Scholar] [CrossRef]
- Qi, S.; Zhang, R.; Zhang, Y.; Liu, X.; Xu, H. Preparation and photocatalytic properties of Bi2WO6/g-C3N4. Inorg. Chem. Commun. 2021, 132, 108761. [Google Scholar] [CrossRef]
- Nguyen Dang, P.; Luc Huy, H.; Guo, P.-C.; Chen, X.-B.; Chou, W.C. Study of photocatalytic activities of Bi2WO6/BiVO4 nanocomposites. J. Sol-Gel Sci. Technol. 2017, 83, 640–646. [Google Scholar] [CrossRef]
- Bai, J.; Ren, X.; Chen, X.; Lu, P.; Fu, M. Oxygen Vacancy-Enhanced Ultrathin Bi2O3-Bi2WO6 Nanosheets’ Photocatalytic Performances under Visible Light Irradiation. Langmuir 2021, 37, 5049–5058. [Google Scholar] [CrossRef]
- Li, Z.S.; Luo, M.H.; Li, B.L.; Lin, Q.C.; Liao, X.C.; Yu, H.Q.; Yu, C.L. 3-D hierarchical micro/nano-structures of porous Bi2WO6: Controlled hydrothermal synthesis and enhanced photocatalytic performances. Microporous Mesoporous Mater. 2021, 313, 110830. [Google Scholar] [CrossRef]
- Cao, P.F.; Ma, S.Y.; Xu, X.L. Novel ultra-sensitive dandelion-like Bi2WO6 nanostructures for ethylene glycol sensing application. Vacuum 2020, 181, 109748. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, K.; Zhao, Y.H.; Zhu, S.Y.; Yuan, X.; Huo, M.X.; Zhang, Y.; Qiu, Y. Bi2WO6/TiO2/Pt nanojunction system: A UV-vis light responsive photocatalyst with high photocatalytic performance. Colloids Surf. A-Physicochem. Eng. Asp. 2015, 481, 252–260. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Zang, J. Bi2MoO6 nanoflower-like microsphere photocatalyst modified by boron-doped carbon quantum dots: Improving the photocatalytic degradation performance of BPA in all directions. J. Alloys Compd. 2023, 962, 171167. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Y.F. Synthesis of square Bi2WO6 nanoplates as high-activity visible-light-driven photocatalysts. Chem. Mater. 2005, 17, 3537–3545. [Google Scholar] [CrossRef]
- Cao, S.W.; Shen, B.J.; Tong, T.; Fu, J.W.; Yu, J.G. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Ali, B.; Abdelkader, E.; Naceur, B.; Houcined, C.; Nadjia, L.; Nourredine, B. Sunlight-driven photocatalytic degradation of Rhodamine B by BiOCl and TiO2 deposited on NiCr-LDH. Int. J. Environ. Anal. Chem. 2021, 103, 6722–6741. [Google Scholar] [CrossRef]
- He, Y.; Zhou, S.; Wang, Y.; Jiang, G.; Jiao, F. Fabrication of g-C3N4@NiFe-LDH heterostructured nanocomposites for highly efficient photocatalytic removal of rhodamine B. J. Mater. Sci.-Mater. Electron. 2021, 32, 21880–21896. [Google Scholar] [CrossRef]
- Suppaso, C.; Pongkan, N.; Intachai, S.; Ogawa, M.; Khaorapapong, N. Magnetically recoverable beta-Ni(OH)2/gamma-Fe2O3/NiFe-LDH composites; isotherm, thermodynamic and kinetic studies of synthetic dye adsorption and photocatalytic activity. Appl. Clay Sci. 2021, 213, 106115. [Google Scholar] [CrossRef]
- Wang, R.; Su, S.; Ren, X.; Guo, W. Polyoxometalate intercalated La-doped NiFe-LDH for efficient removal of tetracycline via peroxymonosulfate activation. Sep. Purif. Technol. 2021, 274, 119113. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, W.; Liu, Y.; Gu, X.; Yang, T.; Hong, Q.; Li, D.; Zhang, D.; Zhou, H.; Huang, H.; et al. Ag-In-Zn-S Quantum Dot-Dominated Interface Kinetics in Ag-In-Zn-S/NiFe LDH Composites toward Efficient Photoassisted Electrocatalytic Water Splitting. Acs Appl. Mater. Interfaces 2021, 13, 42125–42137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ren, J.; Yang, X.; Chang, G.; Bu, Y.; Wei, G.; Han, W.; Yang, D. Interface engineering of 3D BiVO4/Fe-based layered double hydroxide core/shell nanostructures for boosting photoelectrochemical water oxidation. J. Mater. Chem. A 2017, 5, 9952–9959. [Google Scholar] [CrossRef]
- Aboubakr, A.E.A.; El Rouby, W.M.A.; Khan, M.D.; Farghali, A.A.; Revaprasadu, N. ZnCr-CO3 LDH/ruptured tubular g-C3N4 composite with increased specific surface area for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2020, 508, 145100. [Google Scholar] [CrossRef]
- Chen, S.; Liu, R.; Kuai, Z.; Li, X.; Lian, S.; Jiang, D.; Tang, J.; Li, L.; Wu, R.; Peng, C. Facile synthesis of a novel BaSnO3/MXene nanocomposite by electrostatic self-assembly for efficient photodegradation of 4-nitrophenol. Environ. Res. 2022, 204, 111949. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Qin, J.Z.; Yin, Z.F.; Hu, X.; Liu, B.J. 2D MXenes for Photocatalysis. Prog. Chem. 2019, 31, 1729–1736. [Google Scholar]
- Adomavičiūtė-Grabusovė, S.; Ramanavičius, S.; Popov, A.; Šablinskas, V.; Gogotsi, O.; Ramanavičius, A. Selective Enhancement of SERS Spectral Bands of Salicylic Acid Adsorbate on 2D Ti3C2Tx-Based MXene Film. Chemosensors 2021, 9, 223. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.-T.; Ma, T.-Y.; Du, A.; Qiao, S.-Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Thomas, A.M.; Vidyasagar, D.; Rao, V.N.; Yoon, S.G.; Kim, Y.-H.; Kim, S.-G.; Kim, M.-D. ZnO@Ti3C2Tx MXene Hybrid Composite-Based Schottky-Barrier-Coated SAW Sensor for Effective Detection of Sub-ppb-Level NH3 at Room Temperature under UV Illumination. ACS Mater. Lett. 2023, 5, 2739–2746. [Google Scholar] [CrossRef]
- Mahar, I.; Memon, F.H.; Lee, J.-W.; Kim, K.H.; Ahmed, R.; Soomro, F.; Rehman, F.; Memon, A.A.; Thebo, K.H.; Choi, K.H. Two-Dimensional Transition Metal Carbides and Nitrides (MXenes) for Water Purification and Antibacterial Applications. Membranes 2021, 11, 869. [Google Scholar] [CrossRef]
- Huang, K.L.; Li, C.H.; Li, H.Z.; Ren, G.M.; Wang, L.; Wang, W.T.; Meng, X.C. Photocatalytic Applications of Two-Dimensional Ti3C2 MXenes: A Review. Acs Appl. Nano Mater. 2020, 3, 9581–9603. [Google Scholar] [CrossRef]
- Huang, Z.; Zhai, H.; Li, M.; Liu, X. Effects of Ti3AlC2 Content on Cu/Ti3AlC2 Composites by Hot Pressing Method. Rare Met. Mater. Eng. 2011, 40, 529–532. [Google Scholar]
- Zhang, G.; Hu, Z.; Sun, M.; Liu, Y.; Liu, L.; Liu, H.; Huang, C.-P.; Qu, J.; Li, J. Formation of Bi2WO6 Bipyramids with Vacancy Pairs for Enhanced Solar-Driven Photoactivity. Adv. Funct. Mater. 2015, 25, 3726–3734. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Li, T.; Li, F. Bi2WO6 Nanosheets Synthesized by a Hydrothermal Method: Photocatalytic Activity Driven by Visible Light and the Superhydrophobic Property with Water Adhesion. Eur. J. Inorg. Chem. 2015, 2015, 2560–2564. [Google Scholar] [CrossRef]
- Song, X.C.; Zhou, H.; Huang, W.Z.; Wang, L.; Zheng, Y.F. Enhanced Photocatalytic Activity of Nano-Bi2WO6 by Tin Doping. Curr. Nanosci. 2015, 11, 627–632. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Y.; Hu, J.; Li, Z.; Lu, Y. Ionic liquid-assisted hydrothermal synthesis of Bi2WO6-reduced graphene oxide composites with enhanced photocatalytic activity. Rsc Adv. 2014, 4, 63238–63245. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, R.; Zhang, J.; Liu, L.; Huang, W.; Wang, Y. LDH/MXene Synergistic Carrier Separation Effects to Improve the Photoelectric Catalytic Activities of Bi2WO6 Nanosheet Arrays. Nanomaterials 2024, 14, 477. https://doi.org/10.3390/nano14050477
Wang Y, Li R, Zhang J, Liu L, Huang W, Wang Y. LDH/MXene Synergistic Carrier Separation Effects to Improve the Photoelectric Catalytic Activities of Bi2WO6 Nanosheet Arrays. Nanomaterials. 2024; 14(5):477. https://doi.org/10.3390/nano14050477
Chicago/Turabian StyleWang, Yuting, Runhua Li, Jiaying Zhang, Liming Liu, Weiwei Huang, and Yajun Wang. 2024. "LDH/MXene Synergistic Carrier Separation Effects to Improve the Photoelectric Catalytic Activities of Bi2WO6 Nanosheet Arrays" Nanomaterials 14, no. 5: 477. https://doi.org/10.3390/nano14050477




